Abstract
OBJECTIVES
Recent evidence suggests higher prevalence of autism spectrum disorder (ASD) in NICU graduates. This aim of this study was to identify retrospectively early behaviors found more frequently in NICU infants who went on to develop ASD.
METHODS
Twenty-eight NICU graduates who later received a diagnosis of ASD were compared with 2169 other NICU graduates recruited from 1994 to 2005. They differed in gender, gestational age, and birth cohort. These characteristics were used to draw a matched control sample (n = 112) to determine which, if any, early behaviors discriminated subsequent ASD diagnosis. Behavioral testing at targeted ages (adjusted for gestation) included the Rapid Neonatal Neurobehavioral Assessment (hospital discharge, 1 month), Arousal-Modulated Attention (hospital discharge, 1 and 4 months), and Bayley Scales of Infant Development (multiple times, 4–25 months).
RESULTS
At 1 month, children with ASD but not control children had persistent neurobehavioral abnormalities and higher incidences of asymmetric visual tracking and arm tone deficits. At 4 months, children with ASD had continued visual preference for higher amounts of stimulation than did control children, behaving more like newborns. Unlike control children, children with ASD had declining mental and motor performance by 7 to 10 months, resembling infants with severe central nervous system involvement.
CONCLUSIONS
Differences in specific behavior domains between NICU graduates who later receive a diagnosis of ASD and matched NICU control children may be identified in early infancy. Studies with this cohort may provide insights to help understand and detect early disabilities, including ASD.
Keywords: autism, neurodevelopment, NICU, outcomes of high-risk infants, visual function, cognitive and motor impairments
Autism spectrum disorder (ASD) is a group of devastating developmental conditions whose prevalence was reported as <1 in 1000 in the 1980s, as ~1 in 1501,2 early in this decade, and most recently as ~1 in 110.3,4 Clinically, identifying precursors to early diagnosis of ASD is of utmost importance because early intervention is especially effective.5–7 Whether attributable to changes in diagnostic criteria,8 its comorbidity with other developmental disabilities,9 or a true increase in cases, the cause of ASD remains largely unknown, although environmental toxins10,11 and genetic effects12–16 have been implicated. Lack of reliable early developmental signs and seeming late emergence have further complicated early detection and treatment. Initially, there were no prospective methods to study early events in children who go on to develop ASD until investigators began recruiting and studying younger siblings of children with ASD, who are at much higher risk for developing ASD.17–21
Higher prevalence of ASD also has been associated with obstetric and neonatal factors that result in NICU admission. 22–34 These include preterm birth and indicators of obstetric (eg, prenatal infection, maternal bleeding, growth restriction), birth (eg, fetal distress, hypoxia, low Apgar scores), and postnatal (eg, respiratory distress, intraventricular hemorrhage) complications. Even after controlling for other developmental disorder outcomes, Schendel and Bhasin34 found a twofold increased ASD risk as a result of lower birth weight and gestational age (GA). Although these studies identified clinical risk factors for later ASD diagnosis, none examined very early behavioral patterns that may more likely be seen in children who later receive a diagnosis of ASD. Theoretically, identification of early behavioral risk factors could be a major contribution toward improving outcome through earlier detection and intervention. We conducted serial behavioral studies from birth on a large number of NICU graduates at high medical risk as part of a prospective project to determine how brain organization interacts with autoregulatory processes over development.35–37 In the total sample, our early measures involving arousal, attention, and motor regulation predicted deficits in a number of domains. This retrospective report examines early developmental differences between infants who later received a diagnosis of ASD and matched control subjects from our sample of NICU graduates.
METHODS
Participants
Health histories and test performance that were collected at each follow-up visit from NICU graduates who were in our prospective studies and born between August 1994 and July 2005 (N = 2197) were reviewed. Twenty-eight children were classified as having ASD: 20 as having ASD by using multiple criteria (Autism Diagnostic Observation Schedule–Generic,38,39 and PDD Behavior Inventory40,41) at our facility and/or by Dr Cohen and 8 as having multiple clinical sources according to parent report (NYC Early Intervention Program, Board of Education, pediatric neurology, developmental pediatrics; Appendix). ASD prevalence was 1.3% (1 of 79) and falls just within published prevalence of ASD in NICU graduates (1.3%–3.2%)26,34 but may represent an underestimation of ASD incidence, especially in the earliest years. Recruitment criteria for our prospective studies of arousal and attention emphasized risk for developmental disabilities as a result of increased probability of central nervous system (CNS) injury and included: low birth weight (<1800 g); fetal distress (Apgar score <5 at 1 minute or <7 at 5 minutes; pH <7.2); intraventricular hemorrhage;42 hypoxic ischemic encephalopathy; assisted ventilation >48 hours; persistent apnea/bradycardia; seizures/coma/signs of increased intracranial pressure; abnormal neurologic signs (eg, poor feeding, persistent hypotonia, spasticity, tremors without metabolic or drug-related cause); multiple gestation; and small for GA (<10th percentile birth weight for GA or dysmature by clinical diagnosis). Exclusion criteria were congenital/ chromosomal defects, HIV, and prenatal exposure to drugs of abuse. The research protocol was approved by the institutional review boards of all participating institutions, and written informed consents were obtained.
APPENDIX.
Individual Characteristics in ASD Sample
| Patient | GA, wk | Gender | Maternal Age, y | Maternal Education, y | Diagnosis | Diagnosis Age, moa | Diagnosis by | Siblings |
|---|---|---|---|---|---|---|---|---|
| 1 | 38 | Male | 35.6 | 12 | PDD | 72 | IBRclinic; Dev Ped | 1 boy, 1 girl, no ASD |
| 2 | 38 | Male | 31.2 | 12 | PDD | 28 | IBRclinic; EI; BOE | 2 girls, no ASD |
| 3 | 34 | Male | 32.3 | 17 | Autism | 46 | ILC | 0 |
| 4 | 25 | Male | 37.1 | 12 | PDD | 34 | IBRclinic | ? |
| 5 | 36 | Female | 38.0 | 13 | Autism | 31 | ILC | 1 girl, suspected |
| 6 | 38 | Male | 16.8 | 9 | PDD | 28 | EI; BOE; Dev Ped | 0 |
| 7 | 32 | Male | 31.3 | 14 | PDD | 42 | IBRclinic; Dev Ped | 1 boy, no ASD |
| 8 | 40 | Male | 33.1 | 16 | Autism | 44 | IBRclinic; autismEI | ? |
| 9 | 28 | Male | 35.2 | 12 | Autism | 34 | Ped Neuro; autismEI | 4, no information |
| 10 | 32 | Male | 33.0 | 14 | Autism | 46 | ILC; autismEI | 1 girl, twin, no ASD |
| 11 | 33 | Male | 34.7 | 16 | Autism | 25 | EI; BOE | 1 girl, twin, suspected, lang; 1 girl, suspected, DD |
| 12 | 26 | Male | 20.5 | 12 | PDD | 67 | IBRclinic; BOE | 1 boy, no ASD |
| 13 | 26 | Female | 34.6 | 18 | Autism | 18 | IBRclinic; EI | 1 girl, no ASD |
| 14 | 32 | Male | 34.6 | 12 | Autism | 42 | IBRclinic | 1 girl, no ASD |
| 15 | 27 | Male | 35.8 | 14 | PDD | 25 | Ped Neuro; EI | 1 girl, twin, anxiety disorder |
| 16b | 30 | Male | 30.3 | 16 | Autism | 16 | IBRclinic; ILC | 2 boys, triplets, autism |
| 17b | 30 | Male | 30.3 | 16 | Autism | 16 | IBRclinic; ILC | 2 boys, triplets, autism |
| 18b | 30 | Male | 30.3 | 16 | Autism | 16 | IBRclinic; ILC | 2 boys, triplets, autism |
| 19 | 36 | Female | 33.0 | 18 | PDD | 22 | IBRclinic; ILC | 1 girl, twin, no ASD |
| 20 | 38 | Male | 34.0 | 14 | PDD | 34 | Ped Neuro; EI; BOE | 2 boys, 1 girl, no ASD |
| 21 | 35 | Male | 33.3 | 14 | Autism | 34 | IBRclinic; ILC | 1 boy, triplet, DD; 1 girl, triplet, no ASD |
| 22 | 34 | Female | 28.7 | 18 | PDD | 22 | IBRclinic; Ped Neuro; EI | 0 |
| 23 | 34 | Male | 34.9 | 16 | PDD | 25 | EI; BOE | 1 girl, twin, 2 girls, no ASD |
| 24 | 29 | Male | 21.1 | 14 | Autism | 25 | IBRclinic; Ped Neuro; EI | 1 boy, no ASD |
| 25 | 37 | Male | 35.2 | 18 | PDD | 28 | ILC; Dev Ped | 1 boy, twin, suspected; 1 boy, no ASD |
| 26 | 37 | Female | 32.5 | 18 | Autism | 28 | IBRclinic; ILC | 1 girl, autism |
| 27 | 28 | Male | 23.0 | 12 | Autism | 27 | EI; BOE | 1 boy, no ASD |
| 28 | 30 | Male | 29.8 | 18 | PDD | 19 | Ped Neuro; EI | 1 girl, no ASD |
PDD indicates pervasive developmental disorder; IBRclinic, diagnostic clinic located at the NYS Institute for Basic Research in Developmental Disabilities; Dev Ped, developmental pediatrician; Ped Neuro, pediatric neurologist; DD, developmental disability; EI, Early Intervention services; autismEI, EI program specifically designed for children with an ASD diagnosis, BOE, Board of Education; ILC, co-author Cohen
Diagnosis age typically is age at clinical diagnosis, although in almost all cases there were strong suspicions and even intervention before clinical diagnosis.
Patients 16, 17, and 18 are triplets, all with an autism diagnosis.
Behavioral Studies
Studies reported here include (1) neurobehavioral characteristics during the neonatal period (Rapid Neonatal Neurobehavioral Assessment [RNNA]43–45) at hospital discharge and 1 month postterm age (PTA); (2) regulation of visual attention by states of arousal (Arousal-Modulated Attention [AModA] procedure35–37) at hospital discharge, 1 and 4 months PTA; and (3) individual developmental trajectories from standardized assessments across age (Bayley Scales of Infant Development, Second Edition [BSID-II]: Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI)46) from 4 through 25 months’ PTA. PTA was used to adjust age for preterm gestation for testing and subsequent analyses.
Rapid Neonatal Neurobehavioral Assessment
The RNNA is a criterion-referenced procedure. On the basis of normal versus abnormal decisions for individual items, it yields scores for categories of sensory and motor behaviors that measure visual and auditory attention and symmetry; head/neck control; trunk tone; extremity movement, tone, and symmetry; state control; feeding; and jitteriness at each age tested. It also yields a composite score that reflects number and/or severity of problems at each age. Infants with no detected CNS injury should perform perfectly (ie, no abnormalities). Concurrent validity43–45 is evidenced by positive relation between severity of CNS injury detected in the neonatal period and the number of abnormalities identified. By 1 month PTA, all infants show fewer abnormalities; infants with more severe CNS injury still show a higher number of abnormalities, whereas the number in infants with less severe CNS injury approaches that of noninjured infants. Predictive validity47 is seen in the relation of RNNA performance to BSID-II46 through 25 months and to Griffiths Mental Development Scales48 from 28 to 42 months.
Arousal-Modulated Attention
The AModA procedure measures an infant’s ability to modulate his or her visual attention to variations in stimulation when tested at higher and lower levels of exogenous or endogenous arousal. A visual stimulus preference is established when the infant looks longer to 1 stimulus than another49 when the stimuli are ordered along some systematic environmental change (eg, frequency, intensity, complexity). Specifically, infants view all possible pairs of stimuli going on and off at 1, 3, or 8 Hz for 6 trials of 15 seconds in each of 3 arousal conditions. Healthy term and preterm neonates are excellent modulators and show greater attention to more stimulating events when less aroused (after feeding) and to less stimulating events when more aroused (before feeding or with added stimulation before each trial). Neonates with acute CNS injury are poor modulators and tend to prefer less stimulation even when less aroused. By 4 months’ PTA, transitions occur such that the AModA effect no longer is apparent and more stimulation tends to be preferred (although attenuated) in all conditions irrespective of arousal. Moreover, typically, there is normalization such that AModA differences no longer are evident from CNS injury.36,37,50 As with RNNA, AModA has both concurrent (associated with CNS injury) and predictive validity to later functioning (associated with BSID-II MDI and PDI and Griffiths Mental Development Scales).37
Bayley Scales of Infant Development, Second Edition
Beginning at 4 months’ PTA, BSID-II MDI and PDI were administered through 25 months’ PTA, targeting tests at 3-month intervals. MDI and PDI scores were used to establish individual developmental trajectories for standardized performance across age.
Statistical Analyses
Univariate or multivariate analysis of variance and covariance (general least-square F), likelihood ratio χ2, logistic regression (logit; Stata51), conditional logistic regression controlled for matching variables (clogit; Stata), or ordered logistic regression (for CNS injury, ologit; Stata) as appropriate were used to study clinical characteristics, RNNA, and AModA. Multivariate analyses of time series using generalized estimating equations (xtgee; Stata) along with clogit, evaluated BSID-II longitudinal performance. Adjustment for GA was carried through all analyses.
RESULTS
Clinical Characteristics
Demographic, prenatal, intrapartum, and postnatal clinical data from infants with ASD were compared with data from other NICU infants who were similarly recruited and tested behaviorally during the same period. From these data, 6 major factors that typically are associated with NICU outcomes or ASD were analyzed: gender, GA, birth cohort (birth before versus after 2000), birth weight, relative intrauterine growth restriction (z score transformation of infant’s birth weight for GA on the basis of growth curves published by Fenton52), and severity of CNS insult. Other potentially associated risk factors also were analyzed: head circumference, length, Apgar score at 1 and 5 minutes, length of hospital stay, multiple gestation, maternal age and education, and race/ethnicity. Appendix provides additional information for each patient with ASD.
Children With Versus Without ASD
Results for children with and without ASD for the aforementioned variables and for ages at testing for early behavioral studies are presented in Table 1. Analysis of major risk factors indicated that children with ASD were more likely to be male, similar to the 4-to-1 male-female ratio typically reported. 2–4 ASD prevalence for infants who were born after the year 2000 was marginally greater, but no difference in proportion of multiple-gestation pregnancies was found. GA and birth weight but not relative intrauterine growth restriction were significantly lower in children with ASD, and children with ASD were significantly more likely to have very low GA (≤32 weeks) or birth weight (≤1500 g), consistent with others’ reports.24,30,34 Degree of CNS injury and most clinical risk factors individually distinguished ASD, but multiple regression analyses indicated that they were highly correlated with gender, GA, and potentially birth cohort.
TABLE 1.
Clinical Risk Factors in NICU Children With Versus Without ASD and Matched Control Subjects
| Risk Factor | ASD | Non-ASD Total | Non-ASD Controls | ASD vs Non-ASD Totala
|
ASD vs Non-ASD Matched Controlsb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | Statistic | Pb | OR | 95% CI | Statistic | Pb | ||||||
| N = 2197 | 28 | 2169 | 112 | ||||||||||
| Male gender, % | 82.1 | 54.4 | 84.8 | 3.85 | 1.46 to 10.16 |
|
.003 | 0.82 | 0.28 to 2.46 |
|
.730 | ||
| GA, wk | |||||||||||||
| Mean (SD) | 32.6 (4.3) | 34.6 (3.8) | 33.1 (4.3) | F1,2195 = 7.65 | .006 | F1,138 = 0.31 | .580 | ||||||
| Range | 25 to 40 | 23 to 42 | 23 to 41 | ||||||||||
| ≤32 wk, % | 50.0 | 26.0 | 34.8 | 2.84 | 1.34 to 5.99 |
|
.008 | 1.87 | 0.81 to 4.32 |
|
.150 | ||
| Birth year (cohort) Jan 2000 or later, % | 64.3 | 48.4 | 59.8 | 1.92 | 0.88 to 4.18 |
|
.092 | 1.20 | 0.51 to 2.86 |
|
.670 | ||
| Birth weight, g | |||||||||||||
| Mean (SD) | 1836 (926) | 2266 (898) | 2045 (931) | F1,2195 = 8.24 | .005 | 0.99 | 0.99 to 1.00 | z = −1.22 | .220 | ||||
| Range | 569 to 4167 | 397 to 5684 | 397 to 4082 | ||||||||||
| ≤1500 g, % | 46.4 | 20.2 | 29.5 | 3.43 | 1.62 to 7.25 |
|
.002 | 2.07 | 0.89 to 4.84 | z =1.69 | .100 | ||
| RIUG, z | |||||||||||||
| Mean (SD) | −0.88 (1.56) | −0.71 (1.44) | −0.52 (1.46) | F1,2195 = 0.37 | .544 | 0.84 | 0.62 to 1.14 | z = −1.11 | .260 | ||||
| Range | −4.92 to 2.70 | −4.94 to 6.65 | −3.69 to 5.61 | ||||||||||
| ≤10%, z ≤ −1.29, %c | 35.7 | 32.8 | 25.4 | 1.14 | 0.52 to 2.48 |
|
.748 | 1.44 | 0.60 to 3.46 | z = 0.81 | .420 | ||
| Head circumference, cm | |||||||||||||
| Mean (SD) | 29.2 (3.9) | 31.2 (3.3) | 30.2 (3.5) | F1,2011 = 9.23 | .003 | 0.86 | 0.64 to 1.16 | z = −0.98 | .330 | ||||
| Range | 22.0 to 36.0 | 19.5 to 39.5 | 20.5 to 36.2 | ||||||||||
| Length, cm | |||||||||||||
| Mean (SD) | 41.6 (6.5) | 44.6 (5.2) | 43.1 (5.6) | F1,2011 = 8.88 | .003 | 0.94 | 0.79 to 1.10 | z = −0.80 | .430 | ||||
| Range | 31.0 to 56.0 | 27.0 to 63.0 | 28.5 to 55.0 | ||||||||||
| Apgar score | |||||||||||||
| 1 min | |||||||||||||
| Mean (SD) | 6.4 (2.5) | 7.2 (1.9) | 6.7 (2.1) | F1,2136 = 4.67 | .031 | 0.96 | 0.78 to 1.17 | z = −0.44 | .660 | ||||
| Range | 0 to 9 | 0 to 9 | 1 to 9 | ||||||||||
| 5 min | |||||||||||||
| Mean (SD) | 7.8 (1.2) | 8.1 (1.2) | 7.9 (1.2) | F1,2136 = 1.44 | .240 | 1.04 | 0.70 to 1.55 | z = 0.21 | .830 | ||||
| Range | 4 to 9 | 0 to 10 | 3 to 9 | ||||||||||
| Days in hospital | |||||||||||||
| Mean (SD) | 36.5 (29.5) | 21.7 (24.1) | 30.1 (31.8) | F1,2137 = 10.30 | .002 | 1.01 | 0.99 to 1.04 | z = 1.13 | .260 | ||||
| Range | 5 to 98 | 2 to 185 | 3 to 178 | ||||||||||
| Singleton, % | 60.7 | 68.8 | 62.5 | 0.70 | 0.33 to 1.50 |
|
.380 | 0.85 | 0.24 to 3.01 | z = −0.25 | .800 | ||
| CNS insult, %d | 0.21 | 1.06 to 3.98 |
|
.034 | 1.17 | 0.72 to 1.93 | z = 0.65 | .650 | |||||
| No identified CNS insult | 25.0 | 44.9 | 33.9 | ||||||||||
| Abnormal ABR, normal CUS | 42.9 | 34.5 | 38.4 | ||||||||||
| Mild CNS insult | 14.3 | 9.5 | 12.5 | ||||||||||
| Moderate/severe CNS insult | 17.9 | 11.1 | 15.2 | ||||||||||
| Maternal age at delivery, y | |||||||||||||
| Mean (SD) | 31.6 (5.4) | 30.6 (6.2) | 31.5 (5.9) | F1,1521 = 0.65 | .430 | 1.00 | 0.93 to 1.08 | z = 0.11 | .910 | ||||
| Range | 16.8 to 41.0 | 14.5 to 50.0 | 16.1 to 41.6 | ||||||||||
| Maternal education, y | |||||||||||||
| Mean (SD) | 14.8 (2.6) | 14.0 (2.7) | 14.4 (2.7) | F1,1292 = 2.24 | .140 | 1.05 | 0.89 to 1.22 | z = 0.54 | .590 | ||||
| Range | 9.0 to 18.0 | 4.0 to 20.0 | 6.0 to 20.0 | ||||||||||
| Maternal education ≥16 y, % | 46.4 | 22.5 | 46.5 | 2.97 | 1.40 to 6.28 |
|
.006 | 1.08 | 0.91 to 1.26 | z = 0.87 | .380 | ||
| Race/ethnicity, %e | |||||||||||||
| White | 85.7 | 60.8 | 51.8 | 3.94 | 1.36 to 11.40 |
|
.003 | 7.71 | 2.10 to 28.30 | z = 3.08 | .002 | ||
| Black | 10.7 | 21.7 | 30.4 | 1.37 | 0.13 to 1.44 |
|
.130 | 0.19 | 0.04 to 0.83 | z = −2.21 | .029 | ||
| Postconceptional age at testing, wk | |||||||||||||
| NICU test, wk | |||||||||||||
| Mean (SD) | 37.5 (2.7) | 37.7 (2.5) | 37.5 (2.5) | F1,2051 = 0.05 | .830 | 1.03 | 0.83 to 1.26 | z = 0.25 | .800 | ||||
| Range | 33.4 to 44.7 | 32.3 to 48.3 | 33.6 to 45.0 | ||||||||||
| 1-Mo PTA, wk | |||||||||||||
| Mean (SD) | 44.6 (2.0) | 44.4 (1.6) | 44.2 (1.9) | F1,1555 = 0.58 | .450 | 1.11 | 0.90 to 1.38 | z = 1.00 | .320 | ||||
| Range | 41.3 to 49.6 | 41.1 to 54.1 | 41.9 to 54.0 | ||||||||||
| 4-Mo PTA, wk | |||||||||||||
| Mean (SD) | 58.9 (1.2) | 58.8 (1.4) | 58.8 (1.3) | F1,1336 = 0.12 | .730 | 1.02 | 0.66 to 1.59 | z = 0.10 | .930 | ||||
| Range | 57.4 to 62.1 | 53.7 to 65.3 | 54.7 to 64.4 | ||||||||||
RIUG indicates relative intrauterine growth restriction; ABR, auditory brainstem response; CUS, cranial ultrasound.
ASD versus total; statistic denotes likelihood ratio χ2 or general least-square F as appropriate (logistic regression: logit; STATA; or for CNS injury, ordered logistic regression: ologit; STATA).
ASD versus matched control; where the matched control is a subsample of total. OR, CI, and likelihood ratio χ2 or log logistic z score (conditional logistic regression: clogit; STATA) controlled for gender, GA, and birth year.
Z score normalized value = (observed birth weight – expected birth weight for GA)/(SD of expected birth weight for GA), where the expected birth weight and expected SD of birth weight for GA are based on growth curves published by Fenton.47
CNS insult was defined as any identified structural abnormality detected primarily by CUS and also by computerized tomography and MRI; or any identified functional abnormality detected primarily by ABR. Abnormal ABR was defined by delayed component latencies of wave I, III, or V or prolonged interlatency interval of waves III to V on first NICU test on the basis of laboratory norms. Mild CNS insult was defined by any suspicious finding, small choroid cyst(s), or grade of intraventricular hemorrhage = 1 according to Papile et al.42 Moderate/severe CNS insult was defined by grade of intraventricular hemorrhage ≥2 and/or evidence of CNS tissue loss.
The proportion of white or black race/ethnicity in the matched control group without ASD differed from the proportion of white or black race/ethnicity in the total NICU infants without ASD studied (white: χ21 = 3.65, P < .056; black: χ21 = 4.33, P < .038), but to a lesser extent, both groups without ASD differed from the group with ASD. Proportions of other races/ethnicities were too sparse for accurate assessment.
Matched Control Group
The aforementioned analyses suggested the need for controlling potential confounds of gender, GA (or birth weight), and cohort effects before intergroup behavioral comparisons associated with later diagnosis of ASD. We selected a 4-to-1 control sample53 (4 children without ASD from total NICU sample for each child with ASD; n = 112) to maximize statistical power, matching for gender, GA, and year of birth (cohort effect). As seen in Table 1, match was verified by logistic regression indicating no differences between children with ASD and control subjects on gender, GA, and birth cohort (P > .57). Conditional logistic regression for remaining clinical risk factors, controlling for many residual effects of matching variables by matched sets, also indicated no differences between children with ASD and control subjects (P > .10). Racial/ethnic minority distribution (higher percentage of white and lower percentage of black in children with ASD) was similar to that reported by the Centers for Disease Control and Prevention4 for children with ASD and does not affect results for behavioral comparisons reported here.
Behavioral Comparisons Between Children With ASD and Control Subjects
First 4 Months
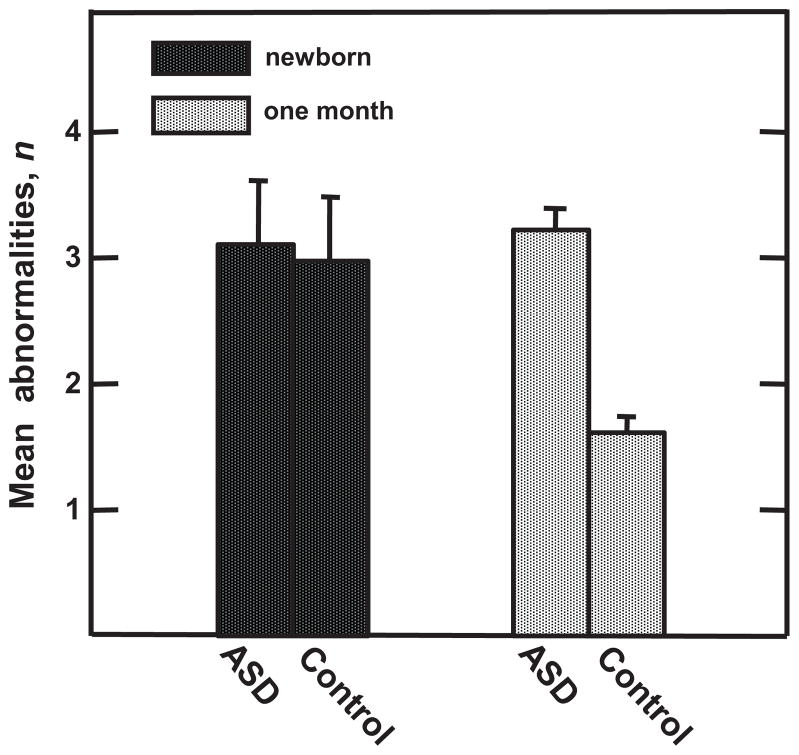
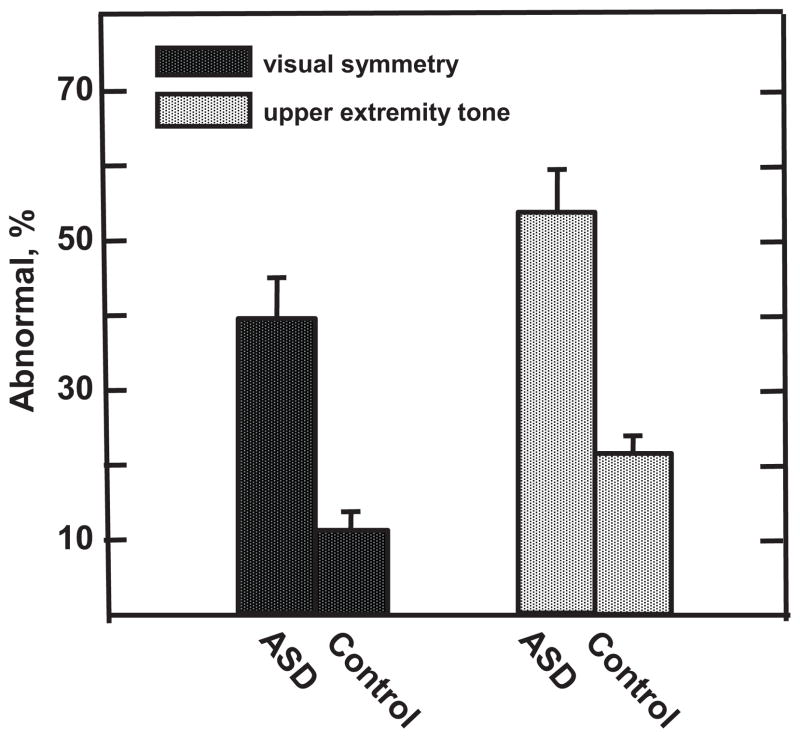
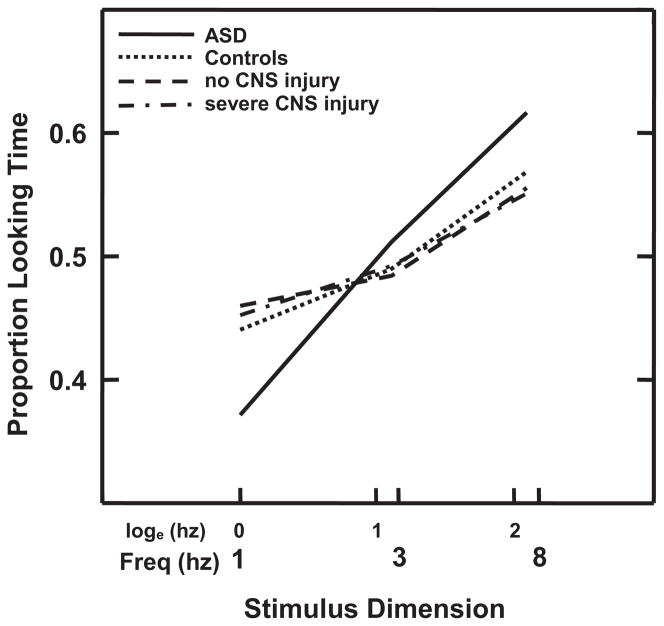
No RNNA or AModA differences were detected at hospital discharge between children with ASD and control subjects (z less than −0.74 to 1.22, P > .22). Unlike the improved performance in the control sample by 1 month PTA, children with ASD did not show significant reduction in number of RNNA abnormalities with age (OR: 1.64 [95% CI: 1.24–2.16]; z = 3.50, P < .0003; Fig 1). Specifically, at 1 month PTA, a higher percentage of children with ASD displayed abnormalities as indicated by asymmetric visual tracking (39.3% vs 10.5%; OR: 15.20 [95% CI: 2.98–77.35]; z = 3.28, P < .001) and by abnormal upper extremity tone (53.6% vs 21.9%; OR: 4.45 [95% CI: 1.70–11.64; z = 3.05, P < .002; Fig 2). Differences in AModA were found between children with ASD and control subjects at 4 months’ PTA but not at younger ages. At this age, children with ASD showed a greater preference for higher frequency stimulation when less aroused than their control subjects (OR: 1.53 [95% CI: 1.02–2.28]; z = 2.10, P < .035), performing more like neonates than 4-month-olds (Fig 3).
FIGURE 1.
Differences in recovery on RNNA from newborn (hospital discharge) to 1 month PTA. Reduction in mean number of RNNA abnormalities in control subjects versus children with ASD reflects improved performance in control subjects but not children with ASD across the neonatal period.
FIGURE 2.
Differences in incidence of specific RNNA abnormalities at 1 month PTA. Higher percentage of children with ASD versus control subjects show visual asymmetry and hypertonicity or hypotonicity in upper extremity tone, which may be early precursors of ASD-associated visual function and motor deficits seen at older ages.
FIGURE 3.
Differences in AModA stimulus preferences at 4 months’ PTA. Higher percentage looking to fastest (8 Hz) frequency in children with ASD versus control subjects, children with no CNS injury, and children with severe CNS injury (no differences among infants without ASD) indicates persistent preference for increased stimulation in ASD, more like newborns than 4-month-olds. Percentage looking plotted as a function of logeHz to equalize stimulus intervals across Hz.
Standardized Performance: 4 to 25 Months’ PTA
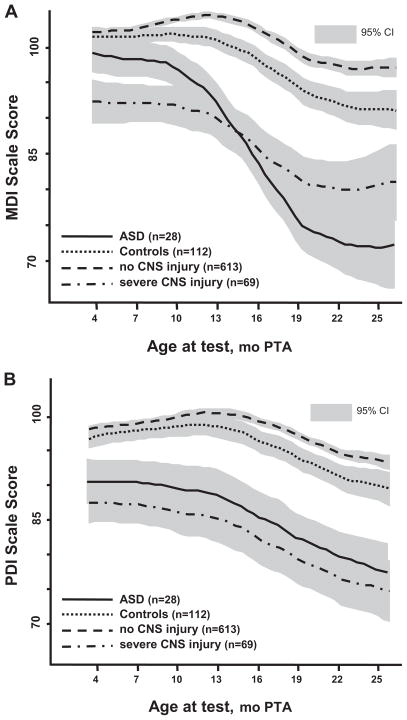
An average of 6.4 tests per infant were used to evaluate performance over age for BSID-II. The best fit polynomials and 95% CI for age curves are plotted for MDI (Fig 4A) and PDI (Fig 4B) for children with ASD and control subjects and, for comparison, no detectable CNS injury and severe CNS injury in the children without ASD. Generalized estimating equations analyses, controlling for gender, GA, and birth cohort, indicated that children with ASD compared with control subjects showed a significantly sharper decline across age for both MDI (95% CI: −8.97 to −5.70; z = −6.05, P < .0001) and PDI (95% CI: −14.68 to −6.46; z = −5.04, P < .0001). These differences were significant as early as 10 months’ PTA for the MDI and 7 months’ PTA for the PDI. Because children with ASD scored significantly higher on the MDI than did infants with CNS injury at younger ages and declined only when language and cognitive skills were required and behavioral issues could interfere with performance, it is unlikely that we were dealing with infants with severe global impairment.
FIGURE 4.
BSID-II MDI (A) and PDI (B) for children with ASD, control subjects, children with no CNS injury, and children with severe CNS injury across ages from 4 to 25 months’ PTA. Nonoverlapping 95% CIs indicate significant differences. A, MDI: Children with ASD versus control subjects and children with no CNS injury show a sharper decline in performance across age, with differences significant by 10 months’ PTA. Children with ASD start higher, similar to control subjects and children with no CNS injury, and end lower than children with severe CNS injury, but apparent crossover effect is not significant. B, PDI: Children with ASD versus control subjects and children with no CNS injury also show a sharper decline across age in performance (but less than for MDIs), with differences significant by 7 months’ PTA. Children with ASD start and end lower than control subjects and children with no CNS injury and are most similar to children with severe CNS injury throughout.
DISCUSSION
Consistent with others’ reports, a substantial subset of our cohort of NICU graduates subsequently received an ASD diagnosis. As generally noted, they were 4 times more likely to be male. They were ~2 weeks younger at birth, but their distribution of types and severity of CNS injury was similar to that in other NICU infants after controlling for GA.
Findings of poor recovery of function between hospital discharge and 1 month PTA, accompanied by asymmetric visual tracking and abnormal upper extremity tone, suggest that such atypical behaviors may be very early markers of ASD, although it is unknown whether they are more specific to ASD or associated similarly with other developmental disorders. We speculate that attention to different stimulus features may stimulate different areas/timing of brain development underlying visual processing, with consequences for subsequent development. 54,55 Continuing higher attention to more stimulating events of infants with ASD at 4 months represents a lack of transition to more mature levels past the neonatal period, providing evidence for early atypical development, including the visual system. Similar conclusions have been reached regarding the importance of abnormally developed/organized neurally mediated visual tracking and attention systems56 and the importance of abnormal transitions in development of pathways that involve visual processing.57 In infants, atypical attention to stimuli and visual regard, impaired disengagement ability, and lack of typical developmental transitions in the visual system have been proposed to result in disruption of the normal bias toward social events, especially those imbedded in complex socially relevant stimuli.18,41,58 Unusual visual function also has been reported in older infants and children with ASD. Klin and associates59 reported that absent preferential looking to the eyes of an approaching adult in 2-year-old toddlers with ASD is related to increased level of social disability and impaired recognition of biological motion60 as early as 15 months.61 That group also recently reported evidence that 20-month-old toddlers with ASD were still bound by physical audiovisual synchrony, whereas control subjects without ASD ignored this synchrony and attended to more socially relevant stimuli.62 Evidence of atypical visual system development also could be reflected in the hypersensitive visual acuity reported for high-functioning adults with ASD/Asperger syndrome.63 The sustained preference for higher frequencies may reflect that higher rates of stimulation are necessary for optimal arousal and reward. One underlying mechanism might involve an endogenous lower arousal level with decreased available dopamine receptors to respond to stimulation, thereby requiring greater stimulation to elicit an adequate dopamine response. 64 Such a mechanism was suggested by increased preference for stimulation in cocaine-exposed neonates65 and reduced cell size in the nucleus accumbens in children with ASD,66 potentially altering reward responses.
Repetitive motor behavior and stereotypy help to define ASD,67 and there are indications of apraxia and abnormal basic motor skills68 possibly related to deficits in connectivity and cerebellar activity.69 Signs of early motor problems during infancy stem mainly from retrospective analyses of video recordings. 70,71 Our findings extend these to the neonatal period. Atypical upper extremity tone may interfere with typical use, especially as reaching to objects and gesturing emerge. We speculate that such deficits in tone, especially in combination with visual tracking errors, could result in suppression of manual exploration or in poor development of fine motor skills, gesturing, or joint attention. 58,72–74 Although these motor deficits are apparent in a number of developmental disorders, it is not known whether their co-occurrence with visual deficits make the path of the developing visual-motor system more or less unique to ASD. Consequences of these very early sensory and motor signs may be an arrest in performance beginning as early as 7 to 10 months’ PTA such that cognitive and motor development as measured by standardized instruments is significantly slowed or suppressed.
Autism is heterogeneous in cause. Our report helps to confirm a substantial prevalence of ASD in NICU graduates, making them important to study. Whether the NICU infants studied are typical of other ASD-affected infants who lack early medical issues is not clear. It also is not clear whether this group is emergent within the increasing cohort of surviving very tiny and preterm infants or identified through more recent changes in diagnostic criteria and better surveillance. Perhaps most important, the early identification of precursor behaviors might lead to greater understanding and insights into the nature of early deficits that lead to later ASD.
The aim of the parent study was to understand development of arousal and attention regulation in infants with high medical risk and how deficits in regulation underlie atypical development in multiple domains not specific to ASD. We did not include studies of social, communication, and language development as might be expected in investigations that are designed to identify early precursors of ASD. The ASD sample size was small, and not all children with ASD received clinical diagnoses; hence, we view results relative to control subjects as conservative. The ASD sample was at risk for multiple developmental problems, creating the necessity to select amatched control sample also at risk for these issues but not having a diagnosis of ASD. Findings may therefore not be specific to all children with ASD and emphasize the need to replicate these retrospective findings with more directed prospective studies.
In summary, this study identified potential early precursor behaviors that are associated with later diagnosis of ASD and could address mechanisms that lead to the emergence of ASD. We have known for some time that a large proportion of infants with developmental disabilities sustained pregnancy and birth complications. In this report, specific behaviors were studied for their link to CNS compromise in NICU infants at high risk for multiple adverse outcomes rather than for their suspected link to ASD. Retrospectively, we found specific behavior patterns that were more frequent in infants who later received a diagnosis of ASD than in matched control subjects. These patterns, although not in the social domain, have features that are consistent with behaviors that are associated with ASD in older children, leading us to speculate that they may be precursors to ASD, if not other developmental disorders. A great deal of additional evidence would be needed to assert that these atypical behaviors are diagnostic for ASD or to prove their connection to later emerging social and communication deficits. We do propose, however, that the association of the behaviors described here with ASD in a medically compromised cohort might establish a group of infants to study prospectively to identify precursors to ASD, along with the more established strategy of study designs involving infant siblings of children with ASD.
CONCLUSIONS
Compared with matched control subjects, NICU graduates who later received a diagnosis of ASD showed persistence of abnormal neonatal neurobehaviors, more visual asymmetry and upper extremity deficits, atypical preference for increased visual stimulation, and early sharp declines in both MDI and PDI scores. Thus, they may be showing a unique behavioral profile starting in early infancy, with slower resolution and development, atypical visual and motor function, and motor as well as cognitive impairment. How this profile might be related to social communication deficits that are the hallmark of ASD requires more research. Whether NICU graduates represent a different phenotype from other children with ASD requires additional confirmation, but, at the least, these findings support the view that studying this cohort prospectively may yield insights into underlying mechanisms and behavioral precursors to ASD.
WHAT’S KNOWN ON THIS SUBJECT
Previous studies found increased prevalence of ASD with obstetric/neonatal risk factors resulting in NICU admission. Although initiating intervention before 2 years is associated with better outcome, behavioral markers typically are identified after 1.0 to 1.5 years, with diagnoses even later.
WHAT THIS STUDY ADDS
The authors identify neonatal/early infant atypical behaviors that occur more frequently in NICU graduates who later receive an ASD diagnosis than matched control subjects. They may serve as markers in areas of visual function, tone, regulation, and inadequate developmental transitions.
Acknowledgments
This work was supported by National Institute of Child Health and Human Development grants P01-HD047281 and R01-HD021784 and by the New York State Office of Mental Retardation and Developmental Disabilities.
We thank the infants and their families who so willingly participated in our studies. We also thank our staff at the Institute for Basic Research in Developmental Disabilities and the doctors, nurses, and staff at Richmond University Medical Center Staten Island for continued help, encouragement, and support.
ABBREVIATIONS
- ASD
autism spectrum disorder
- GA
gestational age
- CNS
central nervous system
- RNNA
Rapid Neonatal Neurobehavioral Assessment
- PTA
postterm age
- AModA
Arousal-Modulated Attention
- BSID-II
Bayley Scales of Infant Development, Second Edition
- MDI
Mental Developmental Index
- PDI
Psychomotor Developmental Index
- OR
odds ratio
- CI
confidence interval
Footnotes
FINANCIAL DISCLOSURE: Dr Cohen receives royalties from the sale of the PDD Behavior Inventory (PDDBI) through an agreement with his employer; the other authors have no financial relationships relevant to this article to disclose.
Portions of these data were presented at the meeting of the American Pediatric Society/Society for Pediatric Research (May 3–6, 2008, Honolulu, HI); the Society for Developmental and Behavioral Pediatrics (October 16–17, 2008; Cincinnati, OH); the International Society on Infant Studies (March 27–29, 2008; Vancouver, British Columbia, Canada); and the International Meeting for Autism Research (May 7–9, 2009; Chicago, IL).
References
- 1.Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children: confirmation of high prevalence. Am J Psychiatry. 2005;162(6):1133–1141. doi: 10.1176/appi.ajp.162.6.1133. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders: Autism and Developmental Disabilities Monitoring Network, United States, 2000–2002. Surveill Summ MMWR. 2007;56(1):1–40. [PubMed] [Google Scholar]
- 3.Kogan MD, Blumberg SJ, Schieve LA, et al. Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics. 2009;124(5):1395–1403. doi: 10.1542/peds.2009-1522. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders: Autism and Developmental Disabilities Monitoring Network, United States. 2006. Surveill Summ MMWR. 2009;58(10):1–20. [PubMed] [Google Scholar]
- 5.Filipek PA, Accardo PJ, Baranek GT, et al. The screening and diagnosis of autistic spectrum disorders. J Autism Dev Disord. 1999;29(6):439–484. doi: 10.1023/a:1021943802493. [DOI] [PubMed] [Google Scholar]
- 6.Bryson SE, Rogers SJ, Fombonne E. Autism spectrum disorders: early detection, intervention, education, and psychopharmacological management. Can J Psychiatry. 2003;48(8):506–516. doi: 10.1177/070674370304800802. [DOI] [PubMed] [Google Scholar]
- 7.Committee on Educational Interventions for Children with Autism, National Research Council. Educating Children With Autism. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 8.Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Jacobsen SJ. The incidence of autism in Olmsted County, Minnesota, 1976–1997: results from a population-based study. Arch Pediatr Adolesc Med. 2005;159(1):37–44. doi: 10.1001/archpedi.159.1.37. [DOI] [PubMed] [Google Scholar]
- 9.Bailey DB, Jr, Hatton DD, Skinner M, Mesibov G. Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. J Autism Dev Disord. 2001;31(2):165–174. doi: 10.1023/a:1010747131386. [DOI] [PubMed] [Google Scholar]
- 10.Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California central valley. Environ Health Perspect. 2007;115(10):1482–1489. doi: 10.1289/ehp.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arndt TL, Stodgell DL, Rodier PM. The teratology of autism. Int J Dev Neurosci. 2005;23(2–3):189–199. doi: 10.1016/j.ijdevneu.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Campbell DB, Sutcliffe JS, Ebert PJ, et al. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci USA. 2006;103(45):16834–16839. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connors SL, Crowell DE, Eberhart CG, et al. B2-adrenergic receptor activation and genetic polymorphisms in autism: data from dizygotic twins. J Child Neurol. 2005;20(11):876–884. doi: 10.1177/08830738050200110401. [DOI] [PubMed] [Google Scholar]
- 14.Brown WT. Genetics of autism. In: Chauhan A, Chauhan V, Brown WT, editors. Autism: Oxidative Stress, Inflammation, and Immune Abnormalities. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2010. pp. 61–72. [Google Scholar]
- 15.Lopez-Rangel E, Lewis ME. Loud and clear evidence for gene silencing by epigenetic mechanisms in autism spectrum and related neurodevelopmental disorders. Clin Genet. 2006;69(1):21–25. doi: 10.1111/j.1399-0004.2006.00543a.x. [DOI] [PubMed] [Google Scholar]
- 16.Serajee FJ, Zhong H, Mahbubul Huq AH. Association of reelin gene polymorphisms with autism. Genomics. 2006;87(1):75–82. doi: 10.1016/j.ygeno.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Rogers SJ. What are infant siblings teaching us about autism in infancy? Autism Res. 2009;2(3):125–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23(2–3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Zwaigenbaum L, Thurm A, Stone W, et al. Studying the emergence of autism spectrum disorders in high risk infants: methodological and practical issues. J Autism Dev Disord. 2007;37(3):466–480. doi: 10.1007/s10803-006-0179-x. [DOI] [PubMed] [Google Scholar]
- 20.Yirmiya N, Gamliel I, Shaked M, Sigman M. Cognitive and verbal abilities of 24- to 36-month-old siblings of children with autism. J Autism Dev Disord. 2007;37(2):218–229. doi: 10.1007/s10803-006-0163-5. [DOI] [PubMed] [Google Scholar]
- 21.Ozonoff S, Losif A, Baguio F, et al. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry. 2010;49(3):256–266. [PMC free article] [PubMed] [Google Scholar]
- 22.Gillberg C, Gillberg IC. Infantile autism: a total population study of reduced optimality in the pre-, peri-, and neonatal period. J Autism Dev Disord. 1983;13(2):153–166. doi: 10.1007/BF01531816. [DOI] [PubMed] [Google Scholar]
- 23.Glasson EJ, Bower C, Petterson B, de Klerk N, Chancy G, Hallmayer JF. Perinatal factors and the development of autism: a population study. Arch Gen Psychiatry. 2004;61(6):618–627. doi: 10.1001/archpsyc.61.6.618. [DOI] [PubMed] [Google Scholar]
- 24.Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Autistic spectrum disorders in extremely premature children [epub ahead of print] J Pediatr. 2010 Jan 5; doi: 10.1016/j.jpeds.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 25.Juul-Dam N, Townsend J, Courchesne E. Prenatal, perinatal, and neonatal factors in autism, pervasive developmental disorder-not otherwise specified, and the general population. Pediatrics. 2001;107(4) doi: 10.1542/peds.107.4.e63. Available at: www.pediatrics.org/cgi/content/full/107/4/e63. [DOI] [PubMed] [Google Scholar]
- 26.Kolevzon A, Gross R, Reichenberg A. Prenatal and perinatal risk factors for autism: a review and integration of findings. Arch Pediatr Adolesc Med. 2007;161(4):326–333. doi: 10.1001/archpedi.161.4.326. [DOI] [PubMed] [Google Scholar]
- 27.Kuban KC, O’Shea TM, Allred EN, Tager-Flusberg H, Goldstein DJ, Leviton A. Positive screening on the Modified Checklist for Autism in Toddlers (M-CHAT) in extremely low gestational age newborns. J Pediatr. 2009;154(4):535–540. doi: 10.1016/j.jpeds.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsson HJ, Eaton WW, Madsen KM, et al. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol. 2005;161(10):916–925. doi: 10.1093/aje/kwi123. [DOI] [PubMed] [Google Scholar]
- 29.Limperopoulos C, Bassan H, Gauvreau K, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-tem cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120(3):584–593. doi: 10.1542/peds.2007-1041. [DOI] [PubMed] [Google Scholar]
- 30.Limperopoulos C, Bassan H, Sullivan NR, et al. Positive screening for autism in expreterm infants: prevalence and risk factors. Pediatrics. 2008;121(4):758–765. doi: 10.1542/peds.2007-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maimburg RD, Vaeth M. Perinatal risk factors and infantile autism. Acta Psychiatr Scand. 2006;114(4):257–264. doi: 10.1111/j.1600-0447.2006.00805.x. [DOI] [PubMed] [Google Scholar]
- 32.Stein D, Weizman A, Ring A, Barak Y. Obstetric complications in individuals diagnosed with autism and in healthy controls. Compr Psychiatry. 2006;47(1):69–75. doi: 10.1016/j.comppsych.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Zwaigenbaum L, Szatmari P, Jones MB, et al. Pregnancy and birth complications in autism and liability to the broader autism phenotype. J Am Acad Child Adolesc Psychiatry. 2002;41(5):572–579. doi: 10.1097/00004583-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Schendel DE, Bhasin TK. Birth weight and gestational age characteristics of children with autism, including a comparison to other developmental disabilities. Pediatrics. 2008;121(6):1155–1164. doi: 10.1542/peds.2007-1049. [DOI] [PubMed] [Google Scholar]
- 35.Gardner JM, Karmel BZ, Magnano CL. Arousal/ visual preference interactions in high-risk neonates. Dev Psychol. 1992;28(5):821–830. [Google Scholar]
- 36.Gardner JM, Karmel BZ. Development of arousal/visual preference interactions in early infancy. Dev Psychol. 1995;31(3):473–482. [Google Scholar]
- 37.Gardner JM, Karmel BZ, Flory MJ. Arousal modulation of neonatal visual attention: implications for development. In: Soraci S Jr, Murata-Soraci K, editors. Perspectives on Fundamental Processes in Intellectual Functioning: Vol 2. Visual Information Processing and Individual Differences. Westport, CT: Praeger Publishers; 2003. pp. 125–154. [Google Scholar]
- 38.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 39.Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule. Los Angeles, CA: Western Psychological Services; 2002. [Google Scholar]
- 40.Cohen IL, Sudhalter V. The PDD Behavior Inventory (PDDBI) Lutz FL: Psychological Assessment Resources; 2005. [Google Scholar]
- 41.Cohen IL, Gomez TR, Gonzales MG, Lennon EM, Karmel BZ, Gardner JM. Parent PDD Behavior Inventory profiles of young children classified according to Autism Diagnostic Observation Schedule-Genetic and Autism Diagnostic Interview revised criteria. J Autism Dev Disord. 2010;40(2):246–354. doi: 10.1007/s10803-009-0863-8. [DOI] [PubMed] [Google Scholar]
- 42.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evaluation of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500 gm. J Pediatr. 1978;92(4):529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 43.Gardner JM, Karmel BZ, Magnano CL, Norton KI, Brown EG. Neurobehavioral indicators of early brain insult in high risk neonates. Dev Psychol. 1990;26(4):563–575. [Google Scholar]
- 44.Gardner JM, Karmel BZ, Freedland RL. Determining functional integrity in neonates: a rapid neurobehavior assessment tool. In: Singer LT, Zeskind PS, editors. Biobehavioral Assessment of the Infant. New York, NY: Guilford Publications; 2001. pp. 398–422. [Google Scholar]
- 45.Karmel BZ, Gardner JM. Neurobehavioral assessment in the neonatal period. Clin Neurosci/Ideggy Szle. 2005;58(9–10):315–323. [PubMed] [Google Scholar]
- 46.Bayley N. Bayley Scales of Infant Development. 2. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 47.Gardner JM, Karmel BZ, Lennon EM, Kittler PM, Flory MJ. Shifts in the Relative Influence of Biological and Environmental Risk Factors on Developmental Outcome of High-risk Infants. Honolulu, HI: American Pediatric Society/Society for Pediatric Research; May, 2008. [Google Scholar]
- 48.Griffiths R. The Abilities of young Children: A Comprehensive System of Mental Measurement for the First Eight Years of Life. High Wycombe, Buckinghamshire, UK: The Test Agency Ltd; 1984. [Google Scholar]
- 49.Fantz RL. Pattern vision in newborn infants. Science. 1963;140(3564):296–297. doi: 10.1126/science.140.3564.296. [DOI] [PubMed] [Google Scholar]
- 50.Karmel BZ, Gardner JM, Freedland RL. Arousal-modulated attention at 4 months as a function of intrauterine cocaine exposure and CNS injury. J Pediatr Psychol. 1996;21(6):821–832. doi: 10.1093/jpepsy/21.6.821. [DOI] [PubMed] [Google Scholar]
- 51.Stata Corp. Stata Statistical Software [computer program]. Release 10. College Station, TX: Stata Corp LP; 2007. [Google Scholar]
- 52.Fenton TR. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatr. 2003:3. doi: 10.1186/1471-2431-3-13. Available at: www.biomedcentral.com/1471-2431/3/13. [DOI] [PMC free article] [PubMed]
- 53.Rothman KJ. Modern Epidemiology. Boston, MA: Little, Brown, & Co; 1986. [Google Scholar]
- 54.Bryson SE, Landry R, Czapinski P, McConnell B, Rombough V, Wainwright A. Autistic spectrum disorders: causal mechanisms and recent findings on attention and emotion. Int J Spec Educ. 2004;19(1):14–22. [Google Scholar]
- 55.Elsabbagh M, Volein A, Holmboe K, et al. Visual orienting in the early broader autism phenotype: disengagement and facilitation. J Child Psychol and Psychiat. 2009;50(5):637–642. doi: 10.1111/j.1469-7610.2008.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brenner LA, Turner KC, Müller RA. Eye movement and visual search: are there elementary abnormalities in autism? J Autism Dev Disord. 2007;37(7):1289–1309. doi: 10.1007/s10803-006-0277-9. [DOI] [PubMed] [Google Scholar]
- 57.McCleery JP, Allman E, Carver LJ, Dobkins KR. Abnormal magnocellular pathway visual processing in infants at risk for autism. Biol Psychiatry. 2007;62(9):1007–1014. doi: 10.1016/j.biopsych.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 58.Mundy P, Sullivan L, Mastergeorge AM. A parallel and distributed-processing model of joint attention, social cognition and autism. Autism Res. 2009;2(1):2–21. doi: 10.1002/aur.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65(8):946–954. doi: 10.1001/archpsyc.65.8.946. [DOI] [PubMed] [Google Scholar]
- 60.Klin A, Lin DJ, Gorrindo P, Ramsey G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klin A, Jones W. Altered face scanning and impaired recognition of biological motion in a 15-month-old infant with autism. Dev Sci. 2008;11(1):40–46. doi: 10.1111/j.1467-7687.2007.00608.x. [DOI] [PubMed] [Google Scholar]
- 62.Northrup JB, Lin D, Ramsey G, Klin A, Jones W. Perception of audiovisual synchrony under varying degrees of social context in infants with autism. Chicago, IL: International Meeting for Autism Research; May, 2009. [Google Scholar]
- 63.Ashwin E, Ashwin C, Rhydderch D, Howells J, Baron-Cohen S. Eagle-eyed visual acuity: an experimental investigation of enhanced perception in autism. Biol Psychiatry. 2009;65(1):17–21. doi: 10.1016/j.biopsych.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 64.Inoue M, Katsumi Y, Hayashi T, et al. Sensory stimulation accelerates dopamine release in the basal ganglia. Brain Res. 2004;1026(2):179–184. doi: 10.1016/j.brainres.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 65.Karmel BZ, Gardner JM. Prenatal cocaine exposure effects on arousal-modulated attention during the neonatal period. Dev Psychobiol. 1996;29(5):463–480. doi: 10.1002/(SICI)1098-2302(199607)29:5<463::AID-DEV5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 66.Weigel J, Wisniewski T, Chauhan A, et al. Type, topology, and sequence of neuropathological changes shaping clinical phenotype of autism. In: Chauhan A, Chauhan V, Brown WT, editors. Autism: Oxidative Stress, Inflammation, and Immune Abnormalities. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2010. pp. 1–34. [Google Scholar]
- 67.Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: comparisons to mental retardation. J Autism Dev Disord. 2000;30(3):237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- 68.Dziuk MA, Gidley Larson JC, Apostu A, Mahone EM, Denckla MB, Mostofsky SH. Dyspraxia in autism: association with motor, social, and communicative deficits. Dev Med Child Neurol. 2007;49(10):734–739. doi: 10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- 69.Mostofsky SG, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132(pt 9):2413–2425. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teitelbaum P, Teitelbaum O, Nye J, Fryman J, Maurer RG. Movement analysis in infancy may be useful for early diagnosis of autism. Proc Natl Acad Sci USA. 1998;95(23):13982–13987. doi: 10.1073/pnas.95.23.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loh A, Soman T, Brian J, et al. Stereotyped motor behaviors associated with autism in high-risk infants: a pilot video tape analysis of a sibling sample. J Autism Dev Disord. 2007;37(1):25–36. doi: 10.1007/s10803-006-0333-5. [DOI] [PubMed] [Google Scholar]
- 72.Ozonoff S, Macari S, Young GS, Goldring S, Thompson M, Rogers SJ. Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism. 2008;12(5):457–472. doi: 10.1177/1362361308096402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Attwood A, Frith U, Hermelin B. The understanding and use of interpersonal gestures by autistic and Down’s syndrome children. J Autism Dev Disord. 1988;18(2):241–257. doi: 10.1007/BF02211950. [DOI] [PubMed] [Google Scholar]
- 74.Mundy P, Sigman M. The theoretical implications of joint-attention deficits in autism. Dev Psychopathol. 1989;1(3):173–183. [Google Scholar]