Abstract
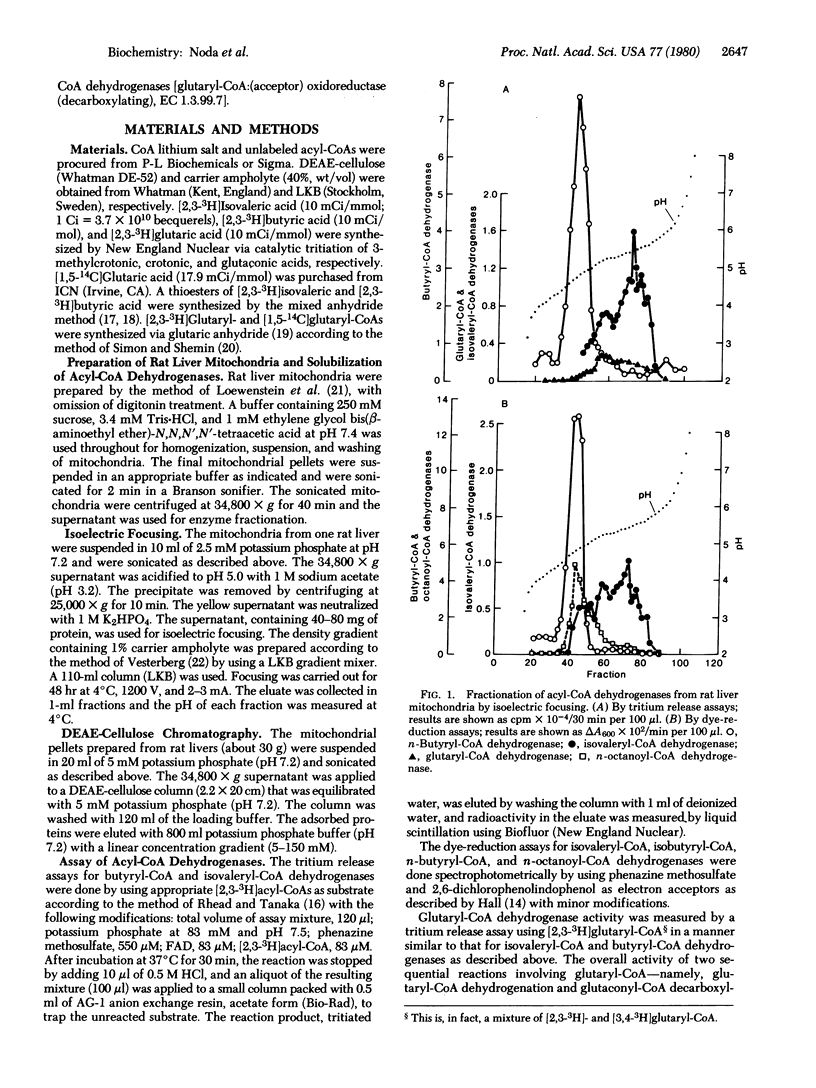
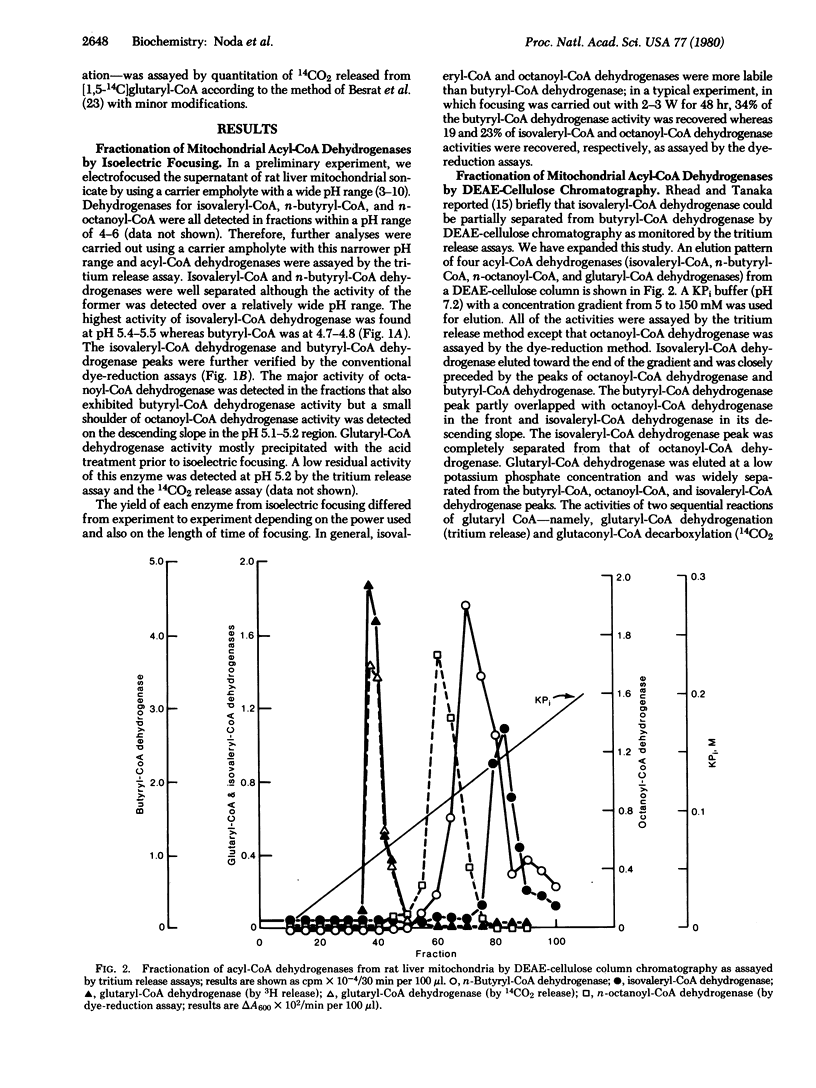
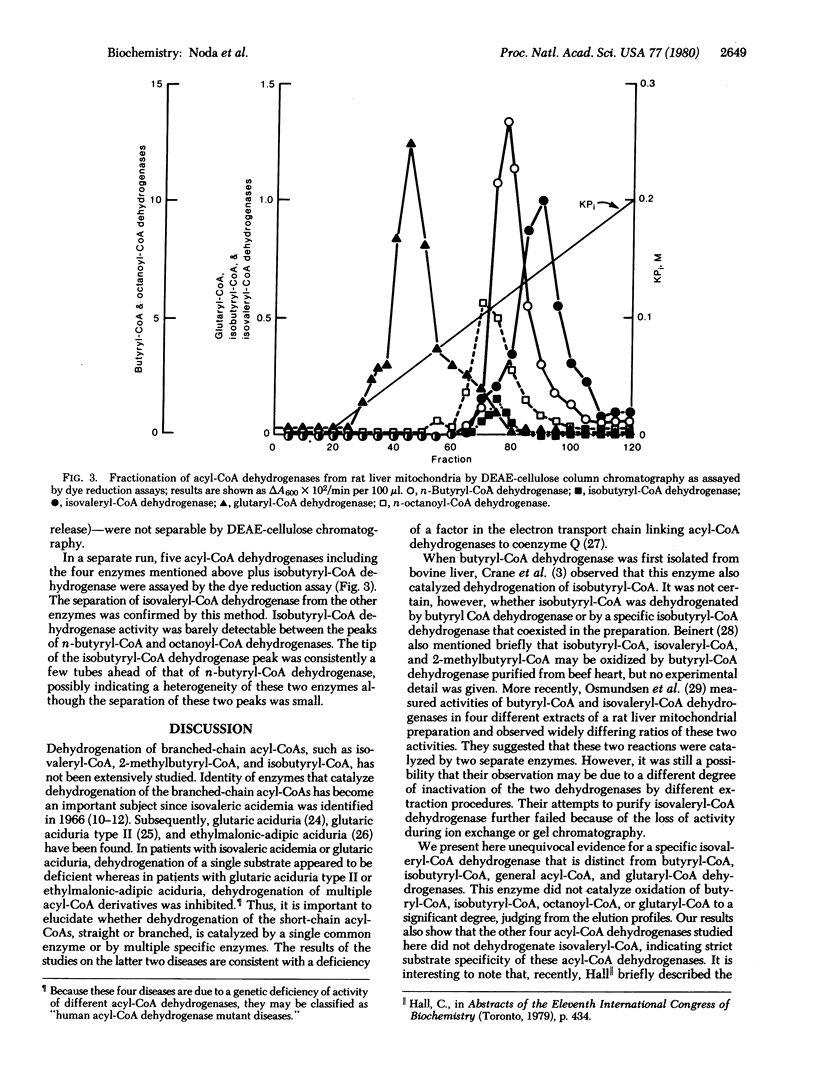
There has been ambiguity concerning the specificity of the enzymes that dehydrogenate short branched-chain acyl-CoAs. It previously had been assumed that isovaleryl-CoA is dehydrogenated by n-butyryl-CoA dehydrogenase [butyryl-CoA:(acceptor) oxidoreductase, EC 1.3.99.2]. To solve this problem, we fractionated five short-chain acyl-CoA dehydrogenases (isovaleryl-CoA, n-butyryl-CoA, isobutyryl-CoA, n-octanoyl-CoA, and glutaryl-CoA dehydrogenases) from rat liver mitochondria by isoelectric focusing and DEAE-cellulose column chromatography. The isovaleryl-CoA dehydrogenase [isovaleryl-CoA:(acceptor) oxidoreductase, EC 1.3.99.10] peak was almost completely separated from the peaks of n-butyryl CoA- and n-octanoyl-CoA dehydrogenases by isoelectric focusing, and it was well separated from glutaryl-CoA dehydrogenase [glutaryl-CoA:(acceptor) oxidoreductase (decarboxylating), EC 1.3.99.7] and n-octanoyl-CoA dehydrogenase by DEAE-cellulose column chromatography. The isovaleryl-CoA dehydrogenase peak partly overlapped that of n-butyryl-CoA and isobutyryl-CoA dehydrogenases in the latter procedure. These results unequivocally demonstrate that isovaleryl-CoA is oxidized by a specific isovaleryl-CoA dehydrogenase. The other dehydrogenase peaks also demonstrated activity toward a single substrate, except that isobutyryl-CoA dehydrogenase activity could not be clearly resolved from n-butyryl-CoA dehydrogenase activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amster J., Tanaka K. Metabolism in rats in vivo of (2S)[3,3,3-2H3]isobutyrate. Identification of (2-pro-S)methyl group as the source of a proton in dehydrogenation. J Biol Chem. 1980 Jan 10;255(1):119–120. [PubMed] [Google Scholar]
- BACHHAWAT B. K., ROBINSON W. G., COON M. J. Enzymatic carboxylation of beta-hydroxyisovaleryl coenzyme A. J Biol Chem. 1956 Apr;219(2):539–550. [PubMed] [Google Scholar]
- Baretz B. H., Lollo C. P., Tanaka K. Metabolism in rats in vivo of RS-2-methylbutyrate and n-butyrate labeled with stable isotopes at various positions. Mechanism of biosynthesis and degradation of ethylmalonyl semialdehyde and ethylmalonic acid. J Biol Chem. 1979 May 10;254(9):3468–3478. [PubMed] [Google Scholar]
- Besrat A., Polan C. E., Henderson L. M. Mammalian metabolism of glutaric acid. J Biol Chem. 1969 Mar 25;244(6):1461–1467. [PubMed] [Google Scholar]
- Budd M. A., Tanaka K., Holmes L. B., Efron M. L., Crawford J. D., Isselbacher K. J. Isovaleric acidemia. Clinical features of a new genetic defect of leucine metabolism. N Engl J Med. 1967 Aug 17;277(7):321–327. doi: 10.1056/NEJM196708172770701. [DOI] [PubMed] [Google Scholar]
- CRANE F. L., MII S., HAUGE J. G., GREEN D. E., BEINERT H. On the mechanism of dehydrogenation of fatty acyl derivatives of coenzyme A. I. The general fatty acyl coenzyme A dehydrogenase. J Biol Chem. 1956 Feb;218(2):701–706. [PubMed] [Google Scholar]
- Christensen E., Brandt N. J. Studies on glutaryl-CoA dehydrogenase in leucocytes, fibroblasts and amniotic fluid cells. The normal enzyme and the mutant form in patients with glutaric aciduria. Clin Chim Acta. 1978 Sep 1;88(2):267–276. doi: 10.1016/0009-8981(78)90431-x. [DOI] [PubMed] [Google Scholar]
- Engel P. C. Possibility of inborn defect in isovalericacidaemia involving altered enzyme specificity rather than total inactivity. Nature. 1974 Mar 8;248(5444):140–142. doi: 10.1038/248140a0. [DOI] [PubMed] [Google Scholar]
- GOLDMAN P., VAGELOS P. R. The specificity of triglyceride synthesis from diglycerides in chicken adipose tissue. J Biol Chem. 1961 Oct;236:2620–2623. [PubMed] [Google Scholar]
- GREEN D. E., MII S., MAHLER H. R., BOCK R. M. Studies on the fatty acid oxidizing system of animal tissues. III. Butyryl coenzyme A dehydrogenase. J Biol Chem. 1954 Jan;206(1):1–12. [PubMed] [Google Scholar]
- Goodman S. I., Kohlhoff J. G. Glutaric aciduria: inherited deficiency of glutaryl-CoA dehydrogenase activity. Biochem Med. 1975 Jun;13(2):138–140. doi: 10.1016/0006-2944(75)90149-0. [DOI] [PubMed] [Google Scholar]
- HAUGE J. G., CRANE F. L., BEINERT H. On the mechanism of dehydrogenation of fatty acyl derivatives of coenzyme A. III. Palmityl coA dehydrogenase. J Biol Chem. 1956 Apr;219(2):727–733. [PubMed] [Google Scholar]
- Hall C. L. Acyl-CoA dehydrogenases and electron-transferring flavoprotein. Methods Enzymol. 1978;53:502–518. doi: 10.1016/s0076-6879(78)53053-x. [DOI] [PubMed] [Google Scholar]
- Hall C. L., Heijkenskjöld L., Bártfai T., Ernster L., Kamin H. Acyl coenzyme A dehydrogenases and electron-transferring flavoprotein from beef hart mitochondria. Arch Biochem Biophys. 1976 Dec;177(2):402–414. doi: 10.1016/0003-9861(76)90453-7. [DOI] [PubMed] [Google Scholar]
- Hall C. L., Kamin H. The purification and some properties of electron transfer flavoprotein and general fatty acyl coenzyme A dehydrogenase from pig liver mitochondria. J Biol Chem. 1975 May 10;250(9):3476–3486. [PubMed] [Google Scholar]
- Hoskins D. D. The electron-transferring flavoprotein as a common intermediate in the mitochondrial oxidation of butyryl coenzyme A and sarcosine. J Biol Chem. 1966 Oct 10;241(19):4472–4479. [PubMed] [Google Scholar]
- Loewenstein J., Scholte H. R., Wit-Peeters E. M. A rapid and simple procedure to deplete rat-liver mitochondria of lysosomal activity. Biochim Biophys Acta. 1970 Dec 8;223(2):432–436. doi: 10.1016/0005-2728(70)90201-x. [DOI] [PubMed] [Google Scholar]
- Mamer O. A., Tjoa S. S., Scriver C. R., Klassen G. A. Demonstration of a new mammalian isoleucine catabolic pathway yielding an Rseries of metabolites. Biochem J. 1976 Dec 15;160(3):417–426. doi: 10.1042/bj1600417a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantagos S., Genel M., Tanaka K. Ethylmalonic-adipic aciduria. In vivo and in vitro studies indicating deficiency of activities of multiple acyl-CoA dehydrogenases. J Clin Invest. 1979 Dec;64(6):1580–1589. doi: 10.1172/JCI109619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przyrembel H., Wendel U., Becker K., Bremer H. J., Bruinvis L., Ketting D., Wadman S. K. Glutaric aciduria type II: report on a previously undescribed metabolic disorder. Clin Chim Acta. 1976 Jan 16;66(2):227–239. doi: 10.1016/0009-8981(76)90060-7. [DOI] [PubMed] [Google Scholar]
- Rhead W. J., Tanaka K. Demonstration of a specific mitochondrial isovaleryl-CoA dehydrogenase deficiency in fibroblasts from patients with isovaleric acidemia. Proc Natl Acad Sci U S A. 1980 Jan;77(1):580–583. doi: 10.1073/pnas.77.1.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEYN-PARVE E. P., BEINERT H. On the mechanism of dehydrogenation of fatty acyl derivatives of coenzyme A. VII. The nature of the green color of butyryl dehydrogenase. J Biol Chem. 1958 Oct;233(4):853–861. [PubMed] [Google Scholar]
- Tanaka K., Budd M. A., Efron M. L., Isselbacher K. J. Isovaleric acidemia: a new genetic defect of leucine metabolism. Proc Natl Acad Sci U S A. 1966 Jul;56(1):236–242. doi: 10.1073/pnas.56.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Isselbacher K. J. The isolation and identification of N-isovalerylglycine from urine of patients with isovaleric acidemia. J Biol Chem. 1967 Jun 25;242(12):2966–2972. [PubMed] [Google Scholar]
- Thorpe C., Matthews R. G., Williams C. H., Jr Acyl-coenzyme A dehydrogenase from pig kidney. Purification and properties. Biochemistry. 1979 Jan 23;18(2):331–337. doi: 10.1021/bi00569a016. [DOI] [PubMed] [Google Scholar]



