Abstract
In vitro cytochrome P4501A1 (CYP1A1) metabolizes omega-3 polyunsaturated fatty acids (n-3 PUFAs); eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), primarily to 17,18-epoxyeicosatetraenoic acid (17,18-EEQ) and 19,20-epoxydocosapentaenoic acid (19,20-EDP), respectively. These metabolites have been shown to mediate vasodilation via increases in nitric oxide (NO) and activation of potassium channels. We hypothesized that genetic deletion of CYP1A1 would reduce vasodilatory responses to n-3 PUFAs, but not the metabolites, and increase blood pressure (BP) due to decreases in NO. We assessed BP by radiotelemetry in CYP1A1 wildtype (WT) and knockout (KO) mice ± NO synthase (NOS) inhibitor. We also assessed vasodilation to acetylcholine (ACh), EPA, DHA, 17,18-EEQ and 19,20-EDP in aorta and mesenteric arterioles. Further, we assessed vasodilation to an NO donor and to DHA ± inhibitors of potassium channels. CYP1A1 KO mice were hypertensive, compared to WT, (mean BP in mmHg, WT 103±1, KO 116±1, n=5/genotype, p<0.05), and exhibited a reduced heart rate (beats per minute, WT 575±5; KO 530±7; p<0.05). However, BP responses to NOS inhibition and vasorelaxation responses to ACh and an NO donor were normal in CYP1A1 KO mice, suggesting that NO bioavailability was not reduced. In contrast, CYP1A1 KO mice exhibited significantly attenuated vasorelaxation responses to EPA and DHA in both the aorta and mesenteric arterioles, but normal vasorelaxation responses to the CYP1A1 metabolites, 17,18-EEQ and 19,20-EDP, and normal responses to potassium channel inhibition. Taken together these data suggest that CYP1A1 metabolizes n-3 PUFAs to vasodilators in vivo and the loss of these vasodilators may lead to increases in BP.
Keywords: Cytochrome P4501A1, omega-3 polyunsaturated fatty acids, vasorelaxation, hypertension
Introduction
Omega-3 polyunsaturated fatty acids (n-3 PUFAs) are metabolized by numerous cytochrome P450s into several products that have potent vasodilatory properties (Schwarz et al., 2004; Schwarz et al., 2005). Some of these metabolites have been recognized to play key roles in contributing to underlying vascular tone, blood pressure (BP), and overall cardiovascular health (Billman et al., 1994; Engler et al., 1999; Menotti et al., 1999; Blanchet et al., 2000). Nonetheless, the contribution of specific P450s to the regulation of vascular tone and BP via n-3 PUFA metabolism remains poorly understood.
Cytochrome P4501A1 (CYP1A1) is one P450 shown to metabolize n-3 PUFAs to vasodilatory products. CYP1A1 is well known to be highly induced in the liver and extrahepatic tissues following exposure to environmental pollutants, such as halogenated aromatic hydrocarbons (HAH). While induction of CYP1A1 might appear to be beneficial based on its ability to metabolize n-3 PUFAs, in fact, studies show HAH exposure reduces hepatic and plasma n-3 PUFAs by >40% (Kakela et al., 2001; Moran et al., 2004). Further, HAH-induced endothelial dysfunction in cultured cells is attenuated by n-3 PUFAs (Hennig et al., 1999; Wang et al., 2008). Thus, these studies suggest that toxicological induction of CYP1A1 may be detrimental and potentially reduce the benefits of n-3 PUFAs.
In addition to HAH-mediated induction, CYP1A1 is also constitutively expressed at low levels in the vascular endothelium with CYP1A1 mRNA and protein detected in human umbilical vein endothelial cells as well as in endothelial cells of the mouse descending thoracic aorta, and human coronary arteries (Eskin et al., 2004; Han et al., 2008; Conway et al., 2009). CYP1A1 is also constitutively expressed in pig coronary arteries (Messina et al., 2012). In addition to its basal expression in the endothelium, CYP1A1 is also induced in the endothelium by physiological levels of shear stress (Han et al., 2008; Conway et al., 2009). Physiological laminar shear stress is considered to be anti-atherogenic (Cunningham and Gotlieb, 2004). It significantly induces antioxidants, antithrombotic factors, and vasodilators, such as nitric oxide (NO) and prostacyclin, and suppresses prothrombotic substances and vasoconstrictors. This raises the possibility, as suggested by Conway et al. (2009) and (De Caterina and Madonna, 2009)(De Caterina and Madonna, 2009)(De Caterina and Madonna, 2009)(De Caterina and Madonna, 2009)(De Caterina and Madonna, 2009)(De Caterina and Madonna, 2009)11(De Caterina and Madonna, March 1, 2009)(De Caterina and Madonna, 2009)(De Caterina and Madonna, 2009)De Caterina and Madonna (2009) that physiological induction of CYP1A1 in the endothelium could contribute to the anti-atherogenic phenotype and that this may be mediated via metabolism of n-3 PUFAs. Parallel increases in CYP1A1 and NO have also been observed in cultured endothelial cells treated with an aryl hydrocarbon receptor agonist (Lim et al., 2007).
As noted above, evidence from several studies show that CYP1A1 metabolizes two major n-3 PUFAs, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), in a stereospecific manner. Human CYP1A1 epoxidizes the 17,18-olefinic bond of EPA in a regiospecific manner to form primarily 17(R),18(S)-epoxyeicosatetraenoic acid [(17(R),18(S) EEQ)] (Schwarz et al., 2004). CYP1A1 also exclusively epoxidizes the 19,20-olefinic bond of DHA, producing 19(R),20(S)-epoxydocosapentaenoic acid [19(R),20(S)-EDP] (Fer et al., 2008; Lucas et al., 2010). Furthermore, both of these metabolites, 17,18-EEQ and 19,20-EDP, are potent vasodilators in the microcirculatory vessels of the pig and/or mouse (Zhang et al., 2001), and 17,18-EEQ also causes relaxation and hyperpolarization of pulmonary artery smooth muscle (Morin et al., 2009). Studies have identified a variety of downstream mechanisms that mediate this vasodilation, including increases in NO signaling (Ma et al., 2004; Li et al., 2007; Stebbins et al., 2008) as well as activation of potassium channels on vascular smooth muscle cells (Lauterbach et al., 2002; Ye et al., 2002; Wang et al., 2011).
Taken together, these data suggest that CYP1A1 could metabolize EPA and DHA as substrates in vivo into vasodilatory metabolites that act via NO or potassium channel activation. Thus, we sought to determine the degree and mechanism by which constitutive CYP1A1 contributes physiologically to vascular responses to n-3 PUFAs and to BP regulation. We used CYP1A1 wildtype (WT) and knockout (KO) mice to test the hypothesis that genetic deletion of CYP1A1 would reduce vasodilatory responses to n-3 PUFAs, but not the metabolites, and increase BP due to decreases in NO.
Materials and Methods
Chemicals
Acetylcholine (ACh), phenylephrine (PE), Nω-nitro-L-arginine (LNNA), S-nitroso-N-acetyl penicillamine (SNAP), 4-aminopyridine, iberiotoxin, and all ingredients of physiological saline solution (PSS) and HEPES-PSS were purchased from Sigma–Aldrich (St. Louis, MO). EPA, DHA, 17,18-EEQ, 19,20-EDP and U46619 were purchased from Cayman Chemical (Ann Arbor, MI). Ionomycin was purchased from EMD Millipore Chemicals (San Diego, CA).
Animals
CYP1A1 KO mice, backcrossed more than eight generations onto the C57BL/6J background, were generously provided by Dr. Daniel Nebert (University of Cincinnati) and were bred at the University of New Mexico (Dalton et al., 2000). Age-matched C57BL/6J mice served as WT controls. Animals were housed in a temperature-controlled environment receiving standard mouse chow (2020X, Teklad Diets, Harlan Laboratories, Madison, WI; n-3 PUFAs constitute 8% of total fatty acids) and water ad libitum. Only male mice were used in subsequent experiments. All animal protocols were approved by the University of New Mexico Animal Care and Use Committee and the investigations conformed to the Guide for the Care and Use of Laboratory Animals published by the U. S. National Institutes of Health (NIH Publication No. 85-23, revised 1996). When tissues were harvested and organ weights determined, mice were anesthetized with ketamine (80 mg/kg)/xylazine (4 mg/kg). The heart was removed, atria were dissected, and the total ventricle weight measured. The right ventricle was dissected and the left ventricle and ventricular septum weight was measured. Kidneys and liver weights were also measured. All tissues were frozen at −80°C.
In vivo analysis of blood pressure
Arterial BP and heart rate (HR) were measured using radiotelemetry in 4–5 month-old CYP1A1 WT and KO male mice (Data Sciences International, St. Paul, MN) as previously described (Lund et al., 2008), using PA-C10 radiotelemeters. Mice were allowed to recover from surgery for 7 d prior to data collection. BP, including systolic, diastolic, mean pressures, and HR were collected for 7 d before drug treatments began. BP was recorded for 10 s every 15 min during baseline measurements. After basal BP was measured, mice were treated with LNNA in the drinking water (250 mg/L) for one week followed by one week of washout (Duling et al., 2006).
Aortic vasoreactivity analysis
Mice were anesthetized with ketamine (80 mg/kg)/xylazine (4 mg/kg) and euthanized by exsanguination. Either the thoracic or abdominal aorta was removed, depending on the study, and placed in ice-cold physiological saline (PSS) containing 0.13 M NaCl, 0.005 M KCl, 0.001 M KH2PO4, 0.001 M MgSO4, 0.015 M NaHCO3, 0.0055 M glucose, 0.026 M CaNa2EDTA, 0.0018 M CaCl2, pH 7.4, and cleaned of connective tissue and adventitial fat. The vessel was cut into 3 mm segments and individual rings were suspended in an organ bath containing PSS at 37°C bubbled with 21% O2, 6% CO2, balanced N2. The rings were attached to a force transducer (Grass Technologies, West Warwick, RI) with steel hangers and resting tension was increased stepwise to 1.5 g over 1 h. In thoracic aorta, after an initial contraction with 10−5 M PE followed by relaxation with 10−5 M ACh, concentration-response curves to PE (10−9 - 10−5 M), or PE following a 30 min preincubation with the NOS inhibitor, LNNA (10−4 M) were performed. An ACh concentration- response (10−9 - 10−5 M) was also conducted in the thoracic aorta following preconstriction with PE (10−5 M). In endothelium-disrupted thoracic aorta, a concentration-response to the NO donor, SNAP (10−9 - 10−5 M) was also conducted. In abdominal aorta, concentration response curves to U46619 (10−9 - 10−5 M) were also conducted. In addition, DHA and EPA concentration-response studies (10−9 - 10−5 M) were conducted following preconstriction with the thromboxane A2 mimetic, U46619 (1.3 × 10−8 M). Lastly, concentration-response curves to 17,18-EEQ, and 19,20-EDP (10−14 to 10−7 M) were conducted in the abdominal aorta preconstricted with U46619. PE was used as the vasoconstrictor in thoracic aorta due to the predominant expression of α1-adrenergic receptors (Russell and Watts, 2000), while U46619 was used at the vasoconstrictor in the abdominal aorta due to the predominant expression of thromboxane A2 receptors.
Mesenteric vasoreactivity analysis
Mice were administered heparin (10 μl/g of 1000 U/ml) by intraperitoneal injection, anesthetized with ketamine (80 mg/kg)/xylazine (4 mg/kg), and euthanized by exsanguination. The intestine together with mesenteric arteries was quickly excised and placed in ice cold HEPES-PSS solution (0.13 M NaCl, 0.006 M KCl, 0.001 M MgCl2, 0.002 M CaCl2, 0.01 M HEPES, 2.6 × 10−5 M EDTA, 0.01 M glucose, and buffered to pH 7.4 with NaOH). The tissue was then pinned to the bottom of a petri dish, and a long segment of a first-order branch of the mesenteric artery was cleared from surrounding adipose tissue and dissected. The artery was transferred to the chamber of a pressure myograph system (DMT -110 systems, Danish Myo Technology, Ann Arbor, MI) for cannulation. The chamber was filled with warm PSS at 37°C bubbled with 21% O2, 6% CO2, balanced N2 (0.13 M NaCl, 0.0047 M KCl, 0.0012 M KH2PO4, 0.0012 M MgSO4, 0.015 M NaHCO3, 0.0055 M glucose, 2.6 × 10−5 M CaNa2EDTA, and 0.0018 M CaCl2, pH 7.4). One end of the vessel was cannulated with the outflow pipette and tied securely with 10-0 ethilon surgical silk thread. The other end of the vessel was then cannulated with the inflow pipette. The pressure was then increased by 10 mmHg increments every 5 min to a maximum of 40 or 60 mmHg, and the artery allowed to equilibrate for 30 min. After equilibration, baseline internal diameter (WT 167.9 ± 3.3, KO 171.7 ± 3.1 μm) was measured using edge detection software (MyoView acquisition software, DMTVAS 6.2.0.59 Danish Myo Technology). In other vessels, passive internal diameter (WT 178.3 ± 5.1, KO 181.4 ± 2.0 μm) was also determined after preincubation with the calcium ionophore, ionomycin (10−5 M) for 15 min. Viability of the arteries was tested by constriction to 0.06 M KCl and vessels that failed to constrict to 30% of baseline internal diameter were discarded. After a PSS wash, the artery was allowed to return to baseline diameter and to rest for at least 15 min. Arteries were then preconstricted to 45% of internal diameter with U46619 (10−8 M), and a concentration-response to EPA (10−8 - 10−4 M) and DHA (10−8 - 10−4 M) was conducted. In other vessels, a DHA concentration-response curve was conducted after preincubation with 10−4 M iberiotoxin (IBTX), an inhibitor of the large conductance, calcium activated potassium channel (BK channel) ± 0.005 M 4-aminopyridine (4-AP), an inhibitor of the voltage-gated potassium channel (Kv). A concentration-response curve to U46619 (0.01–1 μM) was also conducted. Lastly, we assessed concentration-responses to 17,18-EEQ and 19,20-EDP (10−14 - 10−6 M). All experiments were performed without luminal flow.
Analysis of plasma nitrites/nitrates (NOx) and indices of renal function
For plasma NOx, mice were euthanized by exsanguination and plasma was collected from whole blood using heparinized syringes. NOx was measured using the Griess colorimetric assay (Cayman Chemical). To determine plasma indices of renal function, including the concentrations of plasma sodium, potassium, blood urea nitrogen, and creatinine, heparinized whole blood was collected from the mandibular vein and the concentration of analytes were determined using an i-STAT (Abbott Point of Care Inc, Princeton, NJ).
mRNA analysis of potassium channel subunits, CYP2C29, CYP1B1, CYP2E1, CYP2D6 and COX2
Total RNA was isolated from aorta cleaned of connective tissue and adventitial fat, using Trizol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized using iScript Select cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA) with the supplied random primers and 250 ng RNA. PCR amplification was performed using an iCycler (Bio-Rad Laboratories) with a reaction mixture comprised of iQ SYBR Green Supermix (Bio-Rad Laboratories) with 500 μM of each forward and reverse primer (Table 1). Cycle threshold data for both the target gene of interest and control normalization gene, DNA polymerase II (POL2) was used to calculate mean normalized expression as previously described (Simon, 2003).
Table 1.
Real-time PCR primer sequences
| mRNA Target | Sense Primer (5′ to 3′) | Antisense Primer (5′ to 3′) |
|---|---|---|
| BKα subunit | TCACGGAACTCGCTAAGCC | AATGTGCGTCCCACTGTTTTT |
| BKβ4 subunit | GCGAAGCTCAGGGTGTCTTAC | CCGGAGACGATGAGGAACAA |
| Kv1.5 subunit | TCCGACGGCTGGACTCAATAA | GCCTCCTCGGTGATGTTTCT |
| CYP2C29 | TGGTCCACCCAAAAGAAATTGA | GCAGAGAGGCAAATCCATTCA |
| CYP1B1 | AATCAATGCGATTCTCCAGCTTTT | CGACCGTATTCTTGGGGATGTAG |
| CYP2E1 | TGCGGAGGTTTTCCCTAAGTA | TGTGCCTCTCTTTGGATGCG |
| CYP2D6 | CCGCCTTCGCTGACCATAC | CGATCACGTTACACACTGCTT |
| POL2 | TGACTCACAAACTGGCTGACATT | TACATCTTCTGCTATGACATGG |
Statistical analysis
Differences between genotypes were analyzed by Student’s test. The treatment-related changes in BP and vasoreactivity between genotypes were analyzed by repeated measures, two-way analysis of variance with post hoc Holm-Sidak comparisons; p < 0.05 was considered statistically significant.
Results
Body and organ weights and plasma indices of renal function
We compared body and organ weights of CYP1A1 WT and KO mice. CYP1A1 KO mice exhibited significantly lower body weights, compared with age-matched CYP1A1 WT mice (Table 2). All organs weighed from CYP1A1 KO mice, including heart, kidneys and liver, were significantly smaller than WT. When organ weight was normalized to body weight, the liver/body weight and kidney/body weight ratios remained significantly lower in CYP1A1 KO mice, compared to WT mice. Given that the kidney/body weight ratio was significantly lower in CYP1A1 KO mice, we also analyzed plasma indices of renal function. We found that the concentrations of plasma sodium, potassium, blood urea nitrogen, and creatinine did not differ between genotypes (data not shown).
Table 2.
Body and organ weights in 4 mo-old CYP1A1 WT and KO mice.
| Weight | CYP1A1 WT (n=5) | CYP1A1 KO (n=5) |
|---|---|---|
| Body (g) | 29.4 ± 0.4 | 23.6 ± 0.3* |
| Heart (mg) | 129 ± 4 (0. 439 ± 0. 024) † | 107 ± 3* (0. 452 ± 0. 029) |
| LV+S (mg) | 101 ± 4 (0. 343 ± 0. 022) | 84.5 ± 3* (0. 356 ± 0. 024) |
| Kidney (mg) | 425 ± 11 (2.13 ± 0. 024) | 293 ± 10* (1.54 ± 0. 003) * |
| Liver (mg) | 1671 ± 42 (5.6 ± 0. 014) | 1215 ± 31* (5.1 ±0. 089)* |
Values are expressed as mean ± SEM
p < 0.05
(Organ/body weight ratio × 100)
LV+S, Left ventricle + Septum.
Basal blood pressure, heart rate, and activity
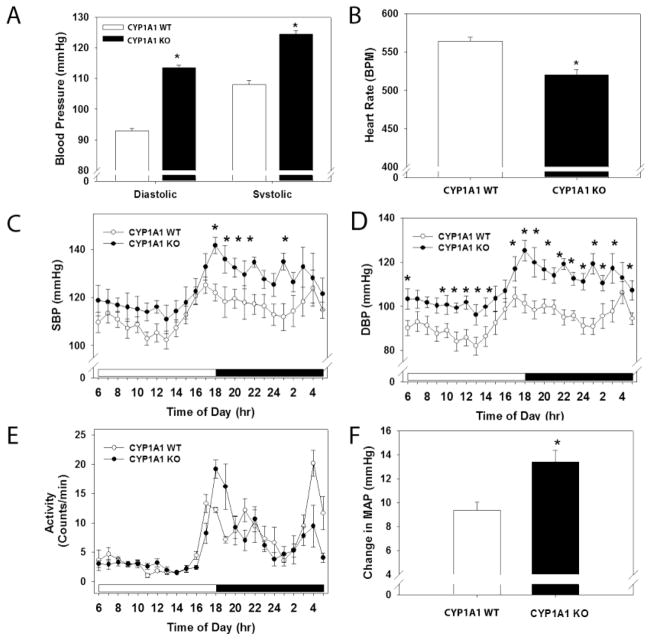
BP of CYP1A1 WT and KO mice was measured by radiotelemetry. The CYP1A1 KO mice exhibited significantly higher systolic and diastolic BP, compared to WT (Fig. 1A). Corresponding HR was significantly lower in CYP1A1 KO mice, compared to WT (Fig. 1B). The increase in systolic BP in CYP1A1 KO mice was evident only at nighttime (Fig. 1C). In contrast, diastolic BP was significantly increased in CYP1A1 KO mice throughout the entire 24 h light/dark cycle, although the normal circadian pattern of an increased BP during the night wakeful period was preserved (Fig. 1D). The level of activity was comparable between CYP1A1 WT and KO mice over the 24 h light/dark cycle (Fig. 1E). Finally, the difference between mean nighttime and mean daytime arterial pressure (MAP) was significantly higher in CYP1A1 KO mice, compared to WT (Fig. 1F).
Figure 1.
Genetic deletion of CYP1A1 increases systolic and diastolic BP, and reduces HR. (A) 24 h mean systolic and diastolic BP, (B) 24 h mean HR, (C) Hourly systolic BP over a 24 h light/dark cycle, (D) Hourly diastolic BP over a 24 h light/dark cycle, (E) Activity over a 24 h light/dark cycle, and (F) Difference in MAP between nighttime and daytime, as measured by radiotelemetry. Data represent mean ± SEM. Data in A, B, and F were analyzed by Student’s t-test; *p < 0.05, and in C, D and E by two-way, repeated measures ANOVA, using post hoc Holm-Sidak comparisons; *p < 0.05 (n=7/genotype).
Plasma NOx and effects of NOS inhibition on blood pressure
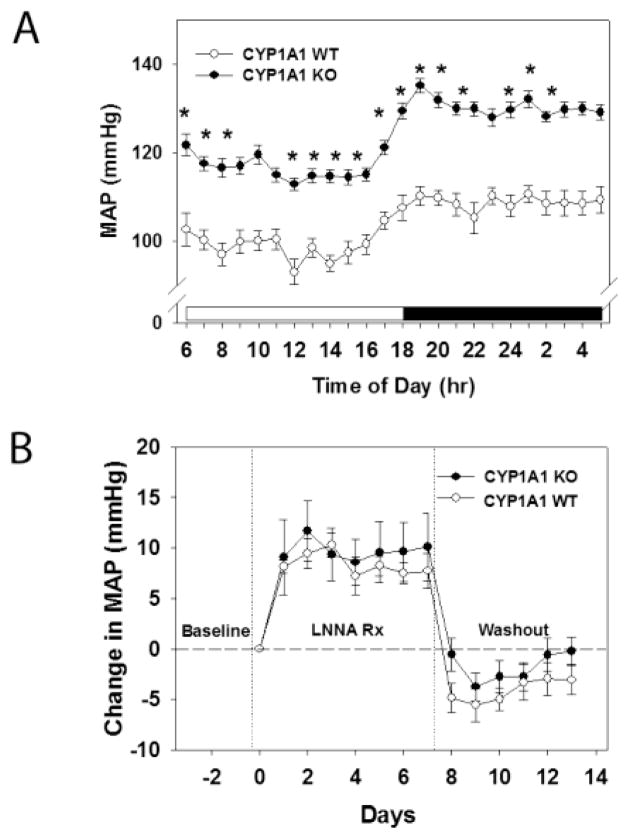
To further assess whether a reduction in NO bioavailability contributed to the hypertension in CYP1A1 KO mice, we measured plasma NOx as an indicator of systemic NO and we treated CYP1A1 WT and KO mice with a NOS inhibitor and measured their BP responses. We found that CYP1A1 KO mice tended to have reduced plasma NOx (p=0.07), although it was not statistically different (data not shown). Further, NOS inhibition failed to normalize BP between CYP1A1 WT and KO mice. Hourly MAP over a 24 h light/dark cycle after NOS inhibition showed that CYP1A1 KO mice remain hypertensive (Fig. 2A) and the relative increase in MAP following NOS inhibition was equivalent between CYP1A1 KO and WT mice (Fig. 2B).
Figure 2.
NOS inhibition by LNNA does not normalize BP in CYP1A1 KO mice in vivo. (A) MAP over a 24 h light/dark cycle after NOS inhibition. (B) Change in MAP from baseline after treatment with 250 mg/L LNNA in drinking water of CYP1A1 WT and KO mice. Data represent mean ± SEM and were analyzed by two-way, repeated measures ANOVA, using post hoc Holm-Sidak comparisons; *p < 0.05, compared to wildtype (A), and by Student’s t-test (B) (n=5/genotype).
Vascular reactivity in aorta and mesenteric resistance arterioles
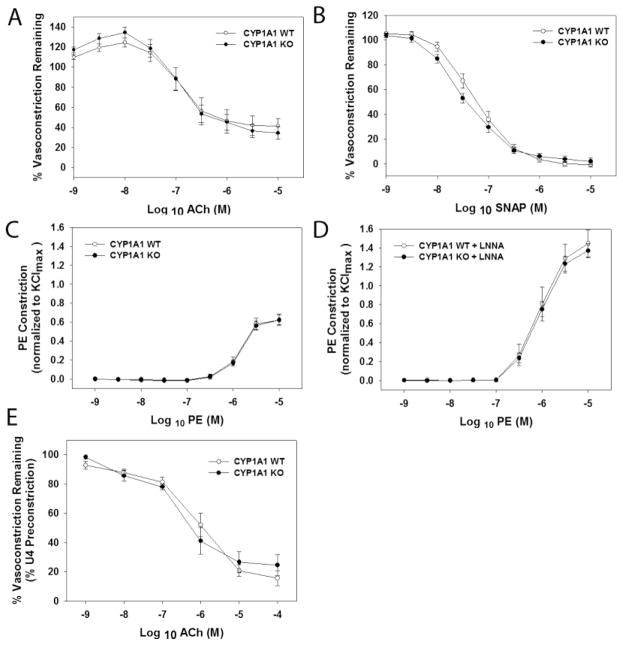
We sought to determine if genetic deletion of CYP1A1 resulted in altered vascular responses indicative of a loss of the endothelial-derived vasodilator, NO. Since ACh-mediated vasorelaxation in the thoracic aorta is dependent solely on NO (Rees et al., 1989), we conducted a concentration-response to ACh in aortic segments preconstricted with PE. We found that CYP1A1 KO mice exhibited completely normal vasorelaxation responses to ACh (Fig 3A). To confirm that NO-mediated signaling was normal in the vascular smooth muscle, we next conducted a concentration-response to the NO donor, SNAP, in endothelium-disrupted aortic segments. Again, CYP1A1 KO mice exhibited completely normal responses to SNAP (Fig. 3B). Lastly, if NO is normal, we would expect constrictor responses to be normal prior to and following treatment with a NOS inhibitor. We found that CYP1A1 KO mice exhibited normal vasoconstriction responses to PE with and without a NOS inhibitor, LNNA (Fig. 3C and D). Since ACh-mediated dilation in resistance vessels also is dependent on NO to a limited degree, we conducted a concentration-response to ACh in first-order pressurized mesenteric arterioles. Again CYP1A1 KO mice exhibited completely normal vasodilation responses to ACh, suggesting that ACh-dependent dilation is also not altered in resistance vessels (Fig. 3E).
Figure 3.
Loss of CYP1A1 does not alter vascular sensitivity to ACh- or SNAP-mediated vasodilation, nor PE-mediated contraction in the absence or presence of the NOS inhibitor, LNNA. (A-D) Thoracic aorta and (E) Mesenteric arterioles. (A) ACh-mediated dilation (% PE preconstriction), (B) SNAP-mediated dilation in endothelium denuded aorta (% PE preconstriction), (C) PE-mediated constriction (% KCl), and (D) PE-mediated constriction in the presence of LNNA, (E) ACh-mediated relaxation (% U46619 preconstriction). Data represent mean ± SEM and were analyzed by two-way, repeated measures ANOVA, using post hoc Holm-Sidak comparisons (n=7–8/genotype).
Vasodilation responses to n-3 PUFAs, EPA and DHA, in abdominal aorta and mesenteric arterioles
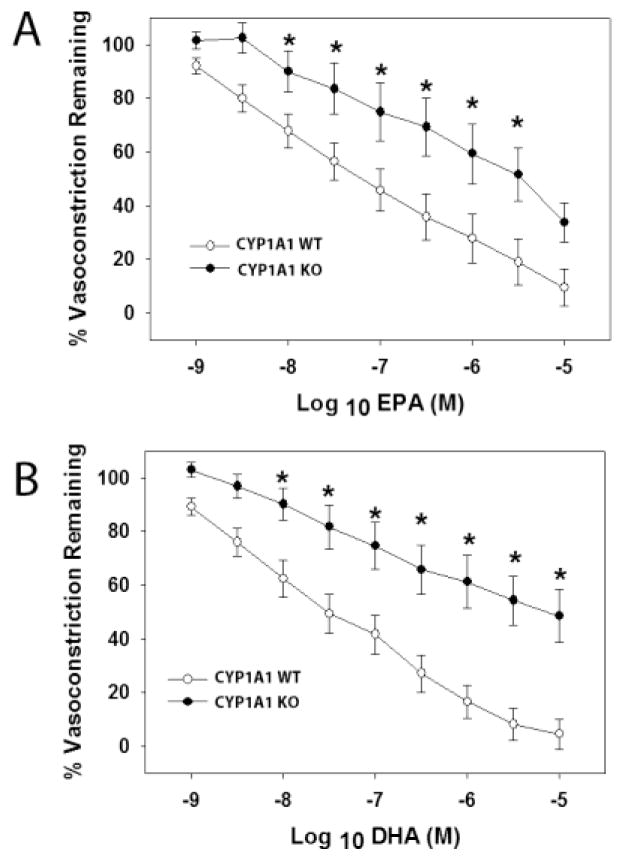
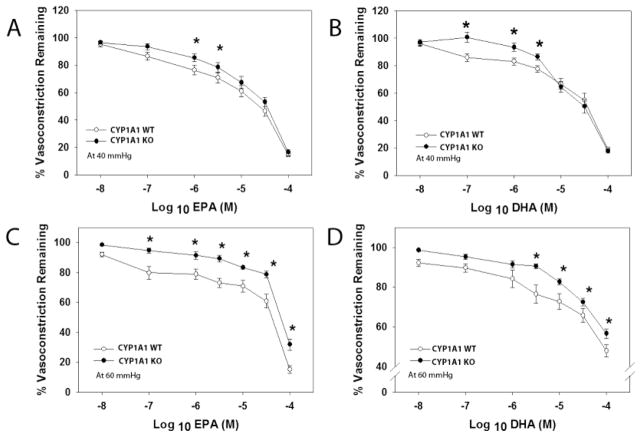
Given that our previous results ruled out a loss of NO bioavailability as a contributor to hypertension in CYP1A1 KO mice, we next sought to determine if vascular reactivity to n-3 PUFAs; EPA and DHA, was altered. We assessed the vasodilation responses in abdominal aorta and pressurized mesenteric arterioles to EPA and DHA following preconstriction with U46619. U46619-mediated vasoconstriction did not differ between CYP1A1 WT and KO mice in either aorta or mesenteric arterioles (See Supplemental Data, Fig. 1S). However, CYP1A1 KO mice exhibited significantly attenuated concentration-dependent vasorelaxation to both EPA and DHA in the aorta (Fig. 4A and B). Similarly, CYP1A1 KO mice exhibited significantly reduced concentration-dependent vasodilation to EPA and DHA in mesenteric arterioles when pressurized at 40 (Fig. 5 A and B) or 60 mmHg (Fig. 5C and D).
Figure 4.
EPA- and DHA-mediated vasodilation is significantly attenuated in abdominal aorta of CYP1A1 KO mice. (A) EPA-mediated relaxation (% U46619 preconstriction), (B) DHA-mediated relaxation (% U46619 preconstriction), in abdominal aorta from CYP1A1 WT and KO mice. Data represent mean ± SEM and were analyzed by two-way, repeated measures ANOVA, using post hoc Holm-Sidak comparisons; *p < 0.05, compared to wildtype ((n=6/genotype).
Figure 5.
EPA- and DHA-mediated vasodilation is significantly attenuated in pressurized mesenteric resistance arterioles at 40 and 60 mmHg. (A) EPA-mediated relaxation at 40 mmHg, (B) DHA-mediated relaxation at 40 mmHg, (C) EPA-mediated relaxation at 60 mmHg, and (D) DHA-mediated relaxation at 60 mmHg. All relaxation experiments were subjected to U46619 preconstriction. Data represent mean ± SEM and were analyzed by two-way, repeated measures ANOVA, using post hoc Holm-Sidak comparisons; *p < 0.05, compared to wildtype (n=8–14/genotype).
Vasodilation responses to CYP1A1 metabolites, 17,18-EEQ and 19,20-EDP, in aorta and mesenteric arterioles
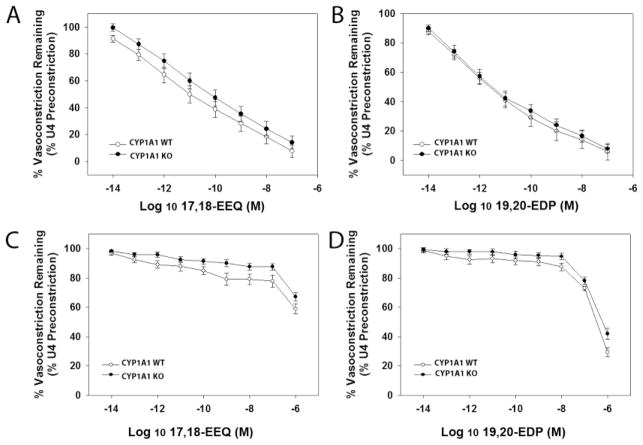
If CYP1A1 mediates n-3 PUFA vasodilation by generating the 17,18-EEQ and 19,20-EDP metabolites as has been demonstrated in vitro, then we would expect that the ability of these metabolites to produce vasodilation when applied directly to the vessel would not differ between genotypes. Thus, we assessed vasodilation of the abdominal aorta and mesenteric arterioles to 17,18-EEQ and 19,20-EDP. We found that 17,18-EEQ and 19,20-EDP elicited potent and highly efficacious vasorelaxation responses in the aorta of both CYP1A1 WT and KO mice at nanomolar concentrations (Fig. 6A and B). In mesenteric arterioles, both metabolites were less potent in inducing vasodilation compared to the aorta, and 17,18-EEQ was significantly less efficacious. Nonetheless, the responses elicited in both CYP1A1 WT and KO mesenteric arterioles were equivalent (Fig. 6C and D).
Figure 6.
P450 metabolites of EPA and DHA, 17,18-EEQ and 19,20-EDP, respectively, induce an equivalent degree of vasodilation in the abdominal aorta and mesenteric arterioles in CYP1A1 WT and KO mice. Vasodilation in abdominal aorta to (A) 17,18-EEQ and (B) 19,20-EDP. Vasodilation in mesenteric resistance arterioles to (C) 17,18-EEQ and (D) 19,20-EDP. Data represent mean ± SEM and were analyzed by two-way, repeated measures ANOVA, using post hoc Holm-Sidak comparisons; *p < 0.05, compared to WT (n=6–8/genotype).
Vasodilation responses to DHA in the presence of potassium channel inhibitors
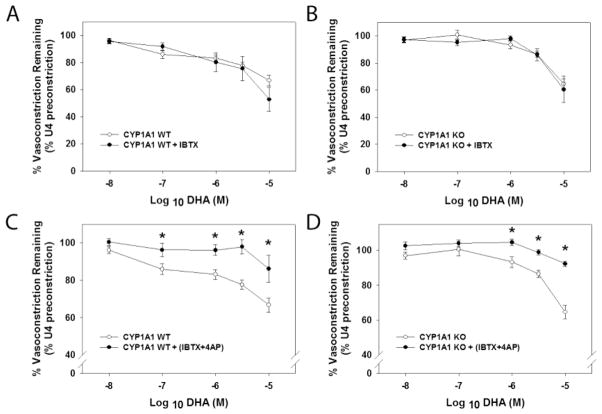
Since previous studies have implicated the activation of potassium ion channels in mediating vasodilation to n-3 PUFAs, we investigated the contribution of two of these channels to vasodilation in CYP1A1 WT and KO mice. We preincubated mesenteric arterioles with a blocker of the BK channel, IBTX, in the absence or presence of an inhibitor of the Kv channel, 4-AP, and then conducted a concentration-response to DHA. We found that both CYP1A1 WT and KO mesenteric arterioles elicited vasodilation to DHA in the presence of the BK channel blocker (Fig. 7A and B). Nonetheless, blockade of both BK and Kv channels significantly inhibited DHA-mediated vasodilation in both genotypes (Fig. 7C and D).
Figure 7.
Responses to potassium channel inhibitors do not differ between CYP1A1 WT and KO mice. (A) DHA-mediated vasodilation after pre-incubation with the BK channel blocker, iberiotoxin (IBTX) in CYP1A1 WT and (B) KO mice. (C) DHA-mediated relaxation after pre-incubation with both IBTX and the voltage gated potassium channel blocker, 4-AP in CYP1A1 WT and (D) KO mice. Data represent mean ± SEM and were analyzed by two-way, repeated measures ANOVA, using post hoc Holm-Sidak comparisons; *p < 0.05, compared to wildtype (n=4–6/genotype).
RNA analysis of BK and Kv channel subunits, and CYP2C29, CYP1B1, CYP2E1, and CYP2D6
To determine if aortic expression of BK and Kv channel subunits, as well as several vascular P450s involved in n-3 PUFA metabolism, CYP2C29, CYP1B1, CYP2E1 and CYP2D6 were altered in their expression, we measured mRNA of BKα, BKβ4, Kv1.5, CYP2C29, CYP1B1, CYP2E1 and CYP2D6 in aortas of CYP1A1 KO and WT mice cleaned of adipose tissue. We did not find any differences in the mRNA expression of these potassium channel subunits or P450s (See Supplemental Data, Table 1S), suggesting that loss of vasodilatory responses to n3- PUFAs in the CYP1A1 KO mice is not a result of changes in other P450s or in potassium channels that mediate the vasodilation.
Discussion
Our study shows for the first time that constitutive expression of CYP1A1 is required to maintain normal levels of BP in vivo and to mediate vasodilation responses to n-3 PUFAs ex vivo. CYP1A1 KO mice are hypertensive with a reduced HR. Additionally, they exhibit attenuated vasodilation to the n-3 PUFAs, EPA and DHA, in both conduit arteries as well as resistance arterioles. Notably, the putative CYP1A1 metabolites of EPA and DHA, 17,18-EEQ and 19,20-EDP, respectively, exhibit equivalent vasodilatory effects in CYP1A1 WT and KO mice, demonstrating that signaling pathways downstream of the production of the metabolites are not altered by loss of CYP1A1. Finally, although DHA-induced vasodilation was attenuated in CYP1A1 KO mice, our results suggest that this vasodilation is mediated by activation of potassium channels in both genotypes, further suggesting that the loss of n-3 PUFA-mediated vasodilation in CYP1A1 KO mice is not a result of changes in downstream signaling.
Despite evidence that CYP1A1 is expressed in the vascular endothelium and highly induced by physiological shear stress (Han et al., 2008; Conway et al., 2009), the role of CYP1A1 in the regulation of blood pressure has never been investigated previously. We found that CYP1A1 KO mice exhibit significantly elevated systolic and diastolic blood pressure. Interestingly, systolic blood pressure is significantly increased only at night when the mice are active. Since physical activity is associated with increased levels of physiological shear stress, this would suggest that CYP1A1 may play a role in the blood pressure responses to exercise. In addition, we found that CYP1A1 KO mice exhibit significantly elevated diastolic blood pressure throughout the entire 24 h light/dark cycle. This would suggest that CYP1A1 also may play a role in the overall regulation of peripheral vascular resistance, which would be consistent with a decrease in HR as a compensatory response. Nonetheless, future studies that assess cardiac output and stroke volume, or use flow probes, would be needed to confirm if peripheral vascular resistance is increased.
Despite evidence that CYP1A1 and NO are induced simultaneously by physiological shear stress in the endothelium (Malek et al., 1999; Boo et al., 2002a; Boo et al., 2002b; Eskin et al., 2004; Han et al., 2008; Conway et al., 2009) and that n-3 PUFAs mediate vasodilation, in part, via increases in NO (Ma et al., 2004; Li et al., 2007; Stebbins et al., 2008), the increase in BP in CYP1A1 KO mice is not a result of a loss of NO. In fact, multiple lines of evidence demonstrate that NO bioavailability and signaling are normal in CYP1A1 KO mice. NO bioavailability could be reduced by decreased production or increased inactivation by superoxide anion. The normal levels of plasma NOx in CYP1A1 KO mice suggest that there is no change in total NO production. Furthermore, our previous work shows that superoxide anion production in the aorta, kidney and heart is not increased in CYP1A1 KO mice, suggesting that NO inactivation is probably not occurring (Kopf et al., 2010). This conclusion is further supported by the data that ACh-mediated dilation in the thoracic aorta, which is exclusively NO-dependent (Rees et al., 1989), is normal in CYP1A1 KO mice as is the dilation response to an NO donor. Most convincingly, treatment of CYP1A1 WT and KO mice with a NOS inhibitor does not normalize BP between the two genotypes, demonstrating that NO contributes equally to BP regulation.
Since constitutive expression of CYP1A1 is regulated by the AHR as is shown in the AHR KO mice, it might be expected that AHR KO and CYP1A1 KO mice would exhibit similar BP phenotypes. However, this is not the case. Our data show that genetic deletion of CYP1A1 is associated with elevated BP, in contrast to reduced blood pressure (i.e. hypotension) observed for global AHR KO mice (Zhang et al., 2010) as well as the endothelial cell-specific AHR conditional KO mice (Agbor et al., 2011). This suggests that multiple downstream targets regulated by the AHR are involved in BP regulation. One of these targets, in addition to CYP1A1, could be CYP1B1. CYP1B1 is constitutively expressed in vascular smooth muscle, and although CYP1B1 KO mice have normal blood pressure, they show a significantly attenuated response to angiotensin II-induced hypertension (Jennings et al., 2010). Our data show that vascular CYP1B1 mRNA expression is not different between genotypes, which is consistent with our previous studies (Kopf et al., 2008); however, it is possible that constitutive expression of CYP1B1 may function in a pro-hypertensive manner, while CYP1A1 may function in a pro-hypotensive manner. Thus, studying BP regulation in a double CYP1A1/1B1 KO model would be useful to elucidate the relative contribution of each P450 to BP regulation.
The involvement of P450s in blood pressure regulation is not unique to CYP1A1. Genetic deletion of CYP2J5 is associated with increased BP and overt afferent arteriolar responses to angiotensin II and endothelin I (Athirakul et al., 2008). In addition, loss of CYP4A10 in mice is associated with a hypertensive phenotype which is salt-sensitive (Nakagawa et al., 2006). Further, mice in which CYP4A14 is deleted are also hypertensive (Holla et al., 2001), and this increase in BP is associated with an increase in renal CYP4A12 expression and an increase in the production of the potent vasoconstrictor, 20-hydroxyeicosatetraenoic acid (Carroll et al., 1996). Nonetheless, to the best of our knowledge, other P450s that have been shown to metabolize n-3 PUFAs in a stereospecific manner in vitro have not been investigated for their contribution to BP regulation in vivo (Lucas et al., 2010; Westphal et al., 2011). It is noteworthy that the percentage of n-3 PUFAs in the diet fed to the mice in this study is relatively low (8% of total fatty acids). Thus, if CYP1A1 is a predominant P450 involved in n-3 PUFA metabolism, the low level of dietary n-3 PUFAs might account for the increase in blood pressure in CYP1A1 KO mice due to the loss of vasodilatory metabolites. Nonetheless, we would predict that augmenting dietary n-3 PUFAs would decrease blood pressure in CYP1A1 KO mice as a result of increasing the substrate availability for other P450s that also metabolize n-3 PUFAs. Studies using diets enriched in n-3 and n-6 PUFAs are currently underway.
Our study also provides the first in vivo evidence that constitutive expression of CYP1A1 mediates, in part, the vasodilatory properties of n-3 PUFAs, EPA and DHA, in both conduit arteries and resistance arterioles. This result is highly consistent with the in vitro studies demonstrating that recombinant human CYP1A1 metabolizes EPA and DHA stereospecifically into products with vasodilatory properties (Fer et al., 2008; Lucas et al., 2010). Schwarz et al. (2004) show that 17(R),18(S)-EEQ represents 70% of the products produced by human CYP1A1 metabolism of EPA, while 19(R),20(S)-EDP is the only product produced by human CYP1A1 metabolism of DHA (Fer et al., 2008). Our preliminary studies suggest that mouse CYP1A1 shows this same degree of stereospecificity in EPA and DHA metabolism as human CYP1A1 (unpublished data) and this would be consistent with our current results showing that mouse CYP1A1 mediates the production of vasodilatory products from EPA and DHA in vivo.
Our data also suggest that the attenuation of vasodilation to EPA and DHA in arteries of CYP1A1 KO mice is not likely a result of altered downstream signaling. There are no differences in the vasodilation responses between CYP1A1 WT and KO mice when the two putative CYP1A1-metabolites, 17,18-EEQ and 19,20-EDP, are applied directly to the arteries. Further, DHA-mediated vasodilation is attenuated by two potassium channel inhibitors in both genotypes, again suggesting that downstream signaling that results in vasodilation is not different between genotypes.
Irrespective of the P450 involved, our data also confirm that 17,18-EEQ and 19,20-EDP are potent and efficacious vasodilators. We found that 17,18-EEQ and 19,20-EDP induce ~50% relaxation of the aorta at sub-nM concentrations and >90% relaxation at nM concentrations. In general, the mesenteric arterioles are less responsive and require nM to μM concentrations to induce vasodilation. The potency and efficacy of 17,18-EEQ and 19,20-EDP in vasorelaxation of the mouse aorta is similar to the vasodilatory responses observed in porcine coronary microvessels (Zhang et al., 2001; Ye et al., 2002). However, the vasodilation responses to 17,18-EEQ in the mouse mesenteric arterioles is more similar to that observed in human pulmonary arteries with ~ 80% relaxation occurring at μM concentrations (Morin et al., 2009). Thus, the vasodilatory responses to these n-3 PUFA metabolites are likely to be both species- and vascular bed-specific.
Studies suggest that there are various downstream mechanisms by which EPA and DHA mediate their vasodilatory responses. Wang et al. (2011) showed that the vasodilatory actions of DHA are mediated by P450 metabolism and downstream activation of BK and Kv channels in rat coronary arteries, while Lauterback et al. (2002) showed that 17,18-EEQ hyperpolarizes vascular smooth muscles from rat cerebral arteries exclusively by BK channel activation. Our data show blockade of BK channels alone does not attenuate DHA-mediated dilation in mouse mesenteric arteries; however, the simultaneous inhibition of Kv channels does significantly attenuate the dilation response, suggesting that BK channels may not play a role in DHA dilation in this microvascular bed.
Finally, CYP1A1 KO mice exhibit phenotypic characteristics that are distinct from age-matched CYP1A1 WT mice. Body weights in CYP1A1 KO mice are significantly lower as are liver and kidney weights when normalized to body weight. The decrease in liver weight is similar to the AHR KO mice, which exhibit decreased liver size resulting from a persistent ductus venosus and reduced hepatocyte size (Lahvis et al., 2000; Lahvis et al., 2005). Despite the decrease in kidney weight in CYP1A1 KO mice, plasma indices of renal function were normal. Further studies are needed to determine the underlying reasons for the differences in body and organ weights between CYP1A1 WT and KO mice.
In summary, our study elucidates for the first time that constitutive CYP1A1 expression has a physiologically important role in the regulation of vascular function and blood pressure, which may involve vasodilatory responses to n-3 PUFAs. Since CYP1A1 is constitutively expressed in the vascular endothelium and physiological levels of shear stress can induce its expression, CYP1A1 metabolism of n-3 PUFAs could represent a novel pathway contributing to an anti-atherogenic phenotype. Future studies will need to determine whether the loss of n-3 PUFA vasodilation and potential increase in peripheral vascular resistance are the proximal mediators of the increase in blood pressure in CYP1A1 KO mice.
Supplementary Material
Highlights.
Assessed blood pressure and vascular reactivity in CYP1A1 WT and KO mice
CYP1A1 KO mice are hypertensive and exhibit reduced vasodilation responses to n-3 PUFAs
Constitutive CYP1A1 expression is required for blood pressure regulation potentially mediated via n-3 PUFA metabolism
Acknowledgments
This study was supported by a grant from the National Institutes of Health [R21 HL107133 to M.K.W.]. The authors thank Drs. Pim Ketsawatsomkron and Nancy L. Kanagy for their technical assistance.
ABBREVIATIONS
- CYP1A1
Cytochrome P4501A1
- PUFAs
Polyunsaturated fatty acids
- EPA
Eicosapentaenoic acids
- DHA
Docosahexaenoic acids
- EDP
Epoxydocosapentaenoic acid
- EEQ
Epoxyeicosatetraenoic acid
- ACh
Acetylcholine
- PE
Phenylephrine
- 4-AP
4-aminopyridine
- IBTX
Iberiotoxin
- SNAP
S-nitroso-N-acetyl penicillamine
- LNNA
Nω-nitro-L-arginine
- MAP
Mean arterial pressure
- BP
Blood pressure
- HR
Heart rate
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- NOx
Nitrates/nitrites
- NO
Nitric oxide
Footnotes
Conflict of interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agbor LN, Elased KM, Walker MK. Endothelial cell-specific aryl hydrocarbon receptor knockout mice exhibit hypotension mediated, in part, by an attenuated angiotensin II responsiveness. Biochem Pharmacol. 2011;82:514–523. doi: 10.1016/j.bcp.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athirakul K, Bradbury JA, Graves JP, DeGraff LM, Ma J, Zhao Y, Couse JF, Quigley R, Harder DR, Zhao X, Imig JD, Pedersen TL, Newman JW, Hammock BD, Conley AJ, Korach KS, Coffman TM, Zeldin DC. Increased blood pressure in mice lacking cytochrome P450 2J5. FASEB J. 2008;22:4096–4108. doi: 10.1096/fj.08-114413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billman GE, Hallaq H, Leaf A. Prevention of ischemia-induced ventricular fibrillation by omega 3 fatty acids. PNAS. 1994;91:4427–4430. doi: 10.1073/pnas.91.10.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet C, Dewailly E, Ayotte P, Bruneau S, Receveur O, Holub BJ. Contribution of selected traditional and market foods to the diet of Nunavik Inuit women. Can J Diet Pract Res. 2000;61:50–59. [PubMed] [Google Scholar]
- Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, Jo H. Shear stress stimulates phosphorylation of eNOS at Ser635 by a protein kinase A-dependent mechanism. Amer J of Phys - H Circ Physio. 2002a;283:H1819–H1828. doi: 10.1152/ajpheart.00214.2002. [DOI] [PubMed] [Google Scholar]
- Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, Jo H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms. J Biol Chem. 2002b;277:3388–3396. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- Carroll MA, Balazy M, Margiotta P, Huang DD, Falck JR, McGiff JC. Cytochrome P-450-dependent HETEs: profile of biological activity and stimulation by vasoactive peptides. Amer J Phys. 1996;271:R863–869. doi: 10.1152/ajpregu.1996.271.4.R863. [DOI] [PubMed] [Google Scholar]
- Conway DE, Sakurai Y, Weiss D, Vega JD, Taylor WR, Jo H, Eskin SG, Marcus CB, McIntire LV. Expression of CYP1A1 and CYP1B1 in human endothelial cells: regulation by fluid shear stress. Cardiovasc Res. 2009;81:669–677. doi: 10.1093/cvr/cvn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2004;85:9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Dieter MZ, Matlib RS, Childs NL, Shertzer HG, Genter MB, Nebert DW. Targeted knockout of Cyp1a1 gene does not alter hepatic constitutive expression of other genes in the mouse [Ah] battery. Biochem Biophys Res Comm. 2000;267:184–189. doi: 10.1006/bbrc.1999.1913. [DOI] [PubMed] [Google Scholar]
- De Caterina R, Madonna R. Cytochromes CYP1A1 and CYP1B1: new pieces in the puzzle to understand the biomechanical paradigm of atherosclerosis. Cardiovasc Res. 2009;81:629–632. doi: 10.1093/cvr/cvp013. [DOI] [PubMed] [Google Scholar]
- Duling LC, Cherng TW, Griego JR, Perrine MF, Kanagy NL. Loss of alpha2B-adrenoceptors increases magnitude of hypertension following nitric oxide synthase inhibition. Amer J Phys. 2006;291:H2403–2408. doi: 10.1152/ajpheart.01066.2005. [DOI] [PubMed] [Google Scholar]
- Engler MM, Engler MB, Goodfriend TL, Ball DL, Yu Z, Su P, Kroetz DL. Docosahexaenoic acid is an antihypertensive nutrient that affects aldosterone production in SHR. Proc Soc Exp Biol Med. 1999;221:32–39. doi: 10.1046/j.1525-1373.1999.d01-51.x. [DOI] [PubMed] [Google Scholar]
- Eskin SG, Turner NA, McIntire LV. Endothelial Cell Cytochrome P450 1A1 and 1B1: Up-Regulation by Shear Stress. Endothelium. 2004;11:1–10. doi: 10.1080/10623320490432434. [DOI] [PubMed] [Google Scholar]
- Fer M, Dreano Y, Lucas D, Corcos L, Salaun JP, Berthou F, Amet Y. Metabolism of eicosapentaenoic and docosahexaenoic acids by recombinant human cytochromes P450. Arch Biochem Biophys. 2008;471:116–125. doi: 10.1016/j.abb.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Han Z, Miwa Y, Obikane H, Mitsumata M, Takahashi-Yanaga F, Morimoto S, Sasaguri T. Aryl hydrocarbon receptor mediates laminar fluid shear stress-induced CYP1A1 activation and cell cycle arrest in vascular endothelial cells. Cardiovasc Res. 2008;77:809–818. doi: 10.1093/cvr/cvm095. [DOI] [PubMed] [Google Scholar]
- Hennig B, Slim R, Toborek M, Robertson LW. Linoleic acid amplifies polychlorinated biphenyl-mediated dysfunction of endothelial cells. J Biochem Mol Toxicol. 1999;13:83–91. doi: 10.1002/(sici)1099-0461(1999)13:2<83::aid-jbt4>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Holla VR, Adas F, Imig JD, Zhao X, Price E, Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. PNAS. 2001;98:5211–5216. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings BL, Sahan-Firat S, Estes AM, Das K, Farjana N, Fang XR, Gonzalez FJ, Malik KU. Cytochrome P450 1B1 contributes to angiotensin II induced hypertension and associated pathophysiology. Hypertension. 2010;56:667–674. doi: 10.1161/HYPERTENSIONAHA.110.154518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakela R, Kinnunen S, Kakela A, Hyvarinen H, Asikainen J. Fatty acids, lipids, and cytochrome p-450 monooxygenase in hepatic microsomes of minks fed fish-based diets and exposed to Aroclor 1242. J Toxicol Environ Health A. 2001;64:427–446. doi: 10.1080/152873901753170759. [DOI] [PubMed] [Google Scholar]
- Kopf P, Huwe J, Walker M. Hypertension, cardiac hypertrophy, and impaired vascular relaxation induced by 2,3,7,8-tetrachlorodibenzo-dioxin are associated with increased superoxide. Cardiovasc Toxicol. 2008;8:181–193. doi: 10.1007/s12012-008-9027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf PG, Scott JA, Agbor LN, Boberg JR, Elased KM, Huwe JK, Walker MK. Cytochrome P4501A1 is required for vascular dysfunction and hypertension induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Tox Sci. 2010;117:537–546. doi: 10.1093/toxsci/kfq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahvis GP, Lindell SL, Thomas RS, McCuskey RS, Murphy C, Glover E, Bentz M, Southard J, Bradfield CA. Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. PNAS. 2000;97:10442–10447. doi: 10.1073/pnas.190256997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahvis GP, Pyzalski RW, Glover E, Pitot HC, McElwee MK, Bradfield CA. The aryl hydrocarbon receptor is required for developmental closure of the ductus venosus in the neonatal mouse. Mol Pharmacol. 2005;67:714–720. doi: 10.1124/mol.104.008888. [DOI] [PubMed] [Google Scholar]
- Lauterbach B, Barbosa-Sicard E, Wang MH, Honeck H, Kargel E, Theuer J, Schwartzman ML, Haller H, Luft FC, Gollasch M, Schunck WH. Cytochrome P450-dependent eicosapentaenoic acid metabolites are novel BK channel activators. Hypertension. 2002;39:609–613. doi: 10.1161/hy0202.103293. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhang Q, Wang M, Liu F, Zhao S, Ma J, Luo N, Li N, Li Y, Xu G, Li J. Docosahexaenoic acid affects endothelial nitric oxide synthase in caveolae. Arch Biochem Biophys. 2007;466:250–259. doi: 10.1016/j.abb.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Lim EJ, Smart EJ, Toborek M, Hennig B. The role of caveolin-1 in PCB77-induced eNOS phosphorylation in human-derived endothelial cells. Amer J Phys. 2007;293:H3340–H3347. doi: 10.1152/ajpheart.00921.2007. [DOI] [PubMed] [Google Scholar]
- Lucas Dl, Goulitquer S, Marienhagen J, Fer M, Dreano Y, Schwaneberg U, Amet Y, Corcos L. Stereoselective epoxidation of the last double bond of polyunsaturated fatty acids by human cytochromes P450. J Lip Res. 2010;51:1125–1133. doi: 10.1194/jlr.M003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund AK, Agbor LN, Zhang N, Baker A, Zhao H, Fink GD, Kanagy NL, Walker MK. Loss of the aryl hydrocarbon receptor induces hypoxemia, endothelin-1, and systemic hypertension at modest altitude. Hypertension. 2008;51:803–809. doi: 10.1161/HYPERTENSIONAHA.107.100586. [DOI] [PubMed] [Google Scholar]
- Ma DWL, Seo J, Davidson LA, Callaway ES, Fan YY, Lupton JR, Chapkin RS. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. FASEB J. 2004;18:1040–1042. doi: 10.1096/fj.03-1430fje. [DOI] [PubMed] [Google Scholar]
- Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- Menotti A, Kromhout D, Blackburn H, Fidanza F, Buzina R, Nissinen A. Food intake patterns and 25-year mortality from coronary heart disease: Cross-cultural correlations in the Seven Countries Study. Eur J Epidemiol. 1999;15:507–515. doi: 10.1023/a:1007529206050. [DOI] [PubMed] [Google Scholar]
- Messina A, Puccinelli E, Gervasi PG, Longo V. Expression and inducibility of CYP1A1, 1A2, 1B1 by beta-naphthoflavone and CYP2B22, CYP3As by rifampicin in heart regions and coronary arteries of pig. Res Vet Sci. 2012 doi: 10.1016/j.rvsc.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Moran FM, Hendrickx AG, Shideler S, Overstreet JW, Watkins SM, Lasley BL. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on fatty acid availability and neural tube formation in cynomolgus macaque, Macaca fascicularis. Birth Defects Res B Dev Reprod Toxicol. 2004;71:37–46. doi: 10.1002/bdrb.10056. [DOI] [PubMed] [Google Scholar]
- Morin C, Sirois M, Echave V, Rizcallah E, Rousseau E. Relaxing effects of 17(18)-EpETE on arterial and airway smooth muscles in human lung. Amer J Phys. 2009;296:L130–L139. doi: 10.1152/ajplung.90436.2008. [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Holla VR, Wei Y, Wang WH, Gatica A, Wei S, Mei S, Miller CM, Cha DR, Price E, Zent R, Pozzi A, Breyer MD, Guan Y, Falck JR, Waterman MR, Capdevila JH. Salt-sensitive hypertension is associated with dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J Clin Invest. 2006;116:1696–1702. doi: 10.1172/JCI27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees DD, Palmer RM, Hodson HF, Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989;96:418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A, Watts S. Vascular reactivity of isolated thoracic aorta of the C57BL/6J mouse. J Pharmacol Exp Ther. 2000;294:598–604. [PubMed] [Google Scholar]
- Schwarz D, Kisselev P, Chernogolov A, Schunck WH, Roots I. Human CYP1A1 variants lead to differential eicosapentaenoic acid metabolite patterns. Biochem Biophys Res Comm. 2005;336:779–783. doi: 10.1016/j.bbrc.2005.08.172. [DOI] [PubMed] [Google Scholar]
- Schwarz D, Kisselev P, Ericksen SS, Szklarz GD, Chernogolov A, Honeck H, Schunck WH, Roots I. Arachidonic and eicosapentaenoic acid metabolism by human CYP1A1: highly stereoselective formation of 17(R),18(S)-epoxyeicosatetraenoic acid. Biochem Pharmacol. 2004;67:1445–1457. doi: 10.1016/j.bcp.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Simon P. Q-Gene: processing quantitative real-time RTPCR data. Bioinformatics. 2003;19:1439–1440. doi: 10.1093/bioinformatics/btg157. [DOI] [PubMed] [Google Scholar]
- Stebbins CL, Stice JP, Hart CM, Mbai FN, Knowlton AA. Effects of dietary docosahexaenoic Acid (DHA) on eNOS in human coronary artery endothelial cells. J Cardiovasc Pharmacol. 2008;13:261–268. doi: 10.1177/1074248408322470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Reiterer G, Toborek M, Hennig B. Changing ratios of omega-6 to omega-3 fatty acids can differentially modulate polychlorinated biphenyl toxicity in endothelial cells. Chem Biol Interact. 2008;172:27–38. doi: 10.1016/j.cbi.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RX, Chai Q, Lu T, Lee HC. Activation of vascular BK channels by docosahexaenoic acid is dependent on cytochrome P450 epoxygenase activity. Cardiovasc Res. 2011;90:344–352. doi: 10.1093/cvr/cvq411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal C, Konkel A, Schunck WH. CYP-eicosanoids--a new link between omega-3 fatty acids and cardiac disease? Prostagl Lip Med. 2011;96:99–108. doi: 10.1016/j.prostaglandins.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Ye D, Zhang D, Oltman C, Dellsperger K, Lee HC, VanRollins M. Cytochrome P-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J Pharmacol Exp Ther. 2002;303:768–776. doi: 10.1124/jpet.303.2.768. [DOI] [PubMed] [Google Scholar]
- Zhang N, Agbor LN, Scott JA, Zalobowski T, Elased KM, Trujillo A, Duke MS, Wolf V, Walsh MT, Born JL, Felton LA, Wang J, Wang W, Kanagy NL, Walker MK. An activated renin angiotensin system maintains normal blood pressure in aryl hydrocarbon receptor heterozygous mice but not in null mice. Biochem Pharmacol. 2010;80:197–204. doi: 10.1016/j.bcp.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Oltman CL, Lu T, Lee HC, Dellsperger KC, VanRollins M. EET homologs potently dilate coronary microvessels and activate BKCa channels. Amer J Phys. 2001;280:H2430–H2440. doi: 10.1152/ajpheart.2001.280.6.H2430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.