Abstract
Chloroplast glycerolipids in a number of higher-plant species, including Arabidopsis thaliana, are synthesized by two distinct pathways termed the prokaryotic and eukaryotic pathways. The molecules of galactolipids produced by the prokaryotic pathway contain substantial amounts of hexadecatrienoic acid fatty acid. Here we describe a new class of mutants, designated gly1, with reduced levels of hexadecatrienoic acid. Lipid fatty acid profiles indicated that gly1 mutants exhibited a reduced carbon flux through the prokaryotic pathway that was compensated for by an increased carbon flux through the eukaryotic pathway. Genetic and biochemical approaches revealed that the gly1 phenotype could not be explained by a deficiency in the enzymes of the prokaryotic pathway. The flux of fatty acids into the prokaryotic pathway is sensitive to changes in glycerol-3-phosphate (G3P) availability, and the chloroplast G3P pool can be increased by exogenous application of glycerol to leaves. Exogenous glycerol treatment of gly1 plants allowed chemical complementation of the mutant phenotype. These results are consistent with a mutant lesion affecting the G3P supply within the chloroplast. The gly1 mutants may therefore help in determining the pathway for synthesis of chloroplast G3P.
Higher plants possess two distinct pathways for the synthesis of chloroplast glycerolipids in leaf cells (Roughan et al., 1980; Browse and Somerville, 1991). The chloroplasts or the plastids of the cell are the sole site of de novo fatty acid synthesis (Ohlrogge et al., 1991). The final products of fatty acid synthesis and of the soluble stearoyl ACP desaturase are 16:0-ACP and 18:1-ACP (McKeon and Stumpf, 1982; Shanklin and Somerville, 1991). These either enter the prokaryotic pathway of the chloroplast inner envelope to produce chloroplastic lipids or they are hydrolyzed to free fatty acids that are exported through the plastid envelope to the cytoplasm as CoA thioesters, thus initiating the eukaryotic pathway. Because of the specificities of the plastid acyltransferases for certain acyl-ACP substrates (Frentzen, 1993), the PA made by the prokaryotic pathway has 16:0 at the sn-2 position and, in most cases, 18:1 at the sn-1 position. This PA is used for the synthesis of PG or is converted to DAG by a PAPase (Joyard and Douce, 1977). This DAG pool is the precursor for the synthesis of MGD, DGD, and SL, the major plastid membrane lipids (Joyard et al., 1993). The PA synthesized in the ER by a different set of acyltransferases than the plastid isozymes is characteristically enriched in 18-carbon fatty acids at the sn-2 position; 16:0, when present, is confined to the sn-1 position. This PA is used to produce phospholipids such as PC, PE, and PI, which are characteristic of the various extrachloroplastic membranes of the cell. In addition, a portion of PC produced by the eukaryotic pathway is returned to the chloroplast and used in the production of chloroplast lipids (Browse and Somerville, 1991).
In the majority of higher plants, PG is the only product of the prokaryotic pathway, and the remaining chloroplast lipids are synthesized entirely by the eukaryotic pathway (Browse et al., 1986b). However, in a number of species, including Arabidopsis, both pathways contribute about equally to the synthesis of MGD, DGD, and SL (Browse and Somerville, 1991), and the leaf lipids of such plants characteristically contain substantial amounts of 16:3, which is found only at the sn-2 position of galactolipid molecules produced by the prokaryotic pathway. These species have been termed 16:3 plants to distinguish them from 18:3 plants, the galactolipids of which contain predominantly α-linolenate (Jamieson and Reed, 1971; Browse and Somerville, 1991).
Mutants of Arabidopsis with altered fatty acid composition have been isolated (Browse and Somerville, 1994). These mutants were identified by direct analysis of leaf or seed fatty acid composition of individual mutagenized plants by GC (Browse et al., 1986a). One of them, act1, is deficient in the activity of chloroplast GPAT, the first enzyme of the prokaryotic pathway, and its leaf fatty acid composition is characterized by greatly reduced amounts of 16:3 because the act1 mutation substantially blocks the flux of carbon into the prokaryotic pathway (Kunst et al., 1988). In this paper we report the isolation and characterization of a second class of mutants with reduced levels of 16:3. These mutants also exhibit reduced carbon flux into the prokaryotic pathway. However, the mutation does not appear to affect a step in lipid synthesis but instead may limit the supply of G3P within the chloroplast.
MATERIALS AND METHODS
Plant Material and Growth Conditions
The lines of Arabidopsis thaliana (L.) Heynh. described here were descended from the Columbia wild type. Mutants were isolated from M2 populations obtained after mutagenesis with ethyl methanesulfonate (Haughn and Somerville, 1986) by directly analyzing the fatty acid compositions of small tissue samples by GC (Browse et al., 1986a). The mutant lines were backcrossed to the wild type three times before being used in any of the experiments reported here. Plants were grown on soil in controlled-environment chambers at 22°C under continuous fluorescent illumination (150 μmol quanta m−2 s−1). Plants used to isolate chloroplasts were grown for 11 d under continuous illumination before being transferred to a growth-type chamber in a day/night cycle at 22°C for another 12 d. The lighting regime in the growth chamber was 150 μmol quanta m−2 s−1 for an 8-h day.
Fatty Acid and Lipid Analysis
The overall fatty acid compositions of leaves and other tissues were determined by GC after derivatization with 2.5% (v/v) H2SO4 in methanol (Miquel and Browse, 1992). When small tissue samples were analyzed, the same procedure was used except that fatty acid methyl esters were extracted into 150 μL (rather than 1 mL) of hexane. Typically, 75 to 100 μL of this extract could be recovered and transferred to a microvial for injection onto the gas chromatograph. Usually, a 1-μL aliquot taken directly from the sample was sufficient for analysis. Samples of leaf tissue were killed rapidly by immersion in liquid N2 and ground under liquid N2 before being extracted and analyzed as described previously (Miquel and Browse, 1992).
Lipase Positional Analysis
The fatty acid compositions at the sn-1 and sn-2 positions of individual lipids were determined by lipase digestion. After TLC lipids were extracted from the silica gel by the method of Bligh and Dyer (1959). The protocol for digestion with Rhizopus sp. lipase, including purification of the lyso-derivatives and fatty acids, was that described by Siebertz and Heinz (1977), except that 50 mm H3BO4 was added to the buffer used for lipase digestion to minimize intramolecular acyl transfer on the lyso-lipids produced. Fatty methyl esters were formed from untreated lipids, lyso-lipids, and fatty acids as described above, and the fatty acid composition of each compound was determined by GC.
Chloroplast Isolation
Leaves of the wild type and mutant were harvested at the end of the night period. Ten grams fresh weight of leaves was homogenized using a Polytron (Kinematica, Lucern, Switzerland; two to three 5-s bursts) in 125 mL of semifrozen buffer containing 0.35 m sorbitol, 25 mm Hepes-KOH, pH 7.8, and 2 mg mL−1 defatted BSA. The homogenates were filtered through two layers of prewetted Miracloth (Calbiochem) and centrifuged at 2,600g for 1 min in an HB-4 swinging-bucket rotor (Beckman). Pellets were gently resuspended with a small volume of cold wash buffer that contained 0.33 m sorbitol, 10 mm Hepes-KOH, pH 7.8, and 2.5 mm EDTA. This plastid suspension was layered on top of a preformed Percoll (Pharmacia) gradient. The gradient was self-generated by mixing 16 mL of 100% Percoll in 0.33 m sorbitol and 16 mL of wash buffer, and centrifuging for 20 min at 27,000g (SS34 rotor, Beckman). The chloroplasts were purified by centrifuging the gradient at 14,000g (HB-4 rotor) for 1 min. The intact chloroplasts (the green band deeper in the gradient) were collected, diluted at least three times with wash buffer, and then centrifuged at 2,600g for 1 min as described previously. Finally, the intact chloroplasts were suspended in wash buffer and used immediately. All operations were carried out at 4°C.
Enzyme Assay
The PAPase was assayed within isolated envelope membranes, according to assay 2 of Malherbe et al. (1992). Radioactive PA was synthesized in situ as purified envelope membranes were loaded with PA synthesized by acylation of sn-[14C]G3P (specific radioactivity, 5.7 × 1012 Bq/mol; NEN Life Sciences Products). PAPase activity was then measured by following PA conversion into DAG. Typically, the reaction mixture contained 100 to 150 μg of fatty acids from envelope membranes and 2 to 3.5 mg from stromal proteins.
Exogenous Glycerol Treatment
Wild-type and mutant plants grown under continuous illumination as described above were 14 d old at the start of the treatment. Glycerol solutions in double-distilled water (10 or 50 mm) were sprayed on plants at different times during a period of 80 h using a perfume atomizer. Control plants were sprayed with water. The volumes used were 500 μL for each treatment of 25 plants during the first 12 h, 650 μL for treatments from 24 to 36 h, and 800 μL after 48 h until the end of the experiment. The surface of the leaves was dry within 30 min after spraying. Thirty minutes after the 36- and 80-h treatments, 6 to 15 plants from each treatment and from each genotype were harvested and weighed individually before being frozen in liquid N2. Total lipids were extracted from each sample and analyzed as described above.
Other Assays
Chloroplast integrity was determined as described by Heber and Santarius (1970). Protein concentration was determined by the method of Bradford (1976) using BSA as a standard.
RESULTS
Genetic Analysis
The mutant lines JB19 and EMS 5 no. 1 were isolated without selection by screening M2 progeny of ethyl methanesulfonate-mutagenized seeds for altered leaf fatty acid composition. The mutant lines were identified as being deficient in 16:3. Genetic complementation tests indicated that the two lines have a lesion at the same locus (data not shown). Therefore, we characterized only one of the lines in detail. The representative mutant line JB19 was normal in appearance and growth characteristics but could be readily distinguished from the wild type by the reduced amount of 16:3 in its leaf lipids (Table I). The mode of inheritance of this altered fatty acid composition was determined by reciprocal crosses between line JB19 plants and wild-type Arabidopsis. Leaves of the F1 progeny showed a slightly decreased amount of 16:3 compared with the wild type (Table I), suggesting that the wild-type allele is incompletely dominant. The frequency of individuals with the mutant phenotype in the F2 population resulting from self-fertilization of F1 plants was also measured by GC of leaf samples. Of 96 F2 plants analyzed, 21 had fatty acid compositions similar to those of plants of the original JB19 line, whereas the remaining individuals had leaf fatty acid compositions similar to those of plants of the wild type or the F1 hybrid. This pattern of segregation is a good fit (χ2 = 0.374, P > 0.6) to the 3:1 hypothesis and indicates that the altered fatty acid composition is caused by a single nuclear mutation at a locus we have designated gly1. Consequently, the two lines JB19 and EMS 5 no. 1 were designated gly1-1 and gly1-2, respectively.
Table I.
Fatty acid composition of total leaf lipids from wild-type (WT) and gly1-1 mutant Arabidopsis
| Fatty Acid | WT | (WT × gly1-1) F1 | gly1-1 |
|---|---|---|---|
| mol % | |||
| 16:0 | 13.9 ± 0.1 | 13.6 ± 0.1 | 13.5 ± 0.1 |
| 16:1 cis | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| 16:1 trans | 2.8 ± 0.1 | 2.7 ± 0.1 | 2.2 ± 0.1 |
| 16:2 | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.5 ± 0.1 |
| 16:3 | 15.5 ± 0.1 | 14.3 ± 0.1 | 5.4 ± 0.1 |
| 18:0 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| 18:1 | 3.0 ± 0.1 | 3.4 ± 0.1 | 8.0 ± 0.2 |
| 18:2 | 13.8 ± 0.1 | 14.5 ± 0.1 | 18.2 ± 0.1 |
| 18:3 | 48.5 ± 0.1 | 49.2 ± 0.1 | 50.8 ± 0.2 |
Homozygous (gly1-1) and heterozygous (F1 of the cross WT × gly1-1) mutant plants were grown together with the wild type. Results are means ± se, n = 24.
An Arabidopsis mutant, act1, with greatly reduced 16:3 amounts in its leaf lipids (1–2% of total fatty acids) has previously been characterized as being deficient in chloroplast GPAT (Kunst et al., 1988). Reciprocal crosses between gly1-1 and act1 produced F1 progeny, the fatty acid composition of which was similar to that of the wild type. This genetic complementation indicates that gly1-1 and act1 are not allelic and that gly1-1 is not deficient in chloroplast GPAT activity.
Biochemical Characterization
In mutant leaves the nearly 3-fold reduction in 16:3 was not accompanied by any increase in the precursors 16:0, 16:1, or 16:2, but was compensated for by increased 18:1, 18:2, and 18:3 amounts (Table I). This lack of precursor accumulation indicates that the mutation in gly1-1 is not attributable to a reduction in desaturation of 16-carbon fatty acids. In plant roots, which contain a predominance of extrachloroplastic membranes, and in seeds, which contain large amounts of triglycerides, the prokaryotic pathway does not contribute significantly to lipid synthesis. Comparison of the overall fatty acid composition of the roots and mature seeds from the mutant and the wild type showed no detectable difference. Although we have shown that the two mutations act1 and gly1-1 are not allelic (see above), the comparison between their respective overall leaf fatty acid composition suggests that the mutation in gly1-1 likely affects the flux of fatty acids into the prokaryotic pathway. Finally, because 16:3 is synthesized exclusively in chloroplasts by sequential desaturation of 16:0 acyl groups of galactolipids (Roughan et al., 1979; Roughan and Slack, 1982), the remaining amount of 16:3 in gly1-1 leaf lipids suggests that the mutation affects a biochemical step that is partially redundant, or that the mutation incompletely blocks a step in the prokaryotic pathway.
The biochemical consequences of the gly1-1 mutation are shown more clearly by an analysis of individual lipids extracted from leaf tissue of wild-type and mutant plants (Table II). The data indicated that the changes in the proportions of the various polar lipids in the mutant affected only the chloroplast lipids MGD, DGD, SL, and PG. In gly1-1 the mole fraction of MGD decreased by 10% compared with that in the wild type, whereas DGD increased by 19%. However, when chloroplast lipids were considered as a whole, there was no difference between gly1-1 and the wild type. The differences in the fatty acid compositions of individual lipids were more informative regarding the nature of the mutation. In MGD, DGD, and SL the reduction in 16-carbon fatty acid amounts was compensated for by higher amounts of 18-carbon fatty acids, as indicated by the ratio C-18/C-16 fatty acids (Table II). This ratio increased by 1.5-, 1.8-, and 2.5-fold for SL, DGD, and MGD, respectively, indicating a reduced synthesis of prokaryotic-type molecules for these lipids. The data on the lipid composition and on the fatty acid compositions of individual lipids showed that the reduced synthesis of galactolipids and SL by the prokaryotic pathway in the mutant was entirely compensated for by increased production of these lipids via the eukaryotic pathway.
Table II.
Fatty acid composition of leaf lipids from wild-type (WT) and gly1-1 mutant Arabidopsis
| Glycerolipid | Total Polar Lipids | Fatty Acid
Composition
|
C-18/C-16 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 16:0 | 16:1a | 16:2 | 16:3 | 18:0 | 18:1 | 18:2 | 18:3 | |||
| % | mol % | |||||||||
| MGD | ||||||||||
| WT | 40.0 | 1.2 | 1.2 | 2.0 | 33.0 | 0.2 | 1.1 | 2.7 | 58.7 | 1.7 |
| gly1-1 | 36.1 | 1.5 | 0.8 | 1.3 | 15.1 | 0.3 | 1.2 | 3.4 | 76.3 | 4.4 |
| DGD | ||||||||||
| WT | 15.6 | 11.6 | –b | 0.7 | 3.0 | 1.4 | 1.7 | 4.3 | 77.4 | 5.6 |
| gly1-1 | 18.6 | 7.4 | – | 0.5 | 0.9 | 0.9 | 1.4 | 3.2 | 85.6 | 10.3 |
| PG | ||||||||||
| WT | 7.5 | 30.0 | 21.4 | – | – | 2.4 | 7.1 | 8.2 | 30.9 | 1.0 |
| gly1-1 | 8.2 | 32.0 | 21.7 | – | – | 2.2 | 5.3 | 9.8 | 29.0 | 0.9 |
| SL | ||||||||||
| WT | 2.0 | 45.5 | – | – | – | 2.5 | 3.8 | 5.4 | 42.8 | 1.2 |
| gly1-1 | 2.3 | 33.0 | – | – | – | 5.0 | 8.2 | 6.7 | 47.1 | 2.0 |
| PC | ||||||||||
| WT | 19.7 | 19.7 | – | – | – | 2.4 | 7.3 | 37.9 | 32.7 | 4.1 |
| gly1-1 | 20.6 | 17.7 | – | – | – | 1.8 | 15.3 | 41.6 | 23.6 | 4.6 |
| PE | ||||||||||
| WT | 12.2 | 28.1 | – | – | – | 2.6 | 4.5 | 37.9 | 27.0 | 2.6 |
| gly1-1 | 11.0 | 27.2 | – | – | – | 2.5 | 8.7 | 43.3 | 18.3 | 2.7 |
| PI | ||||||||||
| WT | 3.0 | 42.3 | – | – | – | 5.7 | 5.2 | 24.0 | 22.8 | 1.4 |
| gly1-1 | 3.2 | 43.1 | – | – | – | 5.5 | 7.4 | 26.3 | 17.7 | 1.3 |
Values represent the averages of three samples.
Sum of 16:1 cis and 16:1 trans fatty acids.
Amounts were <0.1 mol%.
In wild-type Arabidopsis the prokaryotic pathway is responsible for producing approximately 70% of the total leaf MGD, 12% of the DGD, 63% of the SL, and 85% of the PG, as indicated by the amounts of 16-carbon fatty acids at the sn-2 position of the glycerol (Browse et al., 1986b). To quantitate the effect of the mutation on the flux of fatty acids through the prokaryotic pathway, purified lipids were digested with Rhizopus sp. lipase and the fatty acid compositions of the lyso-derivatives and released fatty acids were determined (Table III). This analysis indicated that in the mutant, only 34% of the MGD, 24% of the DGD, and 39% of the SL were synthesized through the prokaryotic pathway. By contrast, the synthesis of PG was not affected. The other polar lipids contained >90% 18-carbon fatty acids at the sn-2 position of the glycerol, indicating that they were produced by the eukaryotic pathway.
Table III.
Mass and fatty acid compositions of wild-type (WT) and gly1-1 mutant Arabidopsis leaf lipids and their lyso-derivatives
| Lipid lyso-Derivative | Mass of
Fatty Acids
|
Fatty Acid Composition
|
||||
|---|---|---|---|---|---|---|
| 16-Carbon
|
18-Carbon
|
|||||
| WT | gly1-1 | WT | gly1-1 | WT | gly1-1 | |
| mol/1000 mol | mol % | |||||
| MGD | 390 | 348 | 37 | 20 | 63 | 80 |
| sn-2 | 70 | 34 | 30 | 66 | ||
| DGD | 152 | 184 | 15 | 8 | 95 | 92 |
| sn-2 | 12 | 24 | 88 | 96 | ||
| PG | 81 | 83 | 52 | 51 | 48 | 49 |
| sn-2 | 83 | 86 | 17 | 14 | ||
| SL | 28 | 23 | 43 | 30 | 57 | 70 |
| sn-2 | 63 | 39 | 37 | 61 | ||
| PC | 218 | 219 | 22 | 22 | 78 | 78 |
| sn-2 | 1 | 2 | 99 | 98 | ||
| PE | 100 | 113 | 29 | 28 | 71 | 72 |
| sn-2 | 1 | 1 | 99 | 99 | ||
Polar lipids were separated by two-dimensional TLC. The mass and fatty acid compositions of the lipids and their lyso-derivatives, resulting from digestion with Rhizopus sp. lipase, were determined by GC analysis as outlined in Methods.
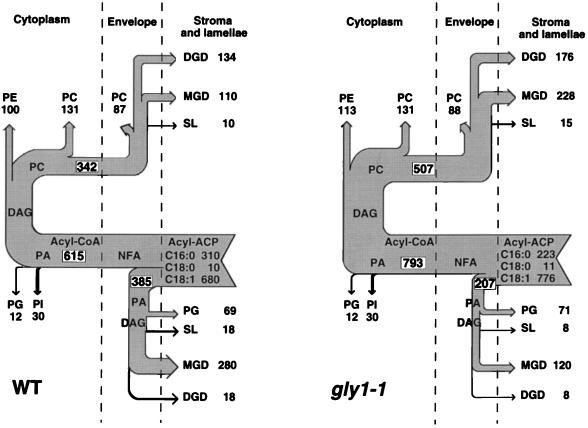
A previous detailed analysis of wild-type Arabidopsis showed that for every 1000 fatty acid molecules made in the chloroplast, 615 enter the eukaryotic pathway (117 as 16-carbon fatty acids and 498 as 18-carbon fatty acids). A similar analysis of gly1-1 indicated a 29% increase in flux through the eukaryotic pathway, which was made up of 85% 18-carbon fatty acid chains (Fig. 1). However, the C-18/C-16 ratio in PC, PE, and PI is the same as in the corresponding lipids of the wild type (Table II). In contrast, the C-18/C-16 ratio in the galactolipids and SL of the mutant in each case is more than the ratio calculated for these lipids synthesized by the eukaryotic pathway in the wild type (table IV of Browse et al., 1986b). Thus, the additional 18-carbon fatty acids entering the eukaryotic pathway in the mutant are found specifically in the additional quantities of chloroplast lipids (galactolipids and SL) that are produced by the eukaryotic pathway in response to the loss of the prokaryotic pathway. This situation is also characteristic of the act1 mutant, in which the loss of the prokaryotic pathway is almost complete except for the synthesis of PG. In act1 there is a 50% increase of the flux of fatty acids through the eukaryotic pathway that is made up of 86% 18-carbon fatty acids (Kunst et al., 1988).
Figure 1.
Flow diagram of fatty acid fluxes (mol/1000 mol) during lipid synthesis by wild-type (WT) and gly1-1 mutant Arabidopsis leaves. NFA, Nonesterified fatty acids.
Another analogy with the act1 mutant is that the mutation gly1-1 causes a significant increase in the amount of 18:1 and a decrease in the amount of 18:3 in all of the extrachloroplastic lipids. In these lipids there is only a slight effect on the amount of 18:2 (Table II). The increase in 18:1 amounts in these lipids in the mutant relative to the wild type reflects a 5 to 7% reduction in the extent of 18:1 desaturation, suggesting that the ER 18:1 desaturase may be unable to completely metabolize the increased flux of lipid through the eukaryotic pathway in the mutant.
PAPase Activity
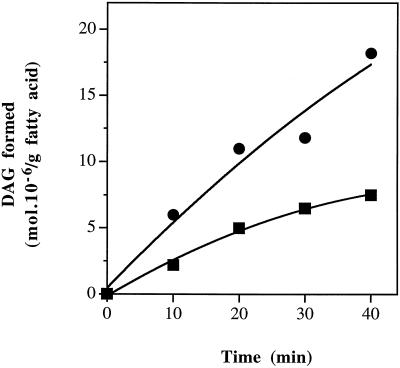
Because the synthesis of PG by the prokaryotic pathway was not affected in the mutant, we first considered the possibility that gly1-1 plants were deficient in the enzyme PAPase. The chloroplast PAPase provides the DAG moieties (Joyard and Douce, 1977) used for the synthesis of the prokaryotic molecular species of MGD, DGD, and SL that characterize 16:3 plants (Browse and Somerville, 1991). In the case of 18:3 plants, the chloroplast PAPase is not functional and the chloroplast lipids are synthesized from eukaryotic DAG molecular species (Browse and Somerville, 1991). Therefore, the gly1-1 phenotype could be explained by a mutation that reduced (but did not eliminate) PAPase activity. We assayed the activity of the chloroplast PAPase (Malherbe et al., 1992) and found that the activity is increased in the mutant compared with wild-type controls (Fig. 2). These results indicate that PAPase is not decreased in the mutant.
Figure 2.
PAPase activity from wild-type and gly1-1 mutant Arabidopsis. PAPase was assayed as described in Methods and data represent a typical experiment. ▪, Wild type; •, gly1-1.
G3P Supply for Lipid Biosynthesis in Chloroplasts
Another hypothesis that could account for the phenotype of gly1-1 plants is a defect in the availability of chloroplast G3P, which provides the glycerol backbone of the lipids synthesized by the prokaryotic pathway (Frentzen, 1993). Experiments with isolated spinach chloroplasts and intact leaf tissues indicate that flux of fatty acids into the prokaryotic pathway is sensitive to changes in G3P availability. Roughan et al. (1980) showed that chloroplasts incubated in a basal medium contained 23% of the incorporated label in glycerolipids (representing the prokaryotic pathway), whereas more than 70% was incorporated into unesterified fatty acids and acyl-CoAs, which are precursors for the eukaryotic pathway. Addition of 0.48 mm G3P to the basal medium increased glycerolipid products to 49% of the total radioactivity and decreased unesterified fatty acids plus acyl-CoAs to 40%. When glycerol was supplied to leaves of spinach plants, it increased the size of the G3P pool and increased the flux of [14C]acetate label into prokaryotic lipids (Gardiner et al., 1982). These results suggest that if the gly1-1 mutation is a lesion reducing the availability of G3P in the chloroplast, then exogenous application of glycerol should result in chemical complementation of the mutant phenotype.
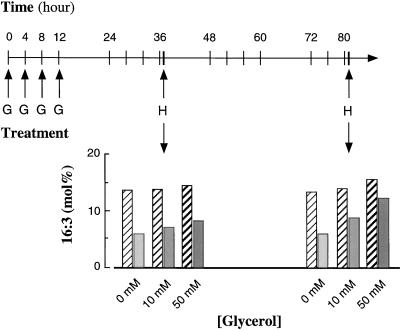
Because 16:3 is produced only by the prokaryotic pathway, it was possible to use this fatty acid to monitor the effects of glycerol treatments. A typical experiment in which glycerol was applied to wild-type and gly1-1 plants is described in Figure 3. The control plants in these experiments received an application of water instead of glycerol solutions. In wild-type plants glycerol caused a small but consistent increase in 16:3 in the total leaf lipids. By contrast, after 80 h of treatment with 50 mm glycerol, gly1-1 plants exhibited a 2-fold increase in the proportion of 16:3 in total lipids to levels that were similar to those of untreated wild-type plants. When the individual lipids were purified and their fatty acid composition analyzed, we determined that the level of 16:3 in MGD of gly1-1 plants after 80 h of 50 mm glycerol application had doubled to 29% compared with that of the control gly1-1 plants. By contrast, in wild-type plants the increase was only to 3%. Therefore, raising the G3P amounts in gly1-1 plants led to a notable alleviation of the altered fatty acid composition. This suggests that the concentration of G3P in gly1-1 chloroplasts is not sufficient to meet the requirement for chloroplast lipid synthesis through the prokaryotic pathway. Application of glycerol is known to inhibit photosynthesis (Leegood et al., 1988), but both the wild-type and mutant plants remained healthy throughout the duration of our experiments.
Figure 3.
Effect of exogenous glycerol treatment of wild-type and gly1-1 mutant Arabidopsis on their leaf fatty acid composition. Wild-type and mutant plants were supplied with exogenous glycerol by repeatedly spraying rosette leaves with water containing 0, 10, or 50 mm glycerol at the times indicated. Thirty minutes after the 36- and 80-h treatments, 6 to 15 plants for each glycerol treatment and from each genotype were harvested and total leaf lipids were extracted from each sample. The bar graphs show 16:3 as a percentage of total fatty acids. Striped bars, Wild type; shaded bars, gly1-1; G, glycerol treatment during the 1st 12 h. Repeat treatments on subsequent days are indicated by tick marks. H, Harvest.
DISCUSSION
Studies of lipid metabolism pathways using a genetic approach have revealed the existence of regulatory mechanisms that coordinate the activity of the two pathways for glycerolipid biosynthesis in higher plants. For example, the deficiency in activity of chloroplast GPAT in act1 mutants is compensated for by increased synthesis of chloroplast glycerolipids via the eukaryotic pathway (Kunst et al., 1988). This and studies of other Arabidopsis mutants (Browse et al., 1989; Kunst et al., 1989; Miquel and Browse, 1992) indicate that lipid metabolism is regulated to ameliorate the consequences of the lesion in each mutant by altering the flux through the two pathways of glycerolipid biosynthesis. Mutant gly1-1 plants are no exception, and the decreased synthesis of prokaryotic species of chloroplast lipids was entirely compensated for by increased production of these lipids via the eukaryotic pathway. This reduced synthesis through the prokaryotic pathway did not result in an increase of 16-carbon chains in the eukaryotic pathway. Instead, the 16:0-ACP was apparently elongated and desaturated to 18:1-ACP before export from the chloroplast. The overall C-18/C-16 ratio was then increased from 1.96 in the wild type to 3.5 in the mutant. The C-18/C-16 ratios of extrachloroplastic lipids were unchanged, suggesting that the amount of 16:0 export was not regulated by the availability of 16:0. Rather, the increase in the overall ratio indicated that elongation is regulated by availability of the substrate (16:0) and that this is determined by competition between alternative pathways of 16-carbon fatty acid metabolism. The mutation in gly1-1 is not an allele of act1, the structural gene for GPAT. Direct assays of PAPase activity in wild-type and mutant plants allowed us to indirectly test for a lesion in a third enzyme of the prokaryotic pathway, LPAAT. Assays of the PAPase activity necessitated a prior loading of chloroplast envelopes with radiolabeled PA and, to this effect, purified chloroplast envelopes were incubated with stromal proteins and the appropriate cofactors (Malherbe et al., 1992). The stroma is the source of the GPAT, which forms lyso-PA, and the envelope contains the LPAAT, which forms PA (Joyard and Douce, 1977). It has been shown that the GPAT possesses a specificity for G3P and exclusively catalyzes the acylation of the sn-1 position of the acyl acceptor (Frentzen, 1986). From this we inferred that the presence of radiolabeled PA in the envelopes resulted from the sole action of the LPAAT on lyso-PA. Thus, the gly1-1 mutation is not a deficiency in LPAAT activity.
The results of the exogenous glycerol treatment and the fact that the gly1-1 phenotype could not be explained by a deficiency in the enzymes of the prokaryotic pathway pointed to a possible defect in the G3P supply for lipid biosynthesis in chloroplasts. The experiments in which exogenous glycerol provides for chemical complementation of the gly1-1 phenotype are consistent with a mutant lesion affecting the supply of G3P within the chloroplast. Because G3P is present in (at least) the chloroplast and cytoplasm of leaf cells it is not possible to measure the chloroplast G3P pool in vivo. In plants G3P can be synthesized via essentially three different reaction sequences (Frentzen, 1993). It can be formed from DHAP by the action of an NAD+-G3P oxidoreductase (EC 1.1.1.8 or DHAP reductase) in both the chloroplast and cytoplasm of leaves from higher plants (Santora et al., 1979; Gee et al., 1988a, 1988b, 1988c, 1989; Kirsch et al., 1992). G3P can also be formed by the pathway DHAP → glyceraldehyde 3-phosphate → glyceraldehyde → glycerol → G3P in the cytoplasm (Ghosh and Sastry, 1988). The last enzyme of the pathway, glycerol kinase, has been detected in all plant organs (Hippman and Heinz, 1976; Sadava and Moore, 1987) and especially in germinating seeds (Huang and Beevers, 1975; Hippman and Heinz, 1976; Finlayson and Dennis, 1980) and appears to be the rate-limiting enzyme of this pathway. Except in developing groundnut seeds (Ghosh and Sastry, 1988), both G3P synthesis pathways are found, therefore raising the question of the respective roles and importance of the different pathways and their regulation. Given the uncertainties surrounding the source of G3P in the chloroplast it is likely that further studies on the gly1-1 mutant will help to resolve the questions in this area of biochemistry.
ACKNOWLEDGMENT
We thank Dr. Jim Tokuhisa for the gift of EMS 5 no. 1.
Abbreviations:
- ACP
acyl carrier protein
- DAG
diacylglycerol
- DGD
digalactosyldiacylglycerol
- DHAP
dihydroxyacetone phosphate
- G3P
glycerol-3-phosphate
- GPAT
acyl-ACP:sn-G3P acyltransferase
- LPAAT
acyl-ACP:sn-1-acylglycerol-3-phosphate acyltransferase
- MGD
monogalactosyldiacylglycerol
- PA
phosphatidic acid
- PAPase
phosphatidate phosphatase
- PC
phosphatidylcholine, PE, phosphatidylethanolamine
- PG
phosphatidylglycerol
- PI
phosphatidylinositol
- SL
sulfoquinovosyldiacylglycerol (sulfolipid)
- X:Y
a fatty acyl group containing X carbon atoms and Y double bonds (cis unless specified)
- 16:0
palmitate
- 16:1
palmitoleate
- 16:2
hexadecadienoic acid
- 16:3
hexadecatrienoic acid
- 18:0
stearate
- 18:1
oleate
- 18:2
linoleate
- 18:3
linolenate
Footnotes
This work was supported by the Centre National de la Recherche Scientifique and the Université Victor Segalen (Bordeaux, France), the National Science Foundation (grant no. IBN-9407902), and the Agricultural Research Center, Washington State University.
LITERATURE CITED
- Bligh EG, Dyer WJ. A rapid method of total extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Browse J, Kunst L, Anderson S, Hugly S, Somerville CR. A mutant of Arabidopsis deficient in the chloroplast 16:1/18:1 desaturase. Plant Physiol. 1989;90:522–529. doi: 10.1104/pp.90.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J, McCourt P, Somerville CR. Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal Biochem. 1986a;152:141–145. doi: 10.1016/0003-2697(86)90132-6. [DOI] [PubMed] [Google Scholar]
- Browse J, Somerville C. Glycerolipids synthesis: biochemistry and regulation. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:467–506. [Google Scholar]
- Browse J, Somerville CR. Lipids. In: Meyerowitz EM, Somerville CR, editors. Arabidopsis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 881–912. [Google Scholar]
- Browse J, Warwick N, Somerville CR, Slack CR. Fluxes through the prokaryotic and the eukaryotic pathways of lipid synthesis in the “16:3” plant Arabidopsis thaliana. Biochem J. 1986b;235:25–31. doi: 10.1042/bj2350025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson SA, Dennis DT. NAD+-specific glycerol 3-phosphate dehydrogenase from developing castor bean endosperm. Arch Biochem Biophys. 1980;199:179–185. doi: 10.1016/0003-9861(80)90271-4. [DOI] [PubMed] [Google Scholar]
- Frentzen M. Biosynthesis and desaturation of the different diacylglycerol moieties in higher plants. J Plant Physiol. 1986;124:193–209. [Google Scholar]
- Frentzen M. Acyltransferases and triacylglycerols. In: Moore TS, editor. Lipid Metabolism in Plants. Boca Raton, FL: CRC Press; 1993. pp. 195–231. [Google Scholar]
- Gardiner SE, Roughan PG, Slack CR. Manipulating the incorporation of [1-14C]acetate into different leaf glycerolipids in several plant species. Plant Physiol. 1982;70:1316–1320. doi: 10.1104/pp.70.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee R, Byerrum RU, Gerber D, Tolbert NE. Changes in the activity of the chloroplastic and cytosolic forms of dihydroxyacetone phosphate reductase during maturation of leaves. Plant Physiol. 1989;89:305–308. doi: 10.1104/pp.89.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee RW, Byerrum RU, Gerber DW, Tolbert NE. Dihydroxyacetone phosphate reductase in plants. Plant Physiol. 1988a;86:98–103. doi: 10.1104/pp.86.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee RW, Byerrum RU, Gerber DW, Tolbert NE. Differential inhibition and activation of two leaf dihydroxyacetone phosphate reductases. Plant Physiol. 1988b;87:379–383. doi: 10.1104/pp.87.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee R, Goyal A, Gerber DW, Byerrum RU, Tolbert NE. Isolation of dihydroxyacetone phosphate reductase from Dunaliella chloroplasts and comparison with isozymes from spinach leaves. Plant Physiol. 1988c;88:896–903. doi: 10.1104/pp.88.3.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Sastry PS. Triacylglycerol synthesis in developing seeds of groundnut (Arachis hypogaea): pathway and properties of enzymes of sn-glycerol 3-phosphate formation. Arch Biochem Biophys. 1988;262:508–516. doi: 10.1016/0003-9861(88)90402-x. [DOI] [PubMed] [Google Scholar]
- Haughn GW, Somerville CR. Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol Gen Genet. 1986;204:430–434. [Google Scholar]
- Heber U, Santarius KA (1970) Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch 25b: 718–728 [DOI] [PubMed]
- Hippman H, Heinz E. Glycerol kinases in leaves. Z Pflanzenphysiol. 1976;79:408–418. [Google Scholar]
- Huang A, Beevers H. Enzymes of glycerol metabolism in the storage tissues of fatty acid seedlings. Plant Physiol. 1975;55:555–558. doi: 10.1104/pp.55.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson GR, Reed EH. The occurrence of hexadeca-7,10,13-trienoic acid in the leaf lipids of angiosperms. Phytochemistry. 1971;10:1837–1843. [Google Scholar]
- Joyard J, Block MA, Malherbe A, Marechal E, Douce R. Origin and synthesis of galactolipid and sulfolipid head groups. In: Moore TS, editor. Lipid Metabolism in Plants. Boca Raton, FL: CRC Press; 1993. pp. 231–258. [Google Scholar]
- Joyard J, Douce R. Site of synthesis of phosphatidic acid and diacylglycerol in spinach chloroplasts. Biochim Biophys Acta. 1977;486:273–285. doi: 10.1016/0005-2760(77)90023-6. [DOI] [PubMed] [Google Scholar]
- Kirsch T, Gerber DW, Byerrum RU, Tolbert NE. Plant dihydroxyacetone phosphate reductases. Plant Physiol. 1992;100:352–359. doi: 10.1104/pp.100.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst L, Browse J, Somerville C. Altered regulation of lipid biosynthesis in a mutant of Arabidopsis deficient in chloroplast glycerol-3-phosphate acyltransferase activity. Proc Natl Acad Sci USA. 1988;85:4143–4147. doi: 10.1073/pnas.85.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst L, Browse J, Somerville C. A mutant of Arabidopsis deficient in desaturation of palmitic acid in leaf lipids. Plant Physiol. 1989;90:943–947. doi: 10.1104/pp.90.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegood RC, Labate CA, Huber SC, Neuhaus HE, Stitt M. Phosphate sequestration by glycerol and its effects on photosynthetic carbon assimilation by leaves. Planta. 1988;176:117–126. doi: 10.1007/BF00392487. [DOI] [PubMed] [Google Scholar]
- Malherbe A, Block MA, Joyard J, Douce R. Feedback inhibition of phosphatidate phosphatase from spinach chloroplast envelope membranes by diacylglycerol. J Biol Chem. 1992;267:23546–23553. [PubMed] [Google Scholar]
- McKeon TA, Stumpf PK. Purification and characterization of the stearoyl-acyl carrier protein desaturase and the acyl-acyl carrier protein thioesterase from maturing seeds of safflower. J Biol Chem. 1982;257:12141–12147. [PubMed] [Google Scholar]
- Miquel M, Browse J. Arabidopsis mutants deficient in polyunsaturated fatty acid synthesis: biochemical and genetic characterization of a plant oleoyl-phosphatidylcholine desaturase. J Biol Chem. 1992;267:1502–1509. [PubMed] [Google Scholar]
- Ohlrogge J, Browse J, Somerville CR. The genetics of plant lipids. Biochim Biophys Acta. 1991;1082:1–26. doi: 10.1016/0005-2760(91)90294-r. [DOI] [PubMed] [Google Scholar]
- Roughan PG, Holland R, Slack CR. On the control of long-chain fatty acid synthesis in isolated intact spinach (Spinacia oleracea) chloroplasts. Biochem J. 1979;184:571–574. doi: 10.1042/bj1840193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan PG, Holland R, Slack CR. The role of chloroplasts and microsomal fractions in polar lipid synthesis from [1-14C]acetate by cell-free preparations from spinach (Spinacia oleracea) leaves. Biochem J. 1980;188:17–24. doi: 10.1042/bj1880017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan PG, Slack CR. Cellular organization of glycerolipid metabolism. Annu Rev Plant Physiol. 1982;33:97–132. [Google Scholar]
- Sadava D, Moore K. Glycerol metabolism in higher plants: glycerol kinase. Biochem Biophys Res Commun. 1987;143:977–983. doi: 10.1016/0006-291x(87)90347-0. [DOI] [PubMed] [Google Scholar]
- Santora G, Gee R, Tolbert NE. Isolation of sn-glycerol 3-phosphate:NAD oxidoreductase from spinach leaves. Arch Biochem Biophys. 1979;196:403–411. doi: 10.1016/0003-9861(79)90291-1. [DOI] [PubMed] [Google Scholar]
- Shanklin J, Somerville CR. The cDNA clones for stearoyl-ACP desaturase from higher plants are not homologous to yeast or mammalian genes encoding stearoyl-CoA desaturase. Proc Natl Acad Sci USA. 1991;88:2510–2514. doi: 10.1073/pnas.88.6.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebertz HP, Heinz E. Labeling experiments on the origin of hexa- and octa-decatrienoic acids in galactolipids of leaves. Z Naturforsch. 1977;32c:193–205. [Google Scholar]