Abstract
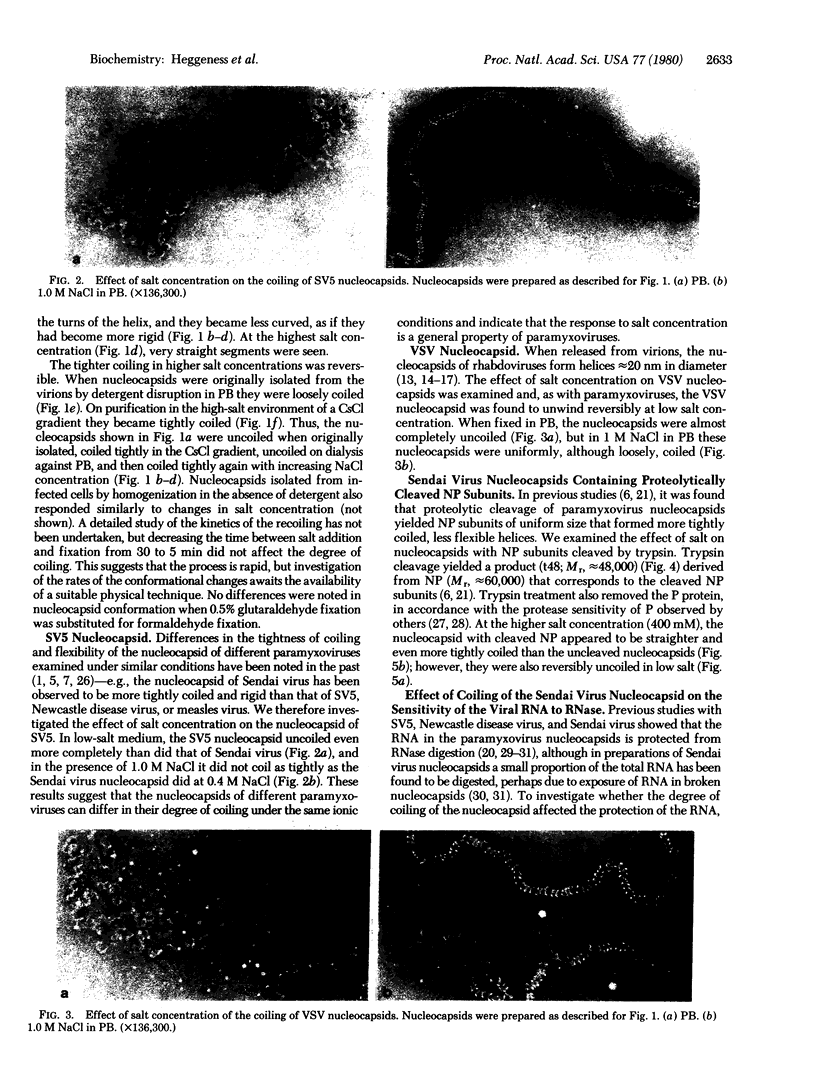
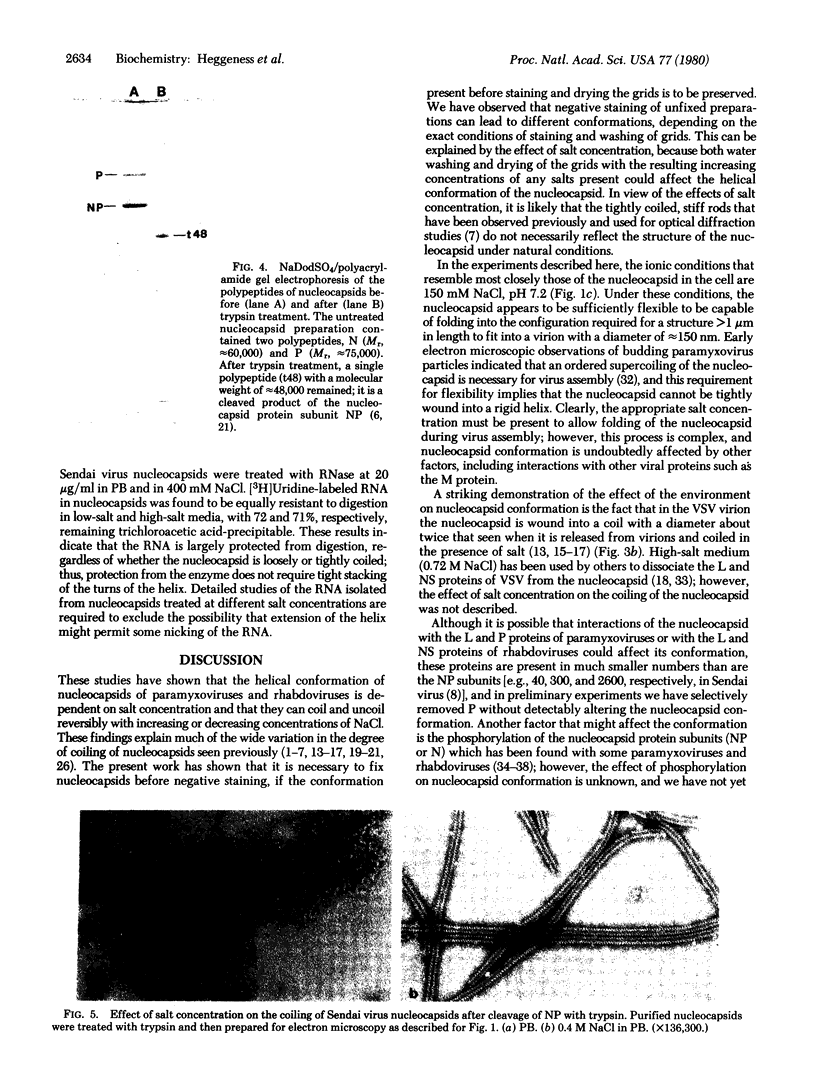
The conformations of the helical nucleocapsids of the paramyxoviruses Sendai virus and simian virus 5, and of a rhabdovirus, vesicular stomatitis virus, have been found to vary extensively with changes in salt concentration. In 10 mM sodium phosphate buffer at pH 7.2, the nucleocapsids are loosely coiled or almost completely extended; with increasing concentrations of NaCl they become more tightly coiled and less flexible. Under isotonic conditions (150 mM) the Sendai virus nucleocapsid is moderately tightly coiled but still curved and apparently flexible, whereas at 400 mM or higher it is very tightly coiled, with the appearance of a rigid rod. These salt-dependent changes in conformation were also found with nucleocapsids composed of proteolytically cleaved protein subunits. Because of the effect of salt concentration, and the fact that it may change during the preparation of negatively stained samples of electron microscopy, it was necessary to fix that nucleocapsids before negative staining to preserve their original conformation. The striking changes in nucleocapsid conformation in response to the ionic milieu indicate the plasticity of its helical structure and suggest that changes in the microenvironment of the nucleocapsid could influence its conformation during viral RNA transcription and replication or during virus assembly by budding, processes in which changes in the coiling of the nucleocapsid or its flexibility could be important.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buetti E., Choppin P. W. The transcriptase complex of the paramyxovirus SV5. Virology. 1977 Oct 15;82(2):493–508. doi: 10.1016/0042-6822(77)90021-6. [DOI] [PubMed] [Google Scholar]
- Bussell R. H., Waters D. J., Seals M. K. Measles, canine distemper and respiratory syncytial virions and nucleocapsids. A comparative study of their structure, polypeptide and nucleic acid composition. Med Microbiol Immunol. 1974;160(2-3):105–124. doi: 10.1007/BF02121718. [DOI] [PubMed] [Google Scholar]
- CHOPPIN P. W., STOECKENIUS W. THE MORPHOLOGY OF SV5 VIRUS. Virology. 1964 Jun;23:195–202. doi: 10.1016/0042-6822(64)90282-x. [DOI] [PubMed] [Google Scholar]
- Cartwright B., Smale C. J., Brown F. Dissection of vesicular stomatitis virus into the infective ribonucleoprotein and immunizing components. J Gen Virol. 1970 Apr;7(1):19–32. doi: 10.1099/0022-1317-7-1-19. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Stone H. O. Isolation of a transcriptive complex from Newcastle disease virions. J Virol. 1976 Sep;19(3):1080–1089. doi: 10.1128/jvi.19.3.1080-1089.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Choppin P. W. Isolation and properties of the helical nucleocapsid of the parainfluenza virus SV5. Proc Natl Acad Sci U S A. 1967 Apr;57(4):949–956. doi: 10.1073/pnas.57.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Choppin P. W. The length of the helical nucleocapsid of Newcastle disease virus. Virology. 1967 Oct;33(2):344–346. doi: 10.1016/0042-6822(67)90153-5. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Choppin P. W. The nucleic acid of the parainfluenza virus SV5. Virology. 1968 Jun;35(2):289–296. doi: 10.1016/0042-6822(68)90269-9. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Holmes K. V., Dales S., Choppin P. W. An electron microscopic study of moderate and virulent virus-cell interactions of the parainfluenza virus SV5. Virology. 1966 Nov;30(3):411–426. doi: 10.1016/0042-6822(66)90119-x. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Mountcastle W. E., Choppin P. W. The sense of the helix of paramyxovirus nucleocapsids. J Mol Biol. 1972 Mar 14;65(1):167–169. doi: 10.1016/0022-2836(72)90499-8. [DOI] [PubMed] [Google Scholar]
- Emerson S. U., Wagner R. R. Dissociation and reconstitution of the transcriptase and template activities of vesicular stomatitis B and T virions. J Virol. 1972 Aug;10(2):297–309. doi: 10.1128/jvi.10.2.297-309.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch J. T., Gibbs A. J. Observations on the structure of the nucleocapsids of some paramyxoviruses. J Gen Virol. 1970 Jan;6(1):141–150. doi: 10.1099/0022-1317-6-1-141. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Lamb R. A., Choppin P. W. The polypeptides of canine distemper virus: synthesis in infected cells and relatedness to the polypeptides of other morbilliviruses. Virology. 1980 Jan 30;100(2):433–449. doi: 10.1016/0042-6822(80)90534-6. [DOI] [PubMed] [Google Scholar]
- Hosaka Y. Isolation and structure of the nucleocapsid of HVJ. Virology. 1968 Jul;35(3):445–457. doi: 10.1016/0042-6822(68)90223-7. [DOI] [PubMed] [Google Scholar]
- Hsu M. C., Scheid A., Choppin P. W. Reconstitution of membranes with individual paramyxovirus glycoproteins and phospholipid in cholate solution. Virology. 1979 Jun;95(2):476–491. doi: 10.1016/0042-6822(79)90502-6. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W., Darlington R. W. Isolation and properties of Newcastle disease virus nucleocapsid. J Virol. 1968 Mar;2(3):248–255. doi: 10.1128/jvi.2.3.248-255.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. W., Portner A., Darlington R. W. Properties of incomplete Sendai virions and subgenomic viral RNAs. Virology. 1970 Dec;42(4):857–871. doi: 10.1016/0042-6822(70)90335-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. The synthesis of Sendai virus polypeptides in infected cells. III. Phosphorylation of polypeptides. Virology. 1977 Sep;81(2):382–397. doi: 10.1016/0042-6822(77)90154-4. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Mahy B. W., Choppin P. W. The synthesis of sendai virus polypeptides in infected cells. Virology. 1976 Jan;69(1):116–131. doi: 10.1016/0042-6822(76)90199-9. [DOI] [PubMed] [Google Scholar]
- Lamb R. A. The phosphorylation of sendai virus proteins by a virus particle-associated protein kinase. J Gen Virol. 1975 Mar;26(3):249–263. doi: 10.1099/0022-1317-26-3-249. [DOI] [PubMed] [Google Scholar]
- Lynch S., Kolakofsky D. Ends of the RNA within Sendai virus defective interfering nucleocapsids are not free. J Virol. 1978 Nov;28(2):584–589. doi: 10.1128/jvi.28.2.584-589.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Compans R. W., Choppin P. W. Proteins of vesicular stomatitis virus and of phenotypically mixed vesicular stomatitis virus-simian virus 5 virions. J Virol. 1971 Nov;8(5):722–729. doi: 10.1128/jvi.8.5.722-729.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Compans R. W., Lackland H., Choppin P. W. Isolation and characterization of the nonglycosylated membrane protein and a nucleocapsid complex from the paramyxovirus SV5. Virology. 1975 Oct;67(2):365–374. doi: 10.1016/0042-6822(75)90438-9. [DOI] [PubMed] [Google Scholar]
- Mountcastle W. E., Compans R. W., Caliguiri L. A., Choppin P. W. Nucleocapsid protein subunits of simian virus 5, Newcastle disease virus, and Sendai virus. J Virol. 1970 Nov;6(5):677–684. doi: 10.1128/jvi.6.5.677-684.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle W. E., Compans R. W., Lackland H., Choppin P. W. Proteolytic cleavage of subunits of the nucleocapsid of the paramyxovirus simian virus 5. J Virol. 1974 Nov;14(5):1253–1261. doi: 10.1128/jvi.14.5.1253-1261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai T., Howatson A. F. The fine structure of vesicular stomatitis virus. Virology. 1968 Jun;35(2):268–281. doi: 10.1016/0042-6822(68)90267-5. [DOI] [PubMed] [Google Scholar]
- Nakai T., Shand F. L., Howatson A. F. Development of measles virus in vitro. Virology. 1969 May;38(1):50–67. doi: 10.1016/0042-6822(69)90127-5. [DOI] [PubMed] [Google Scholar]
- Raghow R., Kingsbury D. W., Portner A., George S. Topography of a flexible ribonucleoprotein helix: protein-protein contacts in Sendai virus nucleocapsids. J Virol. 1979 Jun;30(3):701–710. doi: 10.1128/jvi.30.3.701-710.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghow R., Kingsbury D. W. Protein-RNA contacts in Sendai virus nucleocapsids revealed by photo-crosslinking. Virology. 1979 Oct 15;98(1):267–271. doi: 10.1016/0042-6822(79)90546-4. [DOI] [PubMed] [Google Scholar]
- Scheid A., Caliguiri L. A., Compans R. W., Choppin P. W. Isolation of paramyxovirus glycoproteins. Association of both hemagglutinating and neuraminidase activities with the larger SV5 glycoprotein. Virology. 1972 Dec;50(3):640–652. doi: 10.1016/0042-6822(72)90418-7. [DOI] [PubMed] [Google Scholar]
- Simpson R. W., Hauser R. E. Structural components of vesicular stomatitis virus. Virology. 1966 Aug;29(4):654–667. doi: 10.1016/0042-6822(66)90289-3. [DOI] [PubMed] [Google Scholar]
- Sokol F., Clark H. F. Phosphoproteins, structural components of rhabdoviruses. Virology. 1973 Mar;52(1):246–263. doi: 10.1016/0042-6822(73)90413-3. [DOI] [PubMed] [Google Scholar]
- Sokol F., Schlumberger H. D., Wiktor T. J., Koprowski H. Biochemical and biophysical studies on the nucleocapsid and on the RNA of rabies virus. Virology. 1969 Aug;38(4):651–665. doi: 10.1016/0042-6822(69)90184-6. [DOI] [PubMed] [Google Scholar]
- Stone H. O., Kingsbury D. W., Darlington R. W. Sendai virus-induced transcriptase from infected cells: polypeptides in the transcriptive complex. J Virol. 1972 Nov;10(5):1037–1043. doi: 10.1128/jvi.10.5.1037-1043.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone H. O., Portner A., Kingsbury D. W. Ribonucleic acid transcriptases in Sendai Virions and infected cells. J Virol. 1971 Aug;8(2):174–180. doi: 10.1128/jvi.8.2.174-180.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. C., Snyder R. M., Schnaitman C. A. Protein composition of the structural components of vesicular stomatitis virus. J Virol. 1969 Jun;3(6):611–618. doi: 10.1128/jvi.3.6.611-618.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]