SUMMARY
Microbial pathogens have evolved mechanisms to proactively manipulate innate immunity, thereby improving their fitness in mammalian hosts. We have previously shown that Porphyromonas gingivalis exploits CXC-chemokine receptor-4 (CXCR4) to instigate a subversive crosstalk with Toll-like receptor 2 that inhibits leukocyte killing of this periodontal pathogen. However, whether CXCR4 plays a role in periodontal disease pathogenesis has not been previously addressed. Here, we hypothesized that CXCR4 is required for P. gingivalis virulence in the periodontium and that treatment with AMD3100, a potent CXCR4 antagonist, would inhibit P. gingivalis-induced periodontitis. Indeed, mice administered AMD3100 via osmotic minipumps became resistant to induction of periodontal bone loss following oral inoculation with P. gingivalis. AMD3100 appeared to act in an antimicrobial manner, since mice treated with AMD3100 were protected against P. gingivalis colonization and the associated elevation of the total microbiota counts in the periodontal tissue. Moreover, even when administered two weeks post-infection, AMD3100 halted the progression of P. gingivalis-induced periodontal bone loss. Therefore, AMD3100 can act in both preventive and therapeutic ways and CXCR4 antagonism could be a promising novel approach to treat human periodontitis.
Keywords: P. gingivalis, CXCR4, AMD3100, periodontitis, bone loss
INTRODUCTION
Toll-like receptors (TLRs) detect and respond to microbial infection via rapid activation of inflammatory and antimicrobial responses in cooperation with other innate immune receptors with which they form multireceptor complexes in membrane lipid rafts of front-line defense cells (e.g., neutrophils and macrophages) (Hajishengallis et al., 2006; Triantafilou et al., 2001). However, the tendency of TLRs to functionally associate with heterotypic receptors poses an opportunity for exploitation by microbial pathogens capable of inducing inappropriate lipid raft recruitment of receptors that could subvert host immunity (Hajishengallis & Lambris, 2011).
We have previously shown that Porphyromonas gingivalis, a keystone pathogen in periodontal disease (Hajishengallis et al., 2011), interacts with several innate immune receptors, including complement receptors and the CXC chemokine receptor 4 (CXCR4), in ways that enhance its own adaptive fitness (Hajishengallis & Harokopakis, 2007; Hajishengallis et al., 2008; Liang et al., 2011; Wang et al., 2010; Wang et al., 2007). With regard to CXCR4, we have shown that P. gingivalis uses its surface fimbriae to directly bind and activate CXCR4 to subvert antimicrobial signaling initiated by TLR2 (Hajishengallis et al., 2008; Pierce et al., 2009). Specifically, P. gingivalis induces co-association between CXCR4 and TLR2 in lipid rafts, leading to a subversive crosstalk pathway in which cAMP-dependent protein kinase A signaling inhibits intracellular nitric oxide production. This activity, in turn, impairs the killing function of leukocytes (Hajishengallis et al., 2008) suggesting that P. gingivalis exploits CXCR4 to evade host immunity and, perhaps, to persist in the periodontal tissue and cause disease.
However, in our previous publications we have not examined whether the exploitation of CXCR4 by P. gingivalis enhances its ability to cause periodontitis. To address this hypothesis, we now determined whether a specific and potent antagonist of CXCR4, the bicyclam drug AMD3100 (Donzella et al., 1998), can inhibit P. gingivalis-induced periodontitis in the mouse model. Our current results show that AMD3100 impairs the ability of P. gingivalis to cause bone loss by interfering with its colonization in the murine periodontal tissue. These findings provide proof of concept that CXCR4 antagonists may be promising therapeutics for the treatment of human periodontitis.
METHODS
Bacteria
P. gingivalis ATCC 33277 was used in this study. The bacterium was grown anaerobically at 37°C in hemin- and menadione-containing Gifu anaerobic medium (Nissui Pharmaceuticals).
Periodontitis model
Periodontal bone loss was induced in 10- to 12-week-old BALB/c mice (The Jackson Laboratory) by oral inoculation with P. gingivalis ATCC 33277 as originally described by Baker (Baker et al., 2000) with slight modifications (Wang et al., 2007). Briefly, by means of a ball-ended feeding needle, mice were orally inoculated five times at 2-day intervals with 109 CFU P. gingivalis suspended in 2% carboxy-methylcellulose vehicle. Sham controls received vehicle alone. The mice were euthanized six weeks after the last oral inoculation. Assessment of periodontal bone loss in defleshed maxillae was performed under a dissecting microscope (x40) fitted with a video image marker measurement system (VIA-170K; Boeckeler Instruments). Specifically, the distance from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) was measured on 14 predetermined points on the buccal surfaces of the maxillary molars. To calculate bone loss, the 14-site total CEJ-ABC distance for each mouse was subtracted from the mean CEJ-ABC distance of sham-infected mice (Baker et al., 2000). The results were expressed in mm and negative values indicated bone loss relative to sham controls. All animal procedures described in this study were approved by the institutional animal care and use committee, in compliance with established federal and state policies.
Osmotic minipumps
Alzet osmotic minipumps (model #2004; Alza) were subcutaneously implanted through a mid-scapular incision on the back of the mice. The minipumps were placed slightly posterior to the scapulae. The pumps were filled with 20 mg of AMD3100 (Sigma-Aldrich) in 0.2 ml sterile phosphate-buffered saline (PBS) or PBS alone. The #2004 model pump provides for 4 weeks of continuous infusion and its infusion rate is 0.25 μl/hr. Therefore, when filled with 20 mg of AMD3100 in 0.2 ml PBS, the minipumps would deliver the drug at 600 μg/day, which corresponds to a steady serum level of about 1 μg/ml (Matthys et al., 2001). We found that this concentration effectively blocks CXCR4 in our cell culture experiments (Hajishengallis et al., 2008; Pierce et al., 2009).
Quantitative real-time PCR
Maxillary palatal and buccal gingiva and hard tissue (teeth and immediately surrounding bone) were harvested and placed in ATL lysis buffer from the DNeasy kit (Qiagen). Tissues were lysed overnight at 56°C with occasional agitation. Genomic DNA was isolated using the DNeasy kit and was quantified by NanoDrop spectrometry. The levels of P. gingivalis colonization and the number of total bacteria in the periodontal tissue were determined using quantitative real-time PCR of the ISPg1 gene (P. gingivalis) and the 16S rRNA gene (total oral bacteria) (Hajishengallis et al., 2011). ISPg1 was selected to increase the sensitivity of P. gingivalis detection, since this gene is present in 31 copies in the genome P. gingivalis ATCC 33277 (the gene copy numbers were thus divided by 31 to obtain genome equivalents) (Naito et al., 2008). Real-time PCR was performed using the ABI 7500 Fast System and TaqMan probes, sense primers, and antisense primers used were purchased from Applied Biosystems. The primer sets used to enumerate P. gingivalis copy number and total bacterial load were as follows:
ISPg1 (P. gingivalis) (Hajishengallis et al., 2011)
5′-CGCAGACGACAGAGAAGACA-3′
5′-ACGGACAACCTGTTTTTGATAATCCT-3′
5′-FAM-TCCGCCTCGCTCCGAT-TAMRA-3′
16S rRNA (Universal; total bacterial load) (Kuboniwa et al., 2004)
5′TCCTACGGGA GGCAGCAGT-3′
5′-GGACTACCAGGGTATCTAATCCTGTT-3
5′-FAM-CGTATTACCGCGGCTGCTGGCAC-TAMRA-3′
Statistical Analysis
Data were evaluated by analysis of variance and the Dunnett multiple-comparison test using the InStat program (GraphPad Software, San Diego, CA). Where appropriate (comparison of two groups only), two-tailed t tests were performed. P < 0.05 was taken as the level of significance.
RESULTS
AMD3100 prevents P. gingivalis-induced periodontal bone loss
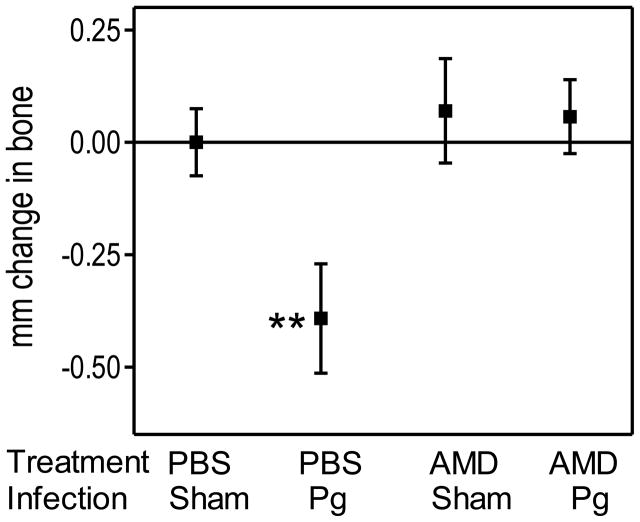
We hypothesized that AMD3100 can interfere with the virulence of P. gingivalis in the periodontal tissue. This hypothesis was based on our previous findings that AMD3100 inhibits the ability of P. gingivalis (or purified fimbriae) to bind CXCR4 and evade leukocyte killing (Hajishengallis et al., 2008; Pierce et al., 2009). Therefore, we investigated whether treatment of BALB/c mice with AMD3100 would protect them against P. gingivalis-induced periodontal bone loss. The study consisted of four groups of mice, which were treated with AMD3100 or vehicle control (PBS) and were infected with P. gingivalis or 2% carboxymethylcellulose vehicle (sham control). AMD3100 was administered systemically by means of osmotic minipumps, which were subcutaneously implanted in the mice 24 hours prior to P. gingivalis infection, involving a total of five oral inoculations at 2-day intervals. Examination of the mice for periodontal bone loss six weeks after the last oral inoculation revealed that only the PBS-treated and P. gingivalis-infected mice developed significant bone loss (P < 0.01; Fig. 1). Strikingly, the AMD3100-treated and P. gingivalis-infected mice were completely protected against bone loss (Fig. 1). Therefore, AMD3100 treatment protects mice from P. gingivalis-induced periodontal bone loss when the drug is administered prior to exposure to the pathogen.
Figure 1. Preventive treatment with AMD3100 abrogates P. gingivalis-induced periodontal bone loss.
BALB/c mice (10–12 weeks of age) were administered AMD3100 (or PBS control) through osmotic minipumps which were implanted subcutaneously 24 hours prior to oral infection with P. gingivalis (or vehicle only; sham) as described in the Methods. The mice were euthanized six weeks after the last inoculation with P. gingivalis, and bone loss measurements were performed in defleshed maxillae. Data are means ± SD (n = 5 mice per group); negative values indicate bone loss in P. gingivalis-infected mice relative to sham-infected controls. **P < 0.01 compared to control and all other experimental groups. AMD, AMD3100; Pg, P. gingivalis.
AMD3100 eliminates P. gingivalis from the murine periodontal tissue
We next hypothesized that the protective effect of AMD3100 against P. gingivalis-induced bone loss involved interference with the capacity of P. gingivalis to enhance its survival through CXCR4 exploitation (Hajishengallis et al., 2008). If this notion were true in the context of periodontitis, AMD3100 would be expected to inhibit the establishment of P. gingivalis in the periodontal tissue. In this regard, we recently showed that P. gingivalis stably colonizes the murine periodontal tissue by day 7 post-infection (Hajishengallis et al., 2011). Therefore, mice were treated with AMD3100 (or PBS control) and infected (or not) with P. gingivalis, as performed in the Fig. 1 study, and were sacrificed 7 days later. The periodontal tissue was harvested to determine the numbers of P. gingivalis and of total periodontal bacteria using quantitative real-time PCR of the ISPg1 gene or the 16S rRNA gene, respectively.
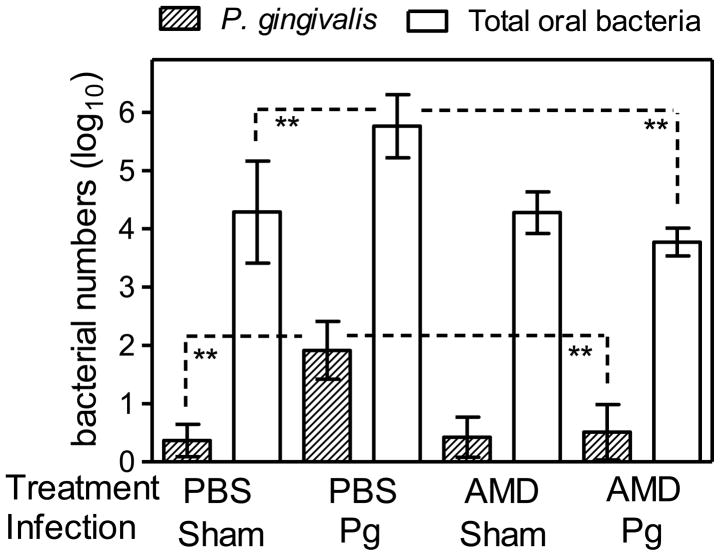
In the absence of AMD3100 treatment, P. gingivalis was readily detected in infected mice at about 4 log10 units lower than total periodontal bacteria (Fig. 2), as seen previously (Hajishengallis et al., 2011). Moreover, in the PBS-treated and P. gingivalis-colonized mice, the levels of total periodontal bacteria were significantly (P < 0.01) higher as compared to those of PBS-treated and sham-infected mice (Fig. 2), confirming the role of P. gingivalis as a keystone pathogen which benefits the entire periodontal biofilm (Hajishengallis et al., 2011). Strikingly, however, treatment with AMD3100 resulted in 97% reduction in the numbers of P. gingivalis (Fig. 2). This virtual elimination of P. gingivalis from the periodontal tissue due to AMD3100 treatment was accompanied by significant (P < 0.01) reduction in the total numbers of periodontal bacteria, which returned to the normal levels seen in mice not colonized by P. gingivalis (sham-infected) (Fig. 2). The reduction in the total bacterial numbers was not a direct effect of AMD3100 on the periodontal microbiota at large, since this antagonist failed to affect the total periodontal bacterial numbers in mice not colonized with P. gingivalis (i.e., the AMD3100-treated and sham-infected mice) (Fig. 2). Moreover, AMD3100 did not have direct killing activity against P. gingivalis (Supporting Fig. 1). Therefore, in the presence of AMD3100, P. gingivalis is not capable of colonizing the periodontal tissue and influencing the resident microbiota.
Figure 2. Effect of AMD3100 on the numbers of P. gingivalis or total bacteria in the murine periodontal tissue.
BALB/c mice (10–12 weeks of age) were treated with AMD3100 (or PBS control) and infected with P. gingivalis (or vehicle only; sham) as described in the legend to Figure 1. The mice were sacrificed 7 days after the last inoculation with P. gingivalis. The numbers of P. gingivalis and of total periodontal bacteria in the periodontal tissue were determined using quantitative real-time PCR of the ISPg1 gene (P. gingivalis) or the 16S rRNA gene (total bacteria). Data are means ± SD (n = 5 mice per group). **P < 0.01 between the indicated groups. AMD, AMD3100; Pg, P. gingivalis.
Therapeutic treatment with AMD3100 halts the progression of P. gingivalis-induced bone loss
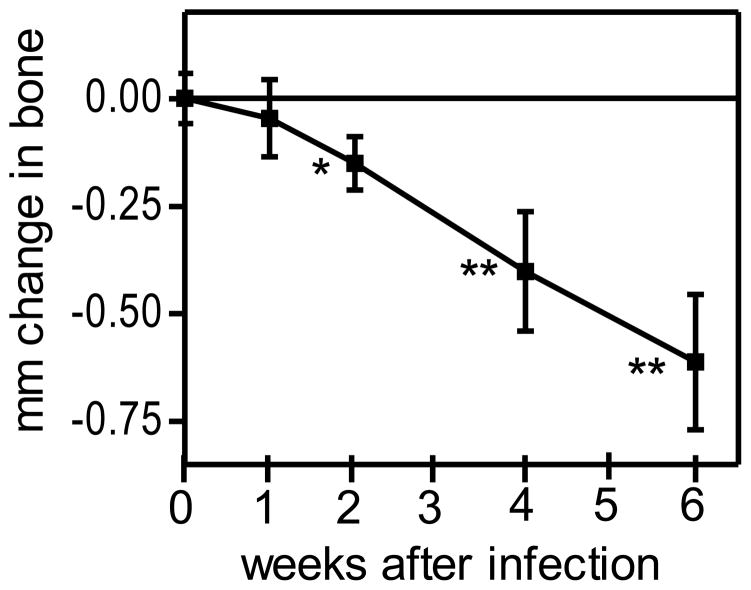
Although treatment with AMD3100 can prevent P. gingivalis-induced bone loss when applied prior to P. gingivalis infection (Fig. 1), this does not necessarily imply that AMD3100 can also be effective when applied in a therapeutic mode. Therefore, a new experiment was designed to determine if AMD3100 can protect against P. gingivalis-induced periodontal bone loss when administered after infection and the onset of bone loss. We first determine the time interval that would be required to observe significant bone loss in P. gingivalis-infected mice. To this end, BALB/c mice were orally inoculated with P. gingivalis using the standard protocol (e.g., as performed in the Fig. 1 study), and groups of mice were sacrificed at 1, 2, 4, and 6 weeks post-infection. We found that 2 weeks represented the minimum time required to observe significant (P < 0.05) P. gingivalis-induced bone loss in BALB/c mice (Fig. 3).
Figure 3. Timecourse of periodontal bone loss induction in BALB/c mice.
10- to 12-week-old BALB/c mice were orally infected with P. gingivalis as described in Methods and euthanized at the indicated times after the last inoculation with P. gingivalis. Bone loss measurements were performed in defleshed maxillae. Data are means ± SD (n = 5 mice per group); negative values indicate bone loss in P. gingivalis-infected mice relative to sham-infected controls. *, P < 0.05 and **, P < 0.01 vs. time 0.
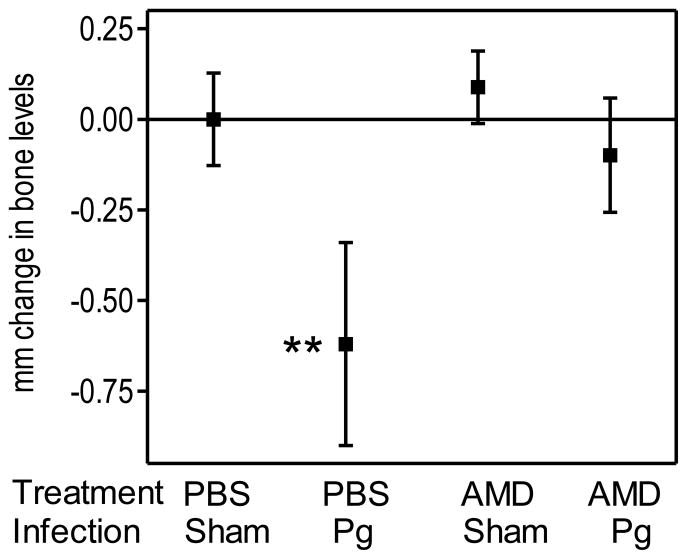
Therefore, in a new bone loss study, the mice were first orally infected or not with P. gingivalis and, 2 weeks after the last inoculating dose, received AMD3100- or PBS-containing osmotic minipumps through subcutaneous implantation. We found that AMD3100-treated and P. gingivalis-infected mice developed significantly (P < 0.01) less bone loss than PBS-treated and P. gingivalis-infected mice (Fig. 4). These data indicate that AMD3100 inhibits the progression of P. gingivalis-induced bone loss and suggest that it could be a promising therapeutic agent against periodontitis.
Figure 4. Therapeutic treatment with AMD3100 inhibits P. gingivalis-induced periodontal bone loss.
BALB/c mice (10–12 weeks of age) were orally infected with P. gingivalis (or vehicle only; sham) as described in Methods. Two weeks after the last inoculation with P. gingivalis, the mice were administered AMD3100 (or PBS control) through subcutaneously implanted osmotic minipumps. The mice were euthanized four weeks later and bone loss measurements were performed in defleshed maxillae. Data are means ± SD (n = 5 mice per group); negative values indicate bone loss in P. gingivalis-infected mice relative to sham-infected controls. **P < 0.01 compared to control and all other experimental or groups. AMD, AMD3100; Pg, P. gingivalis.
DISCUSSION
It has recently been proposed that periodontitis fundamentally represents a disruption of host-microbe homeostasis in the periodontal tissue (Darveau, 2010). This notion is supported by mechanistic studies in the mouse model of periodontitis: Alterations either in the composition of the periodontal microbiota or in local regulatory mechanisms that control leukocyte recruitment can cause disruption of periodontal homeostasis which, in turn, may lead to uncontrolled inflammation and periodontal bone loss (Eskan et al., 2012; Hajishengallis et al., 2011). Currently, there is an urgent need to develop innovative adjunctive therapeutic strategies in chronic periodontitis (Hajishengallis, 2009). Indeed, conventional periodontal treatment is often not sufficient by itself to treat destructive inflammation and, moreover, this oral disease appears to increase the patients’ risk for atherosclerosis, diabetes, chronic obstructive pulmonary disease, adverse pregnancy outcomes, and possibly rheumatoid arthritis (Genco & Van Dyke, 2010; Lalla & Papapanou, 2011; Lundberg et al., 2010; Pihlstrom et al., 2005; Tonetti et al., 2007).
Several approaches have been successfully tested to inhibit periodontitis in preclinical models including anti-cytokine therapy or the use of agents that promote the resolution of inflammation (Assuma et al., 1998; Hajishengallis, 2009; Hasturk et al., 2007). Another approach to treating periodontitis is to counteract immune evasion or subversion by major periodontal pathogens. Periodontal and other microbial pathogens preferentially target and corrupt innate immunity (Finlay & McFadden, 2006; Hajishengallis & Lambris, 2011). Subversion of innate immunity may additionally undermine the overall host defense, given the instructive role of the innate response in the development of adaptive immunity (Pasare & Medzhitov, 2005). Therefore, understanding the molecular mechanisms whereby microbial pathogens interact with and exploit innate immune receptors may facilitate the development of intervention approaches to inhibit immune evasion and disease pathogenesis.
In this paper, we took advantage of our earlier findings that implicated CXCR4 in P. gingivalis immune subversion (Hajishengallis et al., 2008) and showed that a CXCR4 antagonist can protect against P. gingivalis-induced periodontal bone loss in both a preventive and therapeutic way. Since P. gingivalis uses its fimbriae to exploit CXCR4 (Hajishengallis et al., 2008; Pierce et al., 2009), it is likely that the protective effect of AMD3100 is restricted against fimbriated strains of P. gingivalis. The fimbriae of P. gingivalis comprise polymerized fimbrillin (FimA) and accessory proteins (FimCDE) encoded by genes of the fimbrial operon (Wang et al., 2007). Since CXCR4 interacts specifically with the accessory protein components (FimCDE) of the fimbriae (Pierce et al., 2009) which, unlike FimA, are well conserved among different fimbriated strains (Kato et al., 2007), the AMD3100 effect may not be restricted to Type I fimbriated P. gingivalis strains (as is the strain used in this study).
Interestingly, the expression of CXCR4 was shown by independent groups to be elevated in chronic periodontitis as compared to healthy gingiva (Jotwani et al., 2004; Kebschull et al., 2008). However, it has been uncertain whether CXCR4 plays a role in periodontal pathogenesis. In this regard, our study is the first to causally link CXCR4 to periodontitis in a preclinical model. The protective effect of AMD3100 against P. gingivalis-induced periodontitis may be attributed, at least in great part, to the blockade of a host receptor, CXCR4, which is apparently important for P. gingivalis survival in the periodontium. This conclusion is based on the ability of AMD3100 to enhance the killing of P. gingivalis by leukocytes (Hajishengallis et al., 2008) and, moreover, to mediate its elimination from the periodontal tissue in vivo (this study).
CXCR4 affects bone metabolism and, in principle, inhibition of this receptor with AMD3100 might have influenced bone resorption in the periodontitis model used in this study. In this regard, CXCR4 activation is known to induce the chemotactic recruitment, development and survival of osteoclasts (Wright et al., 2005). Conversely, another study showed that it is the disruption of CXCR4 that enhances osteoclastogenesis (Hirbe et al., 2007). Yet, another investigation showed that AMD3100 failed to influence osteoclast formation indicating that CXCR4 may not induce osteoclastogenesis (Matthys et al., 2001). Taken together, these findings suggest that the effects of CXCR4 on osteoclastogenesis may be variable, perhaps depending on environmental context. In a similar vein, AMD3100 has complex effects on cell trafficking, since it can block CXCR4-mediated chemotaxis but, on the other hand, can stimulate the mobilization of hematopoietic stem/progenitor cells and granulocytes from the bone marrow (Lee et al., 2009). Since the continuous presence of low colonization levels of P. gingivalis in the mouse periodontium is required for induction of bone loss (Hajishengallis et al., 2011), we conclude that the ability of AMD3100 to inhibit the persistence of P. gingivalis in the periodontium constitutes the main mechanism responsible for the observed inhibition of periodontal bone loss.
The natural ligand for CXCR4 is the chemokine stromal cell-derived factor-1 (SDF-1), although CXCR4 also functions as a coreceptor with CD4 for the HIV-1 envelope gp120/gp41 complex (Oberlin et al., 1996). In this context, AMD3100, which can also potently antagonize human CXCR4 (Hatse et al., 2002), was shown to block CXCR4-dependent HIV-1 entry and replication (De Clercq, 2005; Donzella et al., 1998). Moreover, AMD3100 can protect against several CXCR4-mediated pathophysiological conditions, such as rheumatoid, infectious, allergic, and malignant diseases, both in humans and in experimental mouse models (De Clercq, 2005; Hogaboam et al., 2005; Lukacs et al., 2002; Matthys et al., 2001). This study adds periodontitis to the list of potential therapeutic applications of AMD3100.
The ability of AMD3100 to inhibit periodontitis by apparently targeting P. gingivalis (as this antagonist did not directly influence the periodontal microbiota) has a theoretical basis on the keystone pathogen concept. According to this concept, P. gingivalis– at low colonization levels– impairs innate immunity in ways that alter the growth and development of the entire biofilm resulting in dysbiosis that triggers periodontal disease, at least in the mouse model (Hajishengallis et al., 2011). On the other hand, neither the indigenous murine microbiota alone, nor P. gingivalis by itself (i.e., in germ-free mice) can initiate pathologic bone loss in young healthy mice (Hajishengallis et al., 2011). In this study, in the presence of AMD3100, P. gingivalis failed to support the overgrowth of the total periodontal microbiota which is required for induction of periodontitis. AMD3100 was effective against periodontitis even when the disease was already in progress, suggesting that the continuous presence of P. gingivalis, albeit at very low levels compared to the total bacterial counts, is strictly required to sustain dysbiosis and disease progression.
In humans, P. gingivalis is also a quantitatively minor component of subgingival pathogenic biofilms, despite its high prevalence, and is associated with progressive bone loss in periodontitis patients (Chaves et al., 2000; Doungudomdacha et al., 2000; Kumar et al., 2006; Moore et al., 1982; Moore et al., 1991). It should be noted that adult chronic periodontitis is associated with multiple etiologies and disease modifiers (Hajishengallis, 2010; Kornman, 2006; Lalla & Papapanou, 2011; Pihlstrom et al., 2005) and, therefore, the presence of P. gingivalis may be just one of several etiologic factors. Nevertheless, under favorable environmental conditions, this bacterium has the potential to act as a keystone pathogen to transform an otherwise symbiotic microbiota into a dysbiotic microbial community that can cause periodontitis (Hajishengallis et al., 2011).
In summary, we have established a role for CXCR4 in P. gingivalis-induced periodontitis and showed that CXCR4 antagonism using AMD3100 confers protection against the disease through an antimicrobial effect. AMD3100 was shown to be safe in humans with only minimal side effects (typically gastrointestinal in nature) observed at high concentrations of the drug (Hendrix et al., 2000; Schols, 2004). Importantly, AMD3100 was recently approved by the FDA as a drug for stem cell mobilization (Pusic & DiPersio, 2010). Given its safety record, AMD3100, and perhaps other CXCR4 antagonists, could find application as adjunctive therapeutics for the treatment of human periodontitis.
Supplementary Material
Acknowledgments
This study was supported by U.S. Public Health Service Grants F31 DE021304 (to M.L.M) and DE015254, DE021580, and DE018292 (to G.H.).
References
- Assuma R, Oates T, Cochran D, et al. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–409. [PubMed] [Google Scholar]
- Baker PJ, Dixon M, Roopenian DC. Genetic control of susceptibility to Porphyromonas gingivalis-induced alveolar bone loss in mice. Infect Immun. 2000;68:5864–5868. doi: 10.1128/iai.68.10.5864-5868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves ES, Jeffcoat MK, Ryerson CC, Snyder B. Persistent bacterial colonization of Porphyromonas gingivalis, Prevotella intermedia, and Actinobacillus actinomycetemcomitans in periodontitis and its association with alveolar bone loss after 6 months of therapy. J Clin Periodontol. 2000;27:897–903. doi: 10.1034/j.1600-051x.2000.027012897.x. [DOI] [PubMed] [Google Scholar]
- Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Potential clinical applications of the CXCR4 antagonist bicyclam AMD3100. Mini Rev Med Chem. 2005;5:805–824. doi: 10.2174/1389557054867075. [DOI] [PubMed] [Google Scholar]
- Donzella GA, Schols D, Lin SW, et al. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- Doungudomdacha S, Rawlinson A, Douglas CW. Enumeration of Porphyromonas gingivalis, Prevotella intermedia and Actinobacillus actinomycetemcomitans in subgingival plaque samples by a quantitative-competitive PCR method. J Med Microbiol. 2000;49:861–874. doi: 10.1099/0022-1317-49-10-861. [DOI] [PubMed] [Google Scholar]
- Eskan MA, Jotwani R, Abe T, et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13:465–473. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay BB, McFadden G. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell. 2006;124:767–782. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Genco RJ, Van Dyke TE. Prevention: Reducing the risk of CVD in patients with periodontitis. Nat Rev Cardiol. 2010;7:479–480. doi: 10.1038/nrcardio.2010.120. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G. Toll gates to periodontal host modulation and vaccine therapy. Periodontology 2000. 2009;51:181–207. doi: 10.1111/j.1600-0757.2009.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. Too old to fight? Aging and its toll on innate immunity. Mol Oral Microbiol. 2010;25:25–37. doi: 10.1111/j.2041-1014.2009.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Harokopakis E. Porphyromonas gingivalis interactions with complement receptor 3 (CR3): innate immunity or immune evasion? Front Biosci. 2007;12:4547–4557. doi: 10.2741/2409. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Lambris JD. Microbial manipulation of receptor crosstalk in innate immunity. Nat Rev Immunol. 2011;11:187–200. doi: 10.1038/nri2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Liang S, Payne MA, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell host & microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Tapping RI, Harokopakis E, et al. Differential interactions of fimbriae and lipopolysaccharide from Porphyromonas gingivalis with the Toll-like receptor 2-centred pattern recognition apparatus. Cell Microbiol. 2006;8:1557–1570. doi: 10.1111/j.1462-5822.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Wang M, Liang S, et al. Pathogen induction of CXCR4/TLR2 crosstalk impairs host defense function. Proc Natl Acad Sci USA. 2008;105:13532–13537. doi: 10.1073/pnas.0803852105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasturk H, Kantarci A, Goguet-Surmenian E, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- Hatse S, Princen K, Bridger G, et al. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 2002;527:255–262. doi: 10.1016/s0014-5793(02)03143-5. [DOI] [PubMed] [Google Scholar]
- Hendrix CW, Flexner C, MacFarland RT, et al. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother. 2000;44:1667–1673. doi: 10.1128/aac.44.6.1667-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirbe AC, Rubin J, Uluckan O, et al. Disruption of CXCR4 enhances osteoclastogenesis and tumor growth in bone. Proc Natl Acad Sci USA. 2007;104:14062–14067. doi: 10.1073/pnas.0705203104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogaboam CM, Carpenter KJ, Schuh JM, et al. The therapeutic potential in targeting CCR5 and CXCR4 receptors in infectious and allergic pulmonary disease. Pharmacol Ther. 2005;107:314–328. doi: 10.1016/j.pharmthera.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Jotwani R, Muthukuru M, Cutler CW. Increase in HIV receptors/co-receptors/alpha-defensins in inflamed human gingiva. J Dent Res. 2004;83:371–377. doi: 10.1177/154405910408300504. [DOI] [PubMed] [Google Scholar]
- Kato T, Kawai S, Nakano K, et al. Virulence of Porphyromonas gingivalis is altered by substitution of fimbria gene with different genotype. Cell Microbiol. 2007;9:753–765. doi: 10.1111/j.1462-5822.2006.00825.x. [DOI] [PubMed] [Google Scholar]
- Kebschull M, Demmer R, Behle JH, et al. Granulocyte chemotactic protein 2 (gcp-2/cxcl6) complements interleukin-8 in periodontal disease. J Periodont Res. 2008;44:465–471. doi: 10.1111/j.1600-0765.2008.01134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornman KS. Interleukin 1 genetics, inflammatory mechanisms, and nutrigenetic opportunities to modulate diseases of aging. Am J Clin Nutr. 2006;83:475S–483S. doi: 10.1093/ajcn/83.2.475S. [DOI] [PubMed] [Google Scholar]
- Kuboniwa M, Amano A, Kimura KR, et al. Quantitative detection of periodontal pathogens using real-time polymerase chain reaction with TaqMan probes. Oral Microbiol Immunol. 2004;19:168–176. doi: 10.1111/j.0902-0055.2004.00135.x. [DOI] [PubMed] [Google Scholar]
- Kumar PS, Leys EJ, Bryk JM, et al. Changes in periodontal health status are associated with bacterial community shifts as assessed by quantitative 16S cloning and sequencing. J Clin Microbiol. 2006;44:3665–3673. doi: 10.1128/JCM.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7:738–748. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- Lee HM, Wysoczynski M, Liu R, et al. Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100-stimulated granulocytes. Leukemia. 2009;24:573–582. doi: 10.1038/leu.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Krauss JL, Domon H, et al. The C5a receptor impairs IL-12-dependent clearance of Porphyromonas gingivalis and is required for induction of periodontal bone loss. J Immunol. 2011;186:869–877. doi: 10.4049/jimmunol.1003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs NW, Berlin A, Schols D, et al. AMD3100, a CxCR4 antagonist, attenuates allergic lung inflammation and airway hyperreactivity. Am J Pathol. 2002;160:1353–1360. doi: 10.1016/S0002-9440(10)62562-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg K, Wegner N, Yucel-Lindberg T, Venables PJ. Periodontitis in RAthe citrullinated enolase connection. Nat Rev Rheumatol. 2010;6:727–730. doi: 10.1038/nrrheum.2010.139. [DOI] [PubMed] [Google Scholar]
- Matthys P, Hatse S, Vermeire K, et al. AMD3100, a potent and specific antagonist of the stromal cell-derived factor-1 chemokine receptor CXCR4, inhibits autoimmune joint inflammation in IFN-γ receptor-deficient mice. J Immunol. 2001;167:4686–4692. doi: 10.4049/jimmunol.167.8.4686. [DOI] [PubMed] [Google Scholar]
- Moore WE, Holdeman LV, Smibert RM, et al. Bacteriology of severe periodontitis in young adult humans. Infect Immun. 1982;38:1137–1148. doi: 10.1128/iai.38.3.1137-1148.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore WE, Moore LH, Ranney RR, et al. The microflora of periodontal sites showing active destructive progression. J Clin Periodontol. 1991;18:729–739. doi: 10.1111/j.1600-051x.1991.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Naito M, Hirakawa H, Yamashita A, et al. Determination of the genome sequence of Porphyromonas gingivalis strain ATCC 33277 and genomic comparison with strain W83 revealed extensive genome rearrangements in P. gingivalis. DNA Res. 2008;15:215–225. doi: 10.1093/dnares/dsn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin E, Amara A, Bachelerie F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol. 2005;560:11–18. doi: 10.1007/0-387-24180-9_2. [DOI] [PubMed] [Google Scholar]
- Pierce DL, Nishiyama S, Liang S, et al. Host adhesive activities and virulence of novel fimbrial proteins of Porphyromonas gingivalis. Infect Immun. 2009;77:3294–3301. doi: 10.1128/IAI.00262-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- Pusic I, DiPersio JF. Update on clinical experience with AMD3100, an SDF-1/CXCL12-CXCR4 inhibitor, in mobilization of hematopoietic stem and progenitor cells. Curr Opin Hematol. 2010;17:319–326. doi: 10.1097/MOH.0b013e328338b7d5. [DOI] [PubMed] [Google Scholar]
- Schols D. HIV co-receptors as targets for antiviral therapy. Curr Top Med Chem. 2004;4:883–893. doi: 10.2174/1568026043388501. [DOI] [PubMed] [Google Scholar]
- Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- Triantafilou K, Triantafilou M, Dedrick RL. A CD14-independent LPS receptor cluster. Nat Immunol. 2001;2:338–345. doi: 10.1038/86342. [DOI] [PubMed] [Google Scholar]
- Wang M, Krauss JL, Domon H, et al. Microbial hijacking of complement-toll-like receptor crosstalk. Sci Signal. 2010;3:ra11. doi: 10.1126/scisignal.2000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Shakhatreh MA, James D, et al. Fimbrial proteins of porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J Immunol. 2007;179:2349–2358. doi: 10.4049/jimmunol.179.4.2349. [DOI] [PubMed] [Google Scholar]
- Wright LM, Maloney W, Yu X, et al. Stromal cell-derived factor-1 binding to its chemokine receptor CXCR4 on precursor cells promotes the chemotactic recruitment, development and survival of human osteoclasts. Bone. 2005;36:840–853. doi: 10.1016/j.bone.2005.01.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.