Abstract
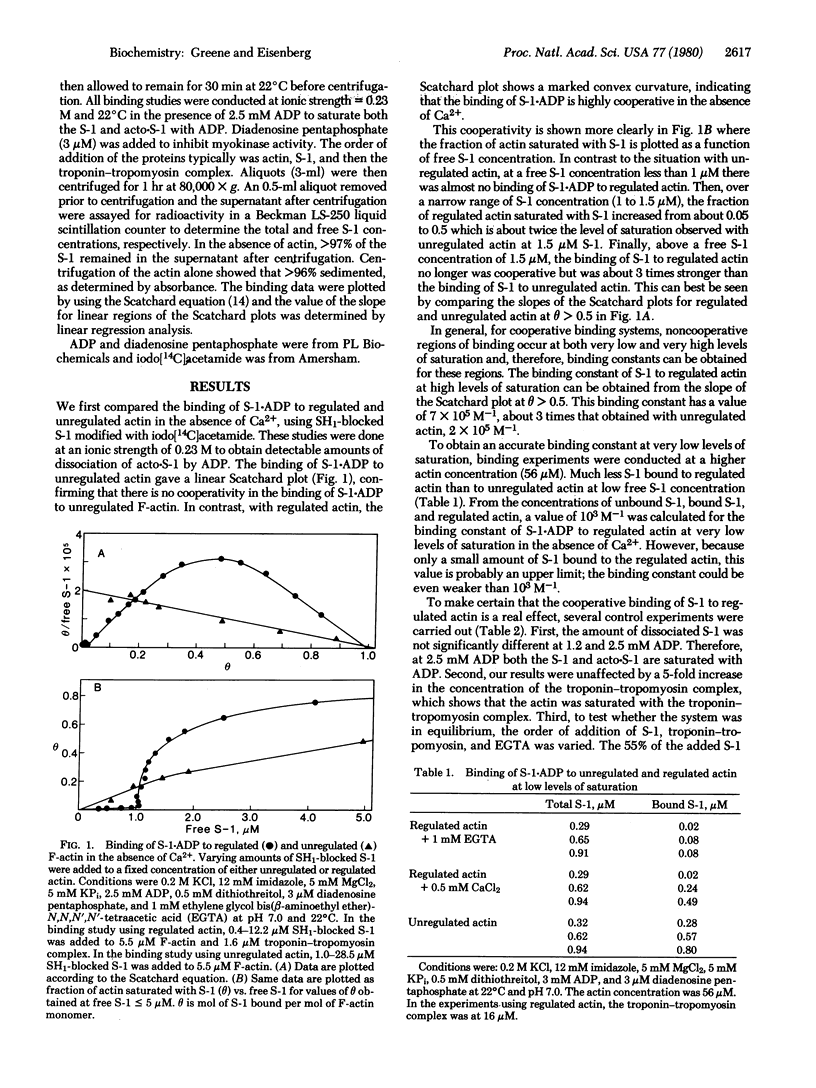
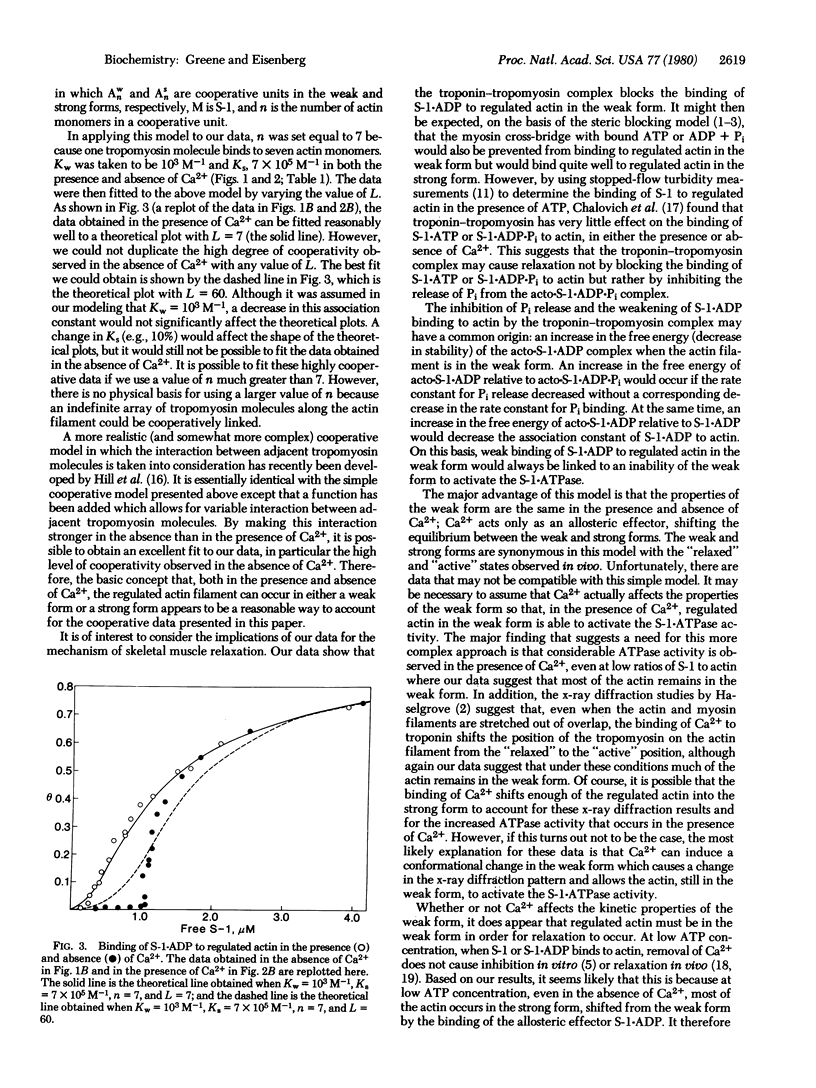
The binding of myosin subfragment-1 (S-1) to the F-actin-troponin-tropomyosin complex (regulated F-actin) was examined in the presence of ADP (ionic strength, 0.23 M; 22 degrees C) by using the ultracentrifuge and S-1 blocked at SH1 with iodo[14C]acetamide. S-1 . ADP binds with positive cooperativity to regulated F-actin, both in the presence and absence of calcium; it binds independently to unregulated actin. With and without Ca2+ at very low levels of occupancy of the regulated actin by S-1 . ADP, S-1 . ADP binds to the regulated actin with less than 1% of the strength that it binds to unregulated actin, whereas at high levels of occupancy of the regulated actin by S-1 . ADP, S-1 . ADP binds about 3-fold more strongly to the regulated actin than it does to unregulated actin. The major difference between the results obtained in the presence and absence of Ca2+ with regulated actin is that, in the absence of Ca2+, the binding of S-1 . ADP remains weak until a higher free S-1 . ADP concentration is reached and the transition to strong binding is much more cooperative. These results are consistent with a model that is basically similar to the cooperative binding model of Hill[Hill, T.L. (1952) J. Chem. Phys. 20, 1259-1273] and of Monod et al. [Monod, J., Wyman, J. & Changeux, J. (1965) J. Mol. Biol. 12, 88-118]: The regulated actin filament can exist in two forms, a weak-binding and a strong-binding form; and Ca2+ and S-1 . ADP, acting as allosteric effectors, shift the equilibrium between the two forms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q Rev Biophys. 1969 Nov;2(4):351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Kielley W. W. Troponin-tropomyosin complex. Column chromatographic separation and activity of the three, active troponin components with and without tropomyosin present. J Biol Chem. 1974 Aug 10;249(15):4742–4748. [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Dissociation of the actin.subfragment 1 complex by adenyl-5'-yl imidodiphosphate, ADP, and PPi. J Biol Chem. 1980 Jan 25;255(2):543–548. [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. The binding of heavy meromyosin to F-actin. J Biol Chem. 1980 Jan 25;255(2):549–554. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Squire J. M. Structural role of tropomyosin in muscle regulation: analysis of the x-ray diffraction patterns from relaxed and contracting muscles. J Mol Biol. 1973 Mar 25;75(1):33–55. doi: 10.1016/0022-2836(73)90527-5. [DOI] [PubMed] [Google Scholar]
- Reuben J. P., Brandt P. W., Berman M., Grundfest H. Regulation of tension in the skinned crayfish muscle fiber. I. Contraction and relaxation in the absence of Ca (pCa is greater than 9). J Gen Physiol. 1971 Apr;57(4):385–407. doi: 10.1085/jgp.57.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein L. A., Schwarz R. P., Jr, Chock P. B., Eisenberg E. Mechanism of actomyosin adenosine triphosphatase. Evidence that adenosine 5'-triphosphate hydrolysis can occur without dissociation of the actomyosin complex. Biochemistry. 1979 Sep 4;18(18):3895–3909. doi: 10.1021/bi00585a009. [DOI] [PubMed] [Google Scholar]
- Weber A., Murray J. M. Molecular control mechanisms in muscle contraction. Physiol Rev. 1973 Jul;53(3):612–673. doi: 10.1152/physrev.1973.53.3.612. [DOI] [PubMed] [Google Scholar]
- White D. C. Rigor contraction and the effect of various phosphate compounds on glycerinated insect flight and vertebrate muscle. J Physiol. 1970 Jul;208(3):583–605. doi: 10.1113/jphysiol.1970.sp009138. [DOI] [PMC free article] [PubMed] [Google Scholar]



