Abstract
IFNG/IFNγ plays a critical role in driving innate and acquired defenses against infectious pathogens. The death-associated protein kinase 1 (DAPK1), originally identified as an activator of IFNG-induced cell death, controls autophagy. Previously, we have shown that transcription factor CEBPB (C/EBP-β) regulates IFNG-induced expression of Dapk1 through a CRE/ATF motif in its enhancer. In this paper we have shown that ATF6, an ER-resident transcription factor regulates IFNG-induced Dapk1 expression through the CRE/ATF site, in association with CEBPB. IFNG-stimulated proteolytic cleavage of ATF6, and MAPK1/3 (ERK2/1)-dependent phosphorylation of CEBPB together control the expression of Dapk1. Consistent with their requirement for DAPK1 expression, IFNG fails to induce autophagy in cells lacking either Atf6 or Cebpb. More importantly, the Atf6−/− mice are highly susceptible to lethal bacterial infections due to a loss of autophagy. This study reported a connection between ER stress and autophagy in mediating antibacterial defenses.
Keywords: signal transduction, gene expression, innate immunity, bacterial infection
Autophagy is now well accepted as a central player in controlling infectious pathogens, development of cancer, immunity and other pathogenic processes. However, the effects of biological response modifiers on autophagy are largely not understood. The interferon family of cytokines executes a frontline defense against pathogens and neoplastic cells in vivo by inducing the expression of several genes, initially by a direct effect on the infected/transformed cell and later by shaping immunity against the invader. Thus, a potential overlap between autophagy and IFNs can be expected. Although a lion’s share of IFN actions are driven through the JAK-STAT pathways, we and others hypothesized that non-STAT pathways also contribute to IFN-actions; and discovered transcription factor CEBPB as a regulator of certain IFN-stimulated genes. Our earlier work demonstrated that the expression of DAPK1, a serine threonine kinase that regulates autophagy and growth suppression, was dependent on transcription factor CEBPB. DAPK1 was originally isolated as an IFNG-induced cell death activator by Adi Kimchi’s lab using a genetic screen. A direct regulation of BECN1 and MAP1LC3B proteins, two critical players in autophagy by DAPK1, was recently reported.
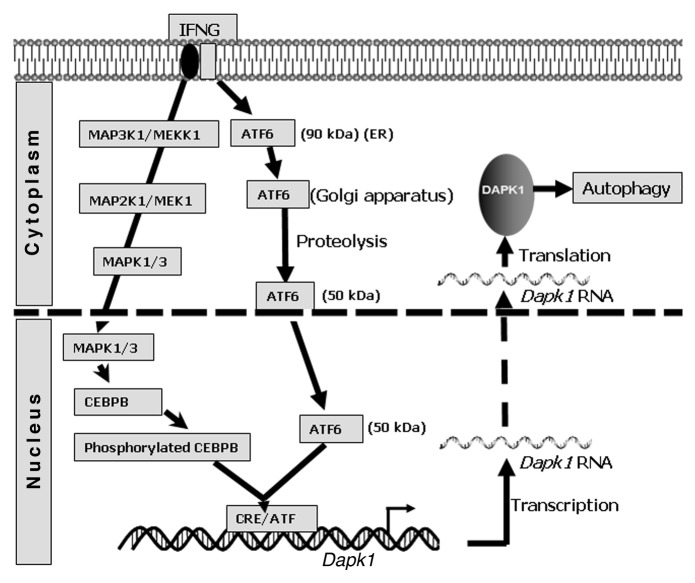
We reported that the IFNG–induced expression of Dapk1 critically depends on CEBPB, via a nonclassical CEBPB–binding site, CRE/ATF. Therefore, we searched for another factor(s) that cooperates with CEBPB in upregulating Dapk1 and identified ATF6, a key ER stress-activated transcription factor, as one such molecule. In its native state, ATF6 is an ER membrane-anchored precursor (90 kDa) held inactive by an inhibitory protein, HSPA5/BiP. Following ER stress, ATF6 dissociates from HSPA5 and translocates to the Golgi apparatus. In the Golgi apparatus, ATF6 is cleaved by endoproteases MBTPS1/S1P and MBTPS2/S2P, leading to the release and nuclear translocation of transcriptionally active ATF6 (50 kDa), and stimulation of target genes (Fig. 1). We showed that ATF6 associates with CEBPB in response to IFNG treatment and binds to the Dapk1 promoter. Ablation of ATF6 expression using either RNAi or by gene knockout suppresses Dapk1 expression. Consistent with a requirement for proteolysis, an ATF6 mutant lacking proteolytic sites, fails to enter the nucleus in response to IFNG treatment and promote Dapk1 expression. This is the first documented IFN-activation of a transcription factor via proteolysis. Previously, we reported that IFN-induced phosphorylation of CEBPB Thr189 by the MAP kinases MAPK1/3 is necessary for Dapk1 expression. Thus, the IFN-induced phosphorylation CEBPB and proteolytic activation of ATF6 are obligatory for Dapk1 expression. More importantly, neither CEBPB nor ATF6 bound to the Dapk1 promoter in the absence of the other.
Figure 1. A model for IFN-induced DAPK1 activated autophagy. In response to IFNG, MAPK1/3 phosphorylate CEBPB through a MAP3K1/MEKK1-MAP2K1/MEK1-MAPK1/3 axis. A second arm involves IFNG-induced translocation of ATF6 from the ER to the Golgi apparatus, where the N terminus containing transcription factor ATF6 is released by proteolysis prior to its entry into the nucleus. In the nucleus, ATF6 associates with phosphorylated CEBPB for inducing Dapk1 expression through a CRE/ATF site.
Since DAPK1 is important for driving certain forms of autophagy, we queried the biological importance of these pathways to autophagy on the basis of the above observations. Deletion of either Cebpb or Atf6 results in a failure to induce autophagy in response to IFNG. Whereas restoration of the corresponding wild-type gene products reinstated autophagy, neither the CEBPB T189A mutant nor the proteolysis-resistant ATF6 mutant supported the development of autophagy in these cells. To address the biological relevance of these observations, we infected wild-type and Atf6−/− mice with a sublethal dose of Bacillus anthracis. The majority of Atf6−/− mice (~72%) died of infection within 6 d, while the wild-type mice were highly resistant even at 10 d. Bacterial loads in the spleen and liver were significantly higher in the Atf6−/− mice compared with the wild-type mice. Peritoneal macrophages from the wild-type, but not Atf6−/−, mice were able to activate autophagy. Thus, ATF6 is essential for the execution of bacterial infection-associated and IFN-induced autophagy. Recently, another group reported the activation of ATF6 in cells infected with Listeria monocytogenes. Previously, Tanaka et al. (1995) reported that deletion of Cebpb caused a susceptibility of mice to infections, similar to what we found with ATF6. Since autophagy was unknown at that time, they concluded that the defective response was due to a loss of macrophage-dependent phagocytosis. However, a careful re-examination of the electron micrographs reported in that paper, suggest defects in autophagy rather than of phagocytosis. Interestingly, the macrophages from these mice also fail to kill heterologous tumors. Whether this is the same case with ATF6 is currently being studied.
IFNG has been reported to induce ER stress in oligodendrocytes. However, how ER stress is coupled to autophagy was not explored. It is likely that while ER membranes support the formation of phagophores and ATF6 controls DAPK1 expression, Ca2+ from ER stores activates DAPK1 and promotes autophagosome maturation. In summary, we showed that extracellular signals emitted by cytokines like IFNs, activate the IFNG–ATF6/CEBPB–DAPK1 pathway for promoting defense against bacterial infections. Such a control over DAPK1 expression is critical because high DAPK1 levels kill cells. Our study directly couples ER stress with autophagy.
Acknowledgments
These studies were supported by NIH R01-CA78282 to D.V.K.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/21403