Abstract
The microenvironment of the mammary gland has been shown to exert a deterministic control over cells from different normal organs during murine mammary gland regeneration in transplantation studies. When mouse mammary tumor virus (MMTV)-neu-induced tumor cells were mixed with normal mammary epithelial cells (MECs) in a dilution series and inoculated into epithelium- free mammary fat pads, they were redirected to noncarcinogenic cell fates by interaction with untransformed MECs during regenerative growth. In the presence of nontransformed MECs (50:1), tumor cells interacted with MECs to generate functional chimeric outgrowths. When injected alone, tumor cells invariably produced tumors. Here, the normal microenvironment redirects MMTV-neu- transformed tumorigenic cells to participate in the regeneration of a normal, functional mammary gland. In addition, the redirected tumor cells show the capacity to differentiate into normal mammary cell types, including luminal, myoepithelial and secretory. The results indicate that signals emanating from a normal mammary microenvironment, comprised of stromal, epithelial and host-mediated signals, combine to suppress the cancer phenotype during glandular regeneration. Clarification of these signals offers improved therapeutic possibilities for the control of mammary cancer growth.
Keywords: erbB2/HER2, mammary, microenvironment, regeneration, suppression of tumorigenesis
Introduction
Breast cancer is the most common cancer among women in the United States and is the second leading cause of cancer-related deaths (Jemal et al., 2007). The presence of erbB2, the product of the neu oncogene, in patients with primary breast cancer acts as a prognostic factor and predicts clinical outcomes. Overexpression of the neu oncogene has been implicated in the development of an aggressive form of human breast cancer, and is inversely proportionate to patient survival (Slamon et al., 1987, 1989). Studies have shown that the unactivated neu protein is the primary factor that contributes to human breast cancer and not the activated form of the protein (Slamon et al., 1987). In genetically engineered strains of mice that are highly susceptible to mammary tumorigenesis and exhibit accelerated tumor development in post-partum or multiparous female mice, parity-identified, long-lived mammary epithelial cells (PI-MEC) serve as targets for neoplastic transformation (Wagner et al., 2002; Boulanger et al., 2005). Transgenic mice expressing the wildtype neu oncogene under transcriptional regulation of the mouse mammary tumor virus-long terminal repeat (MMTV-LTR) were bred with WAP-Cre/Rosa26R mice. Using this tri-transgenic model, Henry et al. (2004) showed that only multiparous female mice consistently exhibited accelerated tumorigenesis compared with their nulliparous littermate controls in a mixed genetic background. In both multiparous and nulliparous female mice, mammary tumors arose from WAP-Cre-activated, lacZ+ epithelial cells. The authors interpreted this finding to indicate that the PI-MEC in these glands represented the major target for MMTV-neu malignant transformation. This observation has been confirmed and extended in MMTV-neu mice (Jeselsohn et al., 2010).
It has been recently shown that the mammary gland microenvironment can redirect the cell fates of testicular cells derived from the seminiferous tubules of adult male mice and bona fide neural stem cells, collected from both embryonic and adult brain, during mammary gland regeneration following transplantation (Boulanger et al., 2007; Booth et al., 2008). The experiments reported here were conducted to determine whether carcinogenic mammary cells might be redirected to participate in the normal development of the mammary gland.
The mammary microenvironment is comprised of several required elements: stem cells, neighboring signaling cells, supporting stroma (extracellular matrix) and a range of intercellular signals that regulate stem cell behavior as well as the behavior of the signaling cells in a juxtacrine/paracrine manner. In this study, we asked whether the normal mammary gland microenvironment could direct carcinogenic MMTV-neu-transformed cells to participate in the normal development of the mammary gland. To test this hypothesis, increasing dilutions of MMTV-neu tissue-cultured cells collected from WAP-Cre/Rosa26R/MMTV-neu tumors were mixed with wild-type MECs from primary mammary cell cultures. These mixtures were inoculated into the epithelium-divested mammary fat pads of immunocompromised juvenile female hosts.
The results show that MMTV-neu-initiated tumor cells are capable of interacting with the normal epithelium such that they contribute epithelial progeny to normal mammary gland growth and regeneration. Second transplant generations from these chimeric outgrowths indicate that the tumor cells are capable of self-renewal and further contribution to normal mammary epithelial development. Surprisingly, unsorted cells recovered from secondary tumor/normal cell outgrowths propagated in vitro were incapable of forming tumors when inoculated into epithelium-free mammary fat pads, even though a large percentage continued to express the neu transgene. When the recovered cells are sorted for erbB2 expression, tumor-initiating activity was re-established.
Results
MMTV-neu tumor-derived cells contribute to normal mammary outgrowths
We used two established tumor cell lines derived from mammary carcinomas that arose in WAP-Cre/Rosa26R/MMTV-neu triple transgenic female mice (Henry et al., 2004). One line was developed from one of the rare tumors that arose in a nulliparous female mice, whereas the second line was derived from one of the numerous tumors developing in parous female mice. Each tumor cell population is constitutively lacZ+ (blue after X-gal staining). Thus, constitutive expression of lacZ is a lineal marker for neu tumor-derived mammary cells in our experiments. To establish the tumorigenicity of these two cell lines, each was injected separately into the cleared mammary fat pads of 3-week-old Nu/Nu mice at varying concentrations (100, 10 and 1.0K; Figure 1a). In all instances, the transplanted tumor-derived cells from either cell line produced mammary tumors, with an average latency of 5.5, 7.5 and 7.5 months, respectively (Table 1). Focal tumor development at numerous sites along the needle track was observed at early time points (Figures 1b1 and b2). Cross-sectional histological analysis shows that each small tumor was comprised entirely of lacZ+ cells, indicating that the growths arose from the transplanted tumor cells (Figure 1b2). The small focal tumors eventually progressed to form large mammary tumors comprised entirely of lacZ+ cells if left unattended (not shown).
Figure 1.
Tumor-derived cells give rise to normal mammary structures. (a) Schematic outlining the experimental protocol used (modified from Bissell and Inman, 2008). Cells were isolated from mammary tumors from WAP-Cre/Rosa26/MMTV-neu female mice. These were transplanted into the cleared mammary fat pads of 3-week-old virgin Nu/Nu female mice alone (100, 10 or 1.0 K) or mixed with 50K normal FVB/N mammary epithelial cells in ratios of 2:1, 1:5 or 1:50. These transplants were allowed to grow for 6–8 weeks or until tumors arose. The mature transplanted gland was removed and 80% was used for tissue analyses and the remaining 20% was transplanted into new 3-week-old virgin Nu/Nu female mice. After 8 weeks, these female mice were placed with male mice and allowed to complete a full-term pregnancy, after which the transplanted mammary glands were removed and analyzed or viably frozen as fragments. (b, b1) Tumor-derived cells (50 K) were transplanted and the resulting focal tumors were whole mounted and X-gal stained. (b2) Cross-section of the portion of the tumor outlined in (b1), indicating that the entire tumor is comprised of lacZ+ cells. (b3) Tumor-derived cells (1.0 K) were mixed with 50K normal FVB/N mammary epithelial cells and transplanted. Resulting mammary glands were whole mounted and stained with X-gal. Normal ductal tree and duct termini are evident. (b4) Higher power magnification of whole mount in (b3) showing X-gal-stained endbuds. (b5) Cross-section of chimeric mammary gland illustrating mosaic-staining pattern of β-gal+ cells. (b6) Cross-section of chimeric mammary gland showing, in some instances, normal development is independent of tumor-derived cells. (b7) Second transplant generation mammary outgrowths displaying similar distribution of lacZ+ tumor-derived cells as seen in (b3) and (b4). (b8) Cross-section of (b7). Nuclei in (b2), (b5), (b6) and (b8) were counterstained pink with Nuclear Fast Red. Scale bars, 2.5mm (b1) and (b3); 50 μm (b2), (b5), (b6) and (b8); and 500 μm (b4) and (b7).
Table 1.
Summary of transplantation studies
| No. injected | Result | Normal growth | %Tumors | LacZ+ | Time (months) |
|---|---|---|---|---|---|
| 100K Tumor | 6/6a | No | 100 | Yes | 3–7 (5.5) |
| 10K Tumor | 6/6 | No | 100 | Yes | 5–8 (7.5) |
| 1K Tumor | 6/6a | No | 100 | Yes | 6–8 (7.5) |
| 100K Tumor +50K FVB/N | 6/6a | No | 100 | Yes | 5 |
| 10K Tumor +50K FVB/N | 7/8 | Yes (5/8) | 62.5 | Yes | 5 |
| 1K Tumor +50K FVB/N | 15/16 | Yes (15/15) | 6.67 | Yes | 6 |
| 10K Tumor +50K FVB/N second Tgen | 4/6 | Yes | 0 | Yes | ND |
| 1K Tumor +50K FVB/N second Tgen | 12/14 | Yes | 0 | Yes | ND |
Abbreviations: Tgen, transplantation generation; ND, no tumors developed up to 18 months.
Result indicates the number of fat pads that contained mammary growth out of the total number of fat pads injected.
Indicates that one mouse died after transplantation before any mammary development from that experimental group. Time, time until tumor formation; range is given in months, with average within parentheses.
MMTV-neu tumor-derived cells were mixed with wild-type MECs in ratios of 2:1, 1:5 and 1:50 and transplanted as described above. Mammary outgrowths comprising of lacZ+ and lacZ− portions were observed in recipients who received either the 1:5 or 1:50 mix of cells (Table 1 and Figures 1b3 and b4). The reconstituted glands contained both lacZ+ and lacZ− cells, indicating that both normal and tumorigenic epithelial cells contributed to the outgrowth since only the tumorigenic cells express lacZ (Figure 1b5). The lacZ+ cells were found in groups indicative of local clonal expansion of lacZ+ progeny and also juxtaposed to lacZ− epithelial cells, suggesting interaction between tumor and normal mammary cells during duct development. Some mammary ducts in the chimeras did not contain lacZ+ cells (Figure 1b6) consistent with the absence of lacZ staining in some portions of the X-gal-stained whole-mount preparations. In second transplantation generation chimeric glands derived from the regenerated mammary glands of 1:5 and 1:50 ratios, no tumors formed after 18 months, although lacZ+ cells were present (Figures 1b7 and b8; Table 1).
Both tumor-derived cell lines are tumorigenic and able to contribute to the outgrowths with equivalent frequency when combined with normal MECs. Thus, the data derived from the two cell lines has been pooled in Table 1 and all additional figures. There was no discernible difference between the two lines in any of the studies conducted.
Differentiation potential of tumor cells in chimeras
MMTV-neu-induced mammary hyperplasia and tumors in the C57/Bl6 background are estrogen receptor alpha negative (ERα−) (Cardiff et al., 2000; Henry et al., 2004). In glands from ERα-null mice, ductal formation is impaired, but in experiments in which epithelial cells from ERα−/− mice are mixed with wildtype epithelial cells, the reconstituted glands develop normally and ERα−/− cells contribute to all mammary epithelial subtypes, proving the requirement of ERα expression during normal mammary ductal development (Bocchinfuso et al., 2000; Mueller et al., 2002; Mallepell et al., 2006). When WAP-Cre/Rosa26R/MMTV-neu tumor-derived cells were mixed with normal FVB/N epithelial cells, ERα+ epithelial cells and progesterone receptor-positive (PR+) cells were observed within the resulting chimeric epithelial structures (Figures 2a1 and b1). These nuclear steroid receptor-positive cells were always lacZ−, indicating that they did not arise from tumor-derived cells. When the tumor-derived cells were injected alone, no ERα+ or PR+ cells were detected (Figures 2a2 and b2). The tumors that arose, either from tumorigenic cells injected alone or arising from mixed injections, remained negative for ERα and PR.
Figure 2.
Tumor-derived cells differentiate into different cell lineages and contribute to the formation of a functional mammary gland. Tumor-derived cells (1.0K) were mixed with 50K normal FVB/N mammary epithelial cells and injected into the cleared fat pad of Nu/Nu female hosts. Tissue sections of chimeric glands and mammary tumors were stained for β-gal (green) and ERα or PR (red). (a, a1) Chimeric mammary outgrowth showing ERα expression (red) indicated by arrows only in β-gal− cells. (a2) Cross-section of a β-gal+ mammary hyperplasia that is ERα−. (b, b1) Chimeric mammary outgrowth showing PR expression (arrows) only in β-gal− cells. (b, b2) Cross-section of a β-gal+ mammary hyperplasia that is PR−. (c) Cross-section of a chimeric gland at day 2 post-partum showing that tumor-derived cells, determined by β-gal (green) expression, become functional secretory epithelial cells and produce the milk protein β-casein (red). Arrows indicate tumor-derived cells that are producing β-casein. L – lumen. Sections (a2), (b2) and c) were counterstained with 4′,6-diamidino-2-phenylindole. Scale bars, 20 μm (a) and (b) and 10 μm (c).
LacZ+ cells (tumor cell progeny) were positioned along the mammary ducts and side branches, suggesting that the tumorigenic cells had proliferated in concert with the normal cells during ductal morphogenesis. LacZ+ cells were found both in luminal and in basal positions. Those in basal positions adopted a myoepithelial cell fate as determined by smooth muscle actin expression (Supplementary Figure S1a) and keratin 14 (K14) expression (Supplementary Figure S1c). The lacZ+ cells found in luminal positions expressed the luminal epithelial marker keratin 8 (K8) (Supplementary Figure S1b). In hosts that were allowed to complete a full-term pregnancy, lacZ+ cells were found along the ducts and in secretory lobules. The secretory lobules in lactating and late pregnant hosts carrying second-generation chimeric outgrowths were often comprised of lacZ+ epithelial cells. In contrast, the ducts in the chimeric outgrowth in late pregnant hosts were often scarcely populated with lacZ+ luminal epithelial cells (Supplementary Figure S1d). This finding supports the conclusion that the erbB2/lacZ+ progeny of tumor cells proliferate and adopt secretory phenotypes in pregnant hosts. The assumption of a secretory phenotype by the tumorigenic cells was confirmed by colocalization of β-casein and β-galactosidase (arrows in Figure 2c). These results provide evidence that the tumor-derived cells differentiate into diverse epithelial subtypes during interaction with the normal mammary cells.
Transgenic erbB2 expression in chimeras
ErbB2 expression was visualized by fluorescent immunohistochemistry in the tumor cell/normal cell chimeras. Immunological detection of erbB2 expression was associated with cells also expressing lacZ within chimeric structures (Figure 3a). In the tumors formed following transplantation of tumor-derived cells, all cells express both lacZ and erbB2 (Figure 3b). In contrast, erbB2 expression was nearly undetectable by immunohistochemistry in normal wild-type FVB/N mammary glands (Figure 3c).
Figure 3.
Tumor-derived cells maintain their erbB2+ status following transplantation. Immunofluorescent staining of chimeric cross-section showing (a) erbB2+ expression (red) and β-gal expression (green). (b) Tumor that developed following transplantation that is completely erbB2+ and β-gal+. (c) Section of wild-type FVB/N mammary gland showing non-existent erbB2 and β-gal expression. Far right image is section stained with nuclear fast red. Scale bars, 20 μm.
Absence of erbB2 phosphorylation in chimeric mammary glands
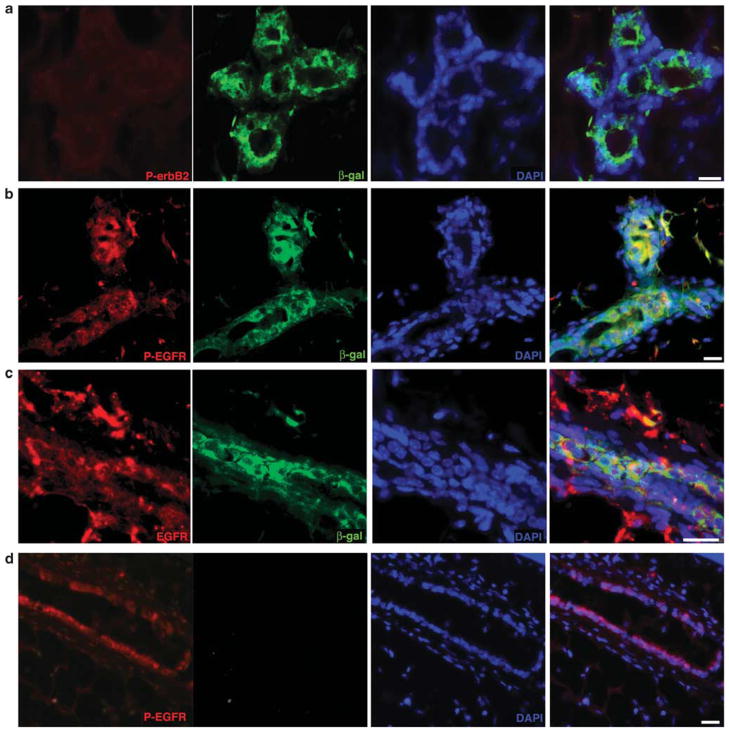
Immunofluorescent images reveal that the MMTV-driven erbB2 expressed in the chimeric mammary glands is not phosphorylated (Figure 4a), suggesting that transgenic erbB2 is not forming dimers with other members of the epidermal growth factor receptor (EGFR) family of receptors; erbB2 generally does not form homodimers and there is no known ligand specific for erbB2. This result indicates that the erbB2 transgenic product does not contribute to EGFR signaling in the chimeric glands. In contrast, erbB2 expressed by the cells within mammary tumors is phosphorylated (Supplementary Figure S2a), indicating that erbB2 is forming heterodimers and initiating signal transduction. EGFR is expressed in the chimeric glands (Figure 4c) and continues to participate in signal transduction as determined by the detection of the phosphorylated receptor (Figure 4b) similar to that observed in wildtype intact mammary glands (Figure 4d). Progressive loss of EGFR expression is observed in a subpopulation of erbB2-overexpressing human breast cancers (Choong et al., 2007). Continued expression of phosphorylated EGFR (Supplementary Figure S2b) and EGFR (Supplementary Figure S2c) was observed within the mammary tumors. No difference was observed with respect to the absence of phosphorylated erbB2 or the presence of EGFR phosphorylation when primary and secondary chimeric transplants were examined. Chimeric mammary outgrowths were dissociated and magnetically sorted into erbB2+ and erbB2− fractions. Western analysis was performed which showed that neither fraction contained cells in which erbB2 was activated (Supplementary Figure S2d). The sorts were not 100% accurate as shown by the presence of β-galactosidase in the erbB2− fraction. Nevertheless, no active erbB2 was found in the chimeric outgrowths. These observations show that interaction with the normal mammary microenvironment influences the signaling capacity of the MMTV-promoter-driven erbB2 and perhaps in turn the ability of the tumor cell progeny to express a tumorigenic phenotype. Whether this influence is through a change in dimerization partnering or is ligand dependent remains to be answered in future studies.
Figure 4.
Tumor-derived cell receptor phosphorylation status following transplantation. (a) Immunofluorescent staining of chimeric cross-section showing that within the chimera, there is no detectable phospho-erbB2 (red). Tumor-derived cells identified by β-gal expression (green). (b) Cross-section showing that within the chimera, tumor-derived cells continue to express phospho-EGFR (phospho-EGFR, red; β-gal, green). (c) Immunofluorescent image of a chimeric cross-section showing that within the chimera, tumor-derived cells continue to express EGFR (EGFR, red; β-gal, green). All sections counterstained with 4′,6-diamidino-2-phenylindole. Scale bars, 30 μm. (d) Section of a normal FVB/N mammary gland stained for P-EGFR and β-galactosidase (phospho-EGFR, red; β-gal, green.
Presence of MMTV-neu DNA and the absence of aneuploidy in the chimeric glands
To further authenticate the contribution of MMTV-neu tumor cells to the normal mammary outgrowths, polymerase chain reaction (PCR) was performed on DNA isolated from the original tumor cells and from the mammary chimeras. The MMTV-neu transgene was detectable in the initial tumor-derived cells and in cell cultures derived from second-generation chimeric outgrowths (Supplementary Figure S3a). WAP-Cre DNA was also present in the chimeras, indicating that the initial tumor-derived triple transgenic tumor cell progeny persisted through a second round of glandular development (not shown). In addition, β-galactosidase protein was found in the lysates of the initial tumor-derived cell lines as well as in cells recovered from second-generation chimeric mammary outgrowths (Supplementary Figure S3b). Similar results were found when erbB2 protein levels were investigated; erbB2 was present in tumor-derived cells before transplantation as well as in cell cultures created from second-generation chimeric outgrowths (Supplementary Figure S3b).
Tumor progression in transgenic mice overexpressing neu is dependent on somatic mutations in the juxta-membrane region of the receptor, resulting in the constitutive activation of the receptor (Muller et al., 1988; Bouchard et al., 1989; Guy et al., 1992, 1996; Siegel et al., 1999). The MMTV-neu transgenes of the original tumor-derived cells and of the recovered cells were sequenced and compared to determine if any new mutations had occurred. No differences were found between the two sequences (not shown). Despite the presence of cells bearing and expressing the MMTV-neu transgene, no tumors appeared in the second transplant generation after 18 months (Table 1). Comparison of ploidy between the initial tumor-derived cells and the recovered chimeric cells revealed no change in the 2N:4N ratio as determined by propidium iodide staining (Supplementary Figure S3c), indicating that fusion between normal and tumor cells did not occur. Cell cycle analysis indicates that there are no significant differences between cells before transplantation and those cells recovered following mammary outgrowth; 65.52% were in G1 before transplantation compared to 56.62% after recovered, 22.0% were in G2 before transplantation compared to 33.29% recovered; and 12.38% were in the S-phase before transplantation compared to 9.69% in the S-phase after recovery (Supplementary Figure S3d).
Tumorigenicity of cells recovered from chimeras
Secondary outgrowths from the chimeric mammary gland were collected, dissociated and subsequently plated in vitro under conditions equivalent to tumor cell propagation. These recovered cells were allowed to proliferate in culture through six passages at which point we postulated that only tumor cell progeny would be expanded significantly. Recipient mice received a range of recovered cells from in vitro passage no. 6 (1, 10, 100, 250 or 500K cells). We found no incidence of mammary tumor formation and only one instance of a normal mammary outgrowth where the lacZ+ cells were limited to the lumens of some ducts, indicating that some growth was occasionally obtained but no tumors.
As the tumor cell progeny in the chimeric mammary outgrowths continue to express erbB2, although its function (absence of phosphorylation) is repressed presumably by signals emanating from the surrounding normal microenvironment, we next attempted to recapture tumor cell progeny from the chimeras by magnetic cell sorting based on the surface expression erbB2. Chimeras were dissociated, cultured for six passages and then magnetically sorted into three fractions: non-sorted, erbB2+ and erbB2−. Each fraction was transplanted into the cleared fat pads of 3-week-old Nu/Nu female mice. No tumors formed from either the non-sorted or the erbB2− fractions. Cells from the erbB2+ fraction produced tumors in Nu/Nu host fat pads that were X-gal+ (Figure 5a). This shows the retention of tumorigenesis in the tumor-derived cells when separated from normal MEC. As in the initial tumors that formed, the recovered erbB2+ fraction produced mammary tumors that comprised of 100% lacZ+ cells (Figure 5b).
Figure 5.
Recovered erbB2+ cells form mammary tumors. Dissociated cells from chimeric outgrowths were magnetically sorted for erbB2 before transplantation. (a) ErbB2+ fraction formed mammary tumors when 1000 cells were transplanted even when fat pad contained host outgrowth (unstained ducts–add arrows). Tumors from two separate mammary recipients are shown. (b) Cross-sections analyses of mammary outgrowths shown in (a) showing entire mammary tumors consist of lacZ+ cells. Sections counterstained with nuclear fast red. Scale bars, 100 μm.
These results indicate that within the initial mammary tumor and the subsequent chimeric outgrowth, a population exists that retains progenitor cell characteristics as well as the capacity to initiate tumorigenesis. These two developmental outcomes, normal development or tumorigenesis, appear to be dictated by interaction with normal epithelial cells in the immediate tissue microenvironment.
Discussion
In previous studies, testicular cells derived from the seminiferous tubules of adult male mice and neural stem cells isolated and propagated from both embryonic and adult brain were redirected to actively participate and provide MEC progeny when mixed with MECs (1:1) before injection into epithelium-divested mammary fat pads (Boulanger et al., 2007; Booth et al., 2008). It is important also that the reprogrammed seminiferous and neural cells possessed the same multipotent capacity as we reported for the bona fide PI-MEC population isolated and characterized from intact mammary tissue (Boulanger et al., 2005). Our current results indicate that the normal mammary microenvironment produced by regenerating mammary outgrowth in epithelium-divested mammary fat pads can suppress the cancer phenotype of erbB2-transformed mammary tumor cells, which apparently arise from transformed PI-MEC (Henry et al., 2004) and redirect these tumor cells to produce progeny capable of contributing to normal mammary development. Suppression of the malignant phenotype by interaction with normal developmental tissues has been shown in earlier studies. In 1975, Mintz and Illmensee (1975) reported that embryonal carcinoma cells from transplantable mouse teratomas were incorporated in many normal adult tissues in genetically mosaic mice produced from blastocysts of fertilized mouse zygotes injected with these cancer cells. After 1 year, these authors showed that single teratocarcinoma cells could produce a clone capable of populating multiple tissue types with normally functioning progeny when injected into genetically marked mouse blastocysts (Illmensee and Mintz, 1976). Later, it was shown that Rous sarcoma virus injected into limb buds in the developing avian embryo fails to produce sarcoma formation (Dolberg and Bissell, 1984). Further, malignant melanoma of the mouse reverted to a normal phenotype when transplanted to embryonic skin during a narrow developmental window (Gerschenson et al., 1986). McCullough et al. (1998) showed that tumorigenic liver cells, which consistently form tumors at ectopic sites, differentiate to form normal hepatocytes in young rat livers, but not in aged rat livers. In these experiments, tumorigenic MECs interact with normal MECs throughout mammary gland regeneration and differentiate into functional mammary epithelial progeny during this process and are inhibited from expressing their tumorigenic phenotype. To our knowledge, this is the first demonstration that epithelial tumorigenic cells from an adult somatic tissue are induced to form progeny capable of differentiating into diverse epithelial cell types during organ morphogenesis.
Alveolar development cannot occur in the absence of PR+ mammary epithelium and normal branching ductal morphogenesis cannot proceed without ERα+ mammary epithelium (Brisken et al., 1998; Mallepell et al., 2006). We found a distinct absence of PR and ERα+ cells in MMTV-neu-induced mammary tumors and an absence of PR and ERα+ lacZ+ epithelial cells in the normal mammary chimeras. One interpretation of these results is that progression of MMTV-neu-induced hyperplasia to tumor formation results from the accumulation of PR− and ERα−, erbB2-expressing PI-MEC progeny that fail to provide the appropriate regulatory signaling required for normal alveolar cell cycling (expansion, function and apoptosis) during pregnancy, lactation, and subsequently during involution and glandular remodeling. The expansion and proliferation of PI-MEC, the targets of neu transformation, coincides with pregnancy and lactation. Similarly, increased MMTV-neu expression coincides with pregnancy and lactation. The result of this is alveolar hyperplasia and subsequent tumorigenesis. In addition, the failure of MMTV-neu-expressing tumor cells to form normal mammary structures by themselves would be impaired by the absence of PR+ and ERα+ progeny.
Contribution of the tumor cells to normal mammary growth and development in the presence of normal mammary epithelium indicate that transformed PI-MEC remain responsive to the normal regulatory signals provided by non-transformed mammary epithelium. Extension of this reasoning suggests that learning what these signals are could lead to a strategy for controlling MMTV-neu-induced tumor growth. In a conditional expression model, Chodosh and co-workers showed that continued expression of activated neu was essential for tumor growth and maintenance, including metastatic lesions (Moody et al., 2002). In this model, continued expression of c-neu is apparently irrelevant to tumor cell incorporation and contribution to normal mammary growth, development and functional differentiation, perhaps because the expressed erbB2 is not actively phosphorylated. This provides a mechanism through which interaction with the normal mammary microenvironment may suppress tumorigenesis. A second mechanism in the suppression of tumorigenesis in the normal gland may be the differentiation of tumor cell progeny into luminal and myoepithelial cells during mammary gland regeneration.
ErbB2+ cells recovered from the normal chimeric mammary outgrowths by magnetic sorting retained tumor-initiating capacity equivalent to the initial tumor- derived population when removed from the normal mammary microenvironment. Presumably when the recovered erbB2+ cells are transplanted without the wild-type MECs, the erbB2+ cells create a tumor-promoting niche. As the erbB2+ cells are incapable of expressing ERα or PR, the intercellular signals that control MMTV-neu tumorigenesis may originate from nuclear steroid receptor-expressing cells.
There is no known erbB2-specific ligand; therefore, the receptor initiates signal transduction by forming heterodimers with the other EGFR family members (EGFR, erbB3 and erbB4). In the chimeric mammary glands, we discovered a lack of phosphorylated erbB2 when compared to a mammary tumor formed from MMTV-neu cells, indicating that the normal MECs are repressing the erbB2 activation. Whether this is due to an intercellular signal or by some cell–cell adhesion signal is unknown at this time. The absence of phosphorylated erbB2 suggests that tumorigenic signals originating from the transgene-generated erbB2 may not be expressed in the tumor-derived cell progeny in the chimeric outgrowths. It is known that in some breast cancers the expression of EGFR is reduced, allowing for fewer EGFR homodimers and an increase in EGFR-erbB2 heterodimers (Choong et al., 2007). The introduction of the normal MECs into the transformed population allows for an increase in binding of EGFR-specific ligands, leading to the formation of more EGFR homodimers. The increase in EGFR expression due to the presence of the normal MECs in turn depletes the availability of EGFR-specific ligands whose receptor interactions results in EGFR-erbB2 heterodimers, thus reducing the number of activated erbB2 receptors and subsequent erbB2-induced intracellular signaling.
The persistence of mammary tumor-derived epithelial cells within normal mammary epithelial outgrowths shows that substituting normal developmental signals derived from normal wild-type epithelial cells and a normal stroma reverses the tumorigenic potential of the MMTV-neu-expressing cells and permits their direct contribution to normal mammary development, differentiation and function. In addition, the demonstration that the tumor-derived cells within the mammary chimera provide both myoepithelial and secretory epithelial progeny supports the conclusion that MMTV-neu tumors are supported and maintained by mutated multipotent PI-MEC.
Materials and methods
Cell culture
Two tumorigenic cell lines derived from triple transgenic WAP-Cre/Rosa26/MMTV-neu were used in this study (Henry et al., 2004). One line was derived from a tumor that arose in a nulliparous female mice, whereas the second line was derived from a parous female mice’s mammary tumor. The cell lines were grown in Dulbecco’s modied Eagle’s medium supplemented with 2% fetal bovine serum, hydrocortisone, insulin, EGF, bovine pituitary extract and gentamicin at 37 °C with 5% CO2. Both cell lines constitutively expressed intracellular lacZ over multiple passages.
Tissue/cell transplantation
DeOme and co-workers first described the technique of tissue fragment transplantation into mammary fat pads cleared of endogenous mammary epithelium (DeOme et al., 1959; Smith and Medina, 2008). The surgical procedures for clearing the fat pad of 3-week-old female mice have been described previously. Epithelial cells at the desired concentration were injected (10 μl volume) into the cleared fat pads. The recipients were kept as nulliparous virgins for 7–12 weeks to allow the transplanted epithelium to penetrate the host fat pad and form a ductal tree. At this time, the recipients were either placed with male mice to facilitate pregnancy or maintained as virgins to monitor tumor induction.
All mice were housed in Association for Assessment and Accreditation of Laboratory Animal Care-accredited facilities in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The National Cancer Institute Animal Care and Use Committee approved all experimental procedures.
X-gal analysis
Mammary glands were prepared as whole mounts in which the entire mammary inguinal gland (that is, gland 4) was spread on a glass slide and fixed for 1–2 h in 4% paraformaldehyde in phosphate-buffered saline (PBS). Tissues were washed repeatedly in PBS and processed for X-gal as described elsewhere (Wagner et al., 1997). For analysis of tissue sections, the mammary glands were post-fixed in Carnoy’s fixative and dehydrated, embedded in paraffin, and subsequently sectioned and counterstained with Nuclear Fast Red.
Immunochemistry
Immunohistochemistry was performed on paraffin-embedded sections according to standard procedures. Histopathological analysis was performed after hematoxylin and eosin staining. Immunohistochemistry was performed following antigen retrieval by boiling slides for 10 min in 10mM citrate buffer (pH 6.0). The primary antibodies used were anti-smooth muscle actin, anti-K8, anti-K14, anti-erbB2, anti-phosphoerbB2, anti-EGFR, anti-phospho-EGFR, anti-ERα and anti- PR. 3,3′ Diaminobenzidine was the chromagen followed by hematoxylin counterstain. For immunofluorescent detection, the slides were prepared as above and the antibodies used were anti-smooth muscle actin, anti-β-galactosidase, anti-neu, anti-phospho- neu, anti-β-casein, anti-EGFR and anti-phospho- EGFR. Secondary antibodies conjugated to Alexa 568 were then added, except for anti-β-galactosidase, which was conjugated directly to Alexa 488 using the Zenon primary labeling kit (Invitrogen, Carlsbad, CA, USA). Slides were coverslipped using ProLong Gold antifade reagent with 4′,6- diamidino-2-phenylindole (Invitrogen).
DNA extraction and PCR
Genomic DNA was extracted from mammary tissue according to Qiagen DNeasy kit protocol. PCR analysis was performed with WAP, Cre and standard mouse glyceraldehyde 3-phosphate dehydrogenase primers as published previously (Wagner et al., 1997; Kordon and Smith, 1998). Primers for MMTV-neu: forward, TTTCCTGCAGCAGCCTACGC and reverse, CGGAACCCACATCAGGCC. PCR conditions were as follows: an initial 7 min denaturation at 94 °C followed by 30 cycles of 94 °C for 1 min, 55 °C for 1 min and 72 °C for 1 min. PCR products were visualized in a 1.2% agarose gel containing ethidium bromide.
Magnetic sorting
Cells were detached by trypsin, washed once with PBS and resuspended in 100 μl of diluent containing primary antibody (1:50; anti-erbB2) and incubated for 30 min at 4 °C. The cells were then washed 2 × 10 min and resuspended in 100 μl of diluent containing biotinylated secondary antibody (1:100) and incubated for 15 min at 4 °C. The cells were then washed 2 × 10 min and resuspended in 80:20 μl mix of diluent and anti-biotin magnetic beads (Miltenyi, Auburn, CA, USA) and incubated for 15 min at 4 °C. The cells were then washed 2 × 10 min and resuspended in 500 μl of running buffer and separated through an LD column (Miltenyi). The positive and depleted fractions were collected and either used immediately for transplantation studies or cryopreserved for later analyses.
Western analysis
Cell lysates were collected using Cell Lysis Buffer (Cell Signaling Technology, Beverly, MA, USA) supplemented with 1mM phenylmethylsulfonyl fluoride and cleared by centrifugation. Lysates were mixed 1:1 with sample loading buffer and boiled for 10 min. The samples were loaded and run on precast 10 or 5–20% gradient SDS–PAGE gels (BioRad, Hercules, CA, USA) and transferred to nitrocellulose. The blots were blocked with 5% non-fat milk in PBS with 0.1% Tween-20 for 1 h and incubated with primary antibody (anti-erbB2 (1:500), anti-phospho-erbB2 (1:1000), anti-β-galactosidase (1:500) or anti-actin (1:1000)) overnight at 4 °C. The blots were washed with PBS/0.05% Tween-20 and incubated with horseradish peroxidase-linked secondary antibodies. LumiGLO (Cell Signaling Technology) was used as the developer.
Determination of ploidy by propidium iodide staining
Dispersed cell preparations were washed twice in DMEM without serum, resuspended at 1–2 × 107 cells per ml and placed on ice for 15 min. Cells were fixed in 70% ethanol, treated with 100U of RNase (Sigma-Aldrich, St Louis, MO, USA) at 37 °C for 20 min and stained for 30 min at 4 °C in a 50 μg/ml solution of propidium iodide (Molecular Probes, Eugene, OR, USA) in PBS. Cell clumps were removed by filtering through 40 μm nylon mesh before analysis on a Calibur flow cytometer running the CellQuest software (Becton Dickinson, Franklin Lakes, NJ, USA). Subsequent cell cycle and DNA content analyses were performed using the FlowJo software (Tree Star Inc., Ashland, OR, USA).
Supplementary Material
Acknowledgments
We thank KU Wagner for the gift of the triple transgenic WAP-Cre/Rosa26/MMTV-neu cell lines and for providing paraffin blocks from WAP-Cre/Rosa26R/MMTV-neu mouse mammary tissues. BK Vonderhaar, DS Salomon, R Callahan and GW Robinson for critical evaluation of the manuscript. The intramural research program of the Center for Cancer Research, NCI, NIH and the Institute for Biological Interfaces of Engineering of Clemson University contributed support to this work.
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Conflict of interest
The authors declare no conflict of interest.
References
- Bissell MJ, Inman J. Reprogramming stem cells is a microenvironmental task. Proc Natl Acad Sci USA. 2008;105:15637–15638. doi: 10.1073/pnas.0808457105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchinfuso WP, Lindzey JK, Hewitt SC, Clark JA, Myers PH, Cooper R, et al. Induction of mammary gland development in estrogen receptor-alpha knockout mice. Endocrinology. 2000;141:2982–2994. doi: 10.1210/endo.141.8.7609. [DOI] [PubMed] [Google Scholar]
- Booth BW, Mack DL, Androutsellis-Theotokis A, McKay RD, Boulanger CA, Smith GH. The mammary microenvironment alters the differentiation repertoire of neural stem cells. Proc Natl Acad Sci USA. 2008;105:14891–14896. doi: 10.1073/pnas.0803214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard L, Lamarre L, Tremblay PJ, Jolicoeur P. Stochastic appearance of mammary tumors in transgenic mice carrying the MMTV/c-neu oncogene. Cell. 1989;57:931–936. doi: 10.1016/0092-8674(89)90331-0. [DOI] [PubMed] [Google Scholar]
- Boulanger CA, Mack DL, Booth BW, Smith GH. Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proc Natl Acad Sci USA. 2007;104:3871–3876. doi: 10.1073/pnas.0611637104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger CA, Wagner KU, Smith GH. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-beta1 expression. Oncogene. 2005;24:552–560. doi: 10.1038/sj.onc.1208185. [DOI] [PubMed] [Google Scholar]
- Brisken C, Park S, Vass T, Lydon JP, O’Malley BW, Weinberg RA. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci USA. 1998;95:5076–5081. doi: 10.1073/pnas.95.9.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ, et al. The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene. 2000;19:968–988. doi: 10.1038/sj.onc.1203277. [DOI] [PubMed] [Google Scholar]
- Choong LY, Lim S, Loh MC, Man X, Chen Y, Toy W, et al. Progressive loss of epidermal growth factor receptor in a subpopulation of breast cancers: implications in target-directed therapeutics. Mol Cancer Ther. 2007;6:2828–2842. doi: 10.1158/1535-7163.MCT-06-0809. [DOI] [PubMed] [Google Scholar]
- DeOme KB, Faulkin LJ, Jr, Bern HA, Blair PB. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19:515–520. [PubMed] [Google Scholar]
- Dolberg DS, Bissell MJ. Inability of Rous sarcoma virus to cause sarcomas in the avian embryo. Nature. 1984;309:552–556. doi: 10.1038/309552a0. [DOI] [PubMed] [Google Scholar]
- Gerschenson M, Graves K, Carson SD, Wells RS, Pierce GB. Regulation of melanoma by the embryonic skin. Proc Natl Acad Sci USA. 1986;83:7307–7310. doi: 10.1073/pnas.83.19.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy CT, Cardiff RD, Muller WJ. Activated neu induces rapid tumor progression. J Biol Chem. 1996;271:7673–7678. doi: 10.1074/jbc.271.13.7673. [DOI] [PubMed] [Google Scholar]
- Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry MD, Triplett AA, Oh KB, Smith GH, Wagner KU. Parity-induced mammary epithelial cells facilitate tumorigenesis in MMTV-neu transgenic mice. Oncogene. 2004;23:6980–6985. doi: 10.1038/sj.onc.1207827. [DOI] [PubMed] [Google Scholar]
- Illmensee K, Mintz B. Totipotency and normal differentiation of single teratocarcinoma cells cloned by injection into blastocysts. Proc Natl Acad Sci USA. 1976;73:549–553. doi: 10.1073/pnas.73.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Jeselsohn R, Brown NE, Arendt L, Klebba I, Hu MG, Kuperwasser C, et al. Cyclin D1 kinase activity is required for the self-renewal of mammary stem and progenitor cells that are targets of MMTV-ErbB2 tumorigenesis. Cancer Cell. 2010;17:65–76. doi: 10.1016/j.ccr.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordon EC, Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125:1921–1930. doi: 10.1242/dev.125.10.1921. [DOI] [PubMed] [Google Scholar]
- Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci USA. 2006;103:2196–2201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough KD, Coleman WB, Ricketts SL, Wilson JW, Smith GJ, Grisham JW. Plasticity of the neoplastic phenotype in vivo is regulated by epigenetic factors. Proc Natl Acad Sci USA. 1998;95:15333–15338. doi: 10.1073/pnas.95.26.15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B, Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci USA. 1975;72:3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody SE, Sarkisian CJ, Hahn KT, Gunther EJ, Pickup S, Dugan KD, et al. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell. 2002;2:451–461. doi: 10.1016/s1535-6108(02)00212-x. [DOI] [PubMed] [Google Scholar]
- Mueller SO, Clark JA, Myers PH, Korach KS. Mammary gland development in adult mice requires epithelial and stromal estrogen receptor alpha. Endocrinology. 2002;143:2357–2365. doi: 10.1210/endo.143.6.8836. [DOI] [PubMed] [Google Scholar]
- Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54:105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- Siegel PM, Ryan ED, Cardiff RD, Muller WJ. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. EMBO J. 1999;18:2149–2164. doi: 10.1093/emboj/18.8.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Smith GH, Medina D. Re-evaluation of mammary stem cell biology based on in vivo transplantation. Breast Cancer Res. 2008;10:203. doi: 10.1186/bcr1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development. 2002;129:1377–1386. doi: 10.1242/dev.129.6.1377. [DOI] [PubMed] [Google Scholar]
- Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, et al. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 1997;25:4323–4330. doi: 10.1093/nar/25.21.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.