Abstract
The use of conventional cytotoxic agents at metronomic schedules, alone or in combination with targeted agents or immunotherapy, is being explored as a promising anticancer strategy. We previously reported a potent antitumor effect of a single low-dose cyclophosphamide and interleukin-12 (IL-12) gene therapy against advanced gastrointestinal carcinoma, in mice. Here, we assessed whether the delivery of IL-12 by gene therapy together with metronomic cyclophosphamide exerts antitumor effects in a murine model of colorectal carcinoma. This combination therapy was able, at least in part, to reverse immunosuppression, by decreasing the number of regulatory T cells (Tregs) as well as of splenic myeloid-derived suppressor cells (MDSCs). However, metronomic cyclophosphamide plus IL-12 gene therapy failed to increase the number of tumor-infiltrating T lymphocytes and, more importantly, to induce a specific antitumor immune response. With respect to this, cyclophosphamide at a single low dose displayed a superior anticancer profile than the same drug given at a metronomic schedule. Our results may have important implications in the design of new therapeutic strategies against colorectal carcinoma using cyclophosphamide in combination with immunotherapy.
Keywords: cancer immunotherapy, cyclophosphamide, Interleukin 12, metronomic dose, single low-dose
During the last two decades, multiple immunotherapy-based strategies against cancer have been developed, with promising results.1 However, the efficacy of these approaches is deeply hampered by the tumor-intrinsic and tumor-extrinsic pathways that suppress effector immune responses.2 In fact, potent mechanisms of immunosuppression are active during tumor progression, leading to the escape of cancer cells from the immune system3 and limiting the effectiveness of anticancer immunotherapy.4 Combined immunomodulatory approaches appear as attractive strategies to overcome the tolerance induced by tumors and to tip the balance toward the generation of antitumor immune responses.5 In this sense, we have previously demonstrated that the sequential combination of cyclophosphamide (Cy) at a single low dose and sub-therapeutic doses of an adenovirus expressing the interleukin 12 (IL-12) gene (AdIL-12) mediates a potent antitumor effect against colorectal and pancreatic carcinomas in mice.6,7 This combined therapy induced complete tumor regression in > 50% of mice in a synergistic fashion, and it significantly prolonged their survival. This strategy was superior to each single treatment in reducing myeloid-derived suppressor cells (MDSCs) and CD4+CD25+FOXP3+ regulatory T cells (Tregs), both in peripheral blood and in the spleen, as well as in increasing the number of activated dendritic cells (DCs) and interferon γ (IFNγ)-secreting CD4+ T lymphocytes.
The immunomodulatory effects of Cy have been observed not only when administered as a single dose but also upon metronomic schedules. The expression “metronomic chemotherapy” (MCT) refers to the frequent administration of low doses of cytotoxic agents (significantly less than the maximum tolerated dose) over a prolonged period of time, with no or only short drug-free intervals.8 In the past few years, this approach has attracted renewed interest because of its possible association with targeted agents or immunotherapy. Cy is the most widely-explored drug in MCT, both as a single agent or within combination regimens. Results from preclinical models indicate that metronomic Cy would exert anticancer effects by inhibiting angiogenesis and/or by stimulating the immune system.9
The objective of this study was to investigate the antitumor activity of combination regimens including cyclophosphamide-based MCT and AdIL-12. In particular, we aimed at increasing the antitumor efficacy previously achieved using a single low-dose of Cy in combination with AdIL-12 in a murine colorectal carcinoma model.
Results
The combination of AdIL-12 and cyclophosphamide at a single low dose is superior to AdIL-12 plus metronomic cyclophosphamide in inducing tumor regression
The aim of our first experiments was to find the lowest non-toxic doses of Cy to be used in MCT combined with a fixed dose of AdIL-12 (109 TCID50, i.t.), which was previously used in combination with a single low dose of Cy (50 mg/kg, i.p.).6 We tested several doses of Cy, ranging from 25 to 150 mg/kg, administered as different metronomic schedules (data not shown). We decided to use the metronomic administration of the lowest dose of Cy that displayed similar antitumor activities as AdIL-12 to analyze the in vivo antitumor effects of the combined regimen. The metronomic schedule of Cy 25mg/kg i.p., 3 times/week (Cy Me) was chosen. Tumor-bearing animals treated with a single dose of Cy (50 mg/kg) (Cy Mo) alone or in combination with AdIL-12 were also included in the study, as a comparison term.
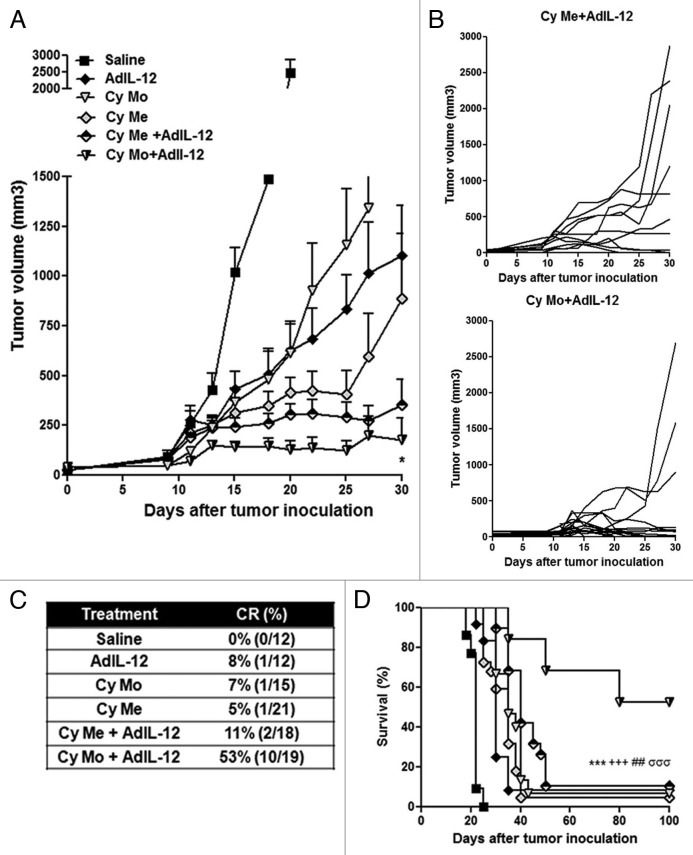
We observed that Cy Me, Cy Mo or AdIL-12 as single agents induce a significant reduction in tumor burden as compared with control conditions (p < 0.001) and complete tumor regression in 5% (1/21), 7% (1/15) and 8% (1/12) of cases, respectively (Fig. 1A-C). Cy Mo + AdIL-12 and Cy Me + AdIL-12 also induced a significant inhibition of tumor growth as compared with control conditions (p < 0,001), but Cy Mo + AdIL-12 was superior than Cy Me + AdIL-12. Cy Mo + AdIL-12 induced a dramatic antitumor effect, leading to complete tumor eradication in 10 out of 19 mice (53%). On the contrary, Cy Me + AdIL-12 induced complete regression in only 11% of animals (2/18) (Fig. 1B, C). In line with these data, the survival rate of mice receiving Cy Mo + AdIL-12 was significantly higher than that of mice treated with Cy Me + AdIL-12, Cy Me alone, Cy Mo alone, AdIL-12 alone or saline (p < 0.001) (Fig. 1D). Analysis of the in vivo interaction between each single agent in both combined treatments was performed by the fractional product method (FTV, fractional tumor volume).10Table 1 summarizes the relative tumor volume of each experimental group, at three distinct time points. These results confirmed that the combination of Cy Me and AdIL-12 exerts less-than-additive antitumor effects.
Figure 1. (A) Antitumor activity of metronomic cyclophosphamide, single low-dose cyclophosphamide, AdIL-12 and their combinations in the CT26 colorectal carcinoma model. Animals were distributed into different groups: saline; metronomic cyclophosphamide (Cy Me, 25 mg/kg i.p., 3 times/week, from day 8); single low-dose cyclophosphamide (Cy Mo, 50 mg/kg i.p., day 8); AdIL-12 (109 TCID50 i.t., day 9); Cy Me + AdIL-12 and Cy Mo + AdIL-12. Data are expressed as mean (bars = SEM) tumor volume. From day 20 onward: Cy Me + AdIL-12 vs. Cy Mo + AdIL-12 p < 0.05. Student's t-test was used to compare tumor sizes among different groups. (B) Antitumor activity of Cy Me + AdIL-12 and Cy Mo + AdIL-12; each line represents one individual tumor. (C) Percentage of complete tumor regressions (CR). (D) Animal survival analysis. Kaplan-Meier, log rank test, *** p < 0.001: saline vs. Cy Me + AdIL-12; +++ p < 0.001: AdIL-12 vs. Cy Me + AdIL-12; ## p < 0.01: Cy Me vs. Cy Me + AdL-12; σσσ p < 0.01: Cy Mo + AdL-12 vs. Cy Me + AdL-12.
Table 1. Analysis of in vivo antitumor synergy by the fractional product method.
| Fractional tumor volume (FTV)* relative to untreated controls | |||||
|---|---|---|---|---|---|
| CY Mo+AdIL-12 or CyMe+AdIL-12 combined treatment | |||||
| Days† | Cy Mo/Cy Me | AdIL-12(109 TCID50) | Expected‡ | Observed | R§ |
| 6 |
0.36/0.46 |
0.51 |
0.184/0.234 |
0.173/0.263 |
1.1/0.9 |
| 12 |
0.27/0.26 |
0.25 |
0.068/0.066 |
0.065/0.145 |
1.1/0.5 |
| 18 | 0.23/0.12 | 0.17 | 0.040/0.020 | 0.032/0.047 | 1.2/0.4 |
* FTV, (experimental mean tumor volume)/(control mean tumor volume). †After treatment onset. ‡Cy Mo/Cy Me mean FTV × (AdIL-12 mean FTV). §R = Expected FTV/Observed FTV for Cy Mo + AdIL-12/Cy Me + AdIL-12. Ratios > 1 indicate a synergistic effect, while ratios < 1 indicate a less-than-additive effect.
The combination of AdIL-12 and either metronomic or single low-dose cyclophosphamide reduced some tumor-elicited immunosuppressive mechanisms
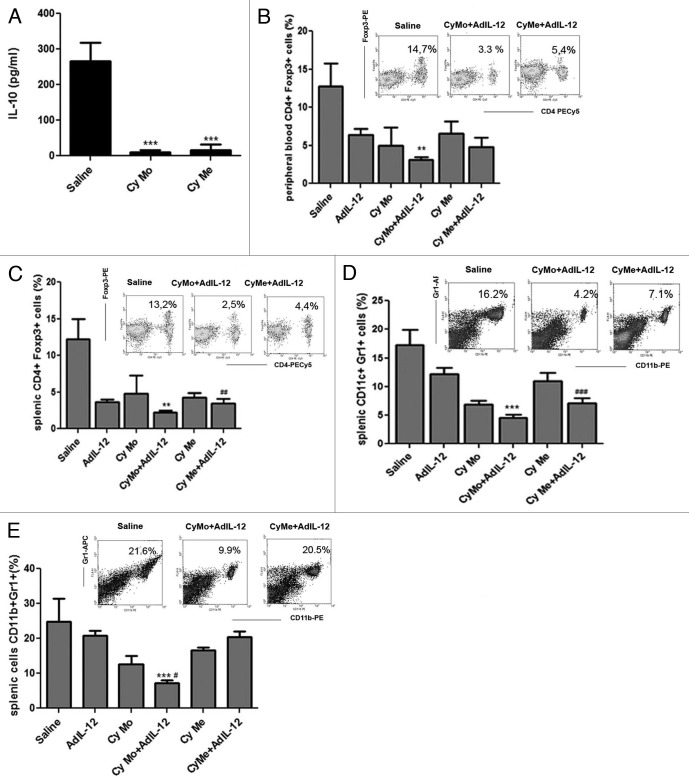
We decided to investigate whether immunosuppressive mechanisms could be involved in the differential antitumoral response obtained after the administration of Cy Mo + AdIL-12 or Cy Me + AdIL-12. To this aim, we measured IL-10 serum levels in mice bearing CT26-derived tumors and receiving wither Cy Mo or Cy Mo, finding that both treatment schedules similarly reduced serum IL-10 (p < 0.001) (Fig. 2A). Then, we evaluated the effect of AdIL-12, Cy Me and Cy Mo, alone or in combination, on the prevalence of regulatory T cells (Tregs) by flow cytometry. We observed that untreated mice showed about 13% and 12% of CD4+FOXP3+ cells in the peripheral blood and spleen, respectively. However, mice treated with Cy Mo + AdIL-12 showed a significant decrease in Tregs levels in both compartments (3.1% and 2.1% in the peripheral blood and spleen, respectively; p < 0.01 vs. saline). Interestingly, we observed that the combination of Cy Me + AdIL-12 also induced a significant reduction in the levels of Tregs in peripheral blood (4.7%; p < 0,05 vs. saline) and spleen (3,5%; p < 0.01 vs. saline) (Fig. 2B, C).
Figure 2. (A) Quantification of IL-10. Serum IL-10 was measured in CT26 tumor-bearing mice treated with saline, metronomic cyclophosphamide (Cy Me, 25 mg/kg i.p., 3 times/week, from day 8), or single low-dose cyclophosphamide (Cy Mo, 50 mg/kg i.p., day 8). Data are expressed as mean (bars = SEM) serum concentration.***p < 0.001: Cy Mo vs. saline ; ### p < 0.001 Cy Me vs. saline. (B) Percentage of CD4+FOXP3+ cells (Tregs) in peripheral blood. ** p < 0.01: Cy Mo + AdIL-12 vs. saline; # p < 0.05: Cy Me + AdIL-12 vs. saline; (C) or spleen ** p < 0.01: Cy Mo + AdIL-12 vs. saline; ## p < 0.01: Cy Me + AdIL-12 vs. saline. (D) Percentage of splenic CD11b+Gr1+ cells at days 7 and (E) at 15 post treatment). Day 7: *** p < 0.001 Cy Mo + AdIL-12 vs. saline; ### Cy Me+AdIL-12 vs. saline. Day 15: *** p < 0.001 Cy Mo + AdIL-12 vs. saline; # p < 0.05 Cy Mo + AdIL-12 vs. Cy Me + AdIL-12. One-way ANOVA and Bonferroni post-test were used to compare differences among groups.
It has previously been demonstrated that myeloid-derived suppressor cells (MDSCs) are key players in the generation of an immunosuppressive cancer microenvironment.11 Therefore, we analyzed the percentage of splenic CD11b+Gr1+ MDSCs in the spleens of tumor-bearing mice treated as above, and observed that both Cy Mo + AdIL12 and Cy Me + AdIL-12 induced a decrease in the levels of MDSCs in comparison with saline (4.2% and 7.6% respectively, vs. 16.5% in saline group; p < 0.001) 7 d after treatment (Fig. 2D). However, we found that mice receiving Cy Mo + AdIL-12 maintained low levels of MDSCs in comparison with saline- and Cy Me + AdIL12-treated animals even 15 d after therapy (p < 0.001) (Fig. 2E).
Metronomic cyclophosphamide has reduced ability to enhance tumor immunogenicity
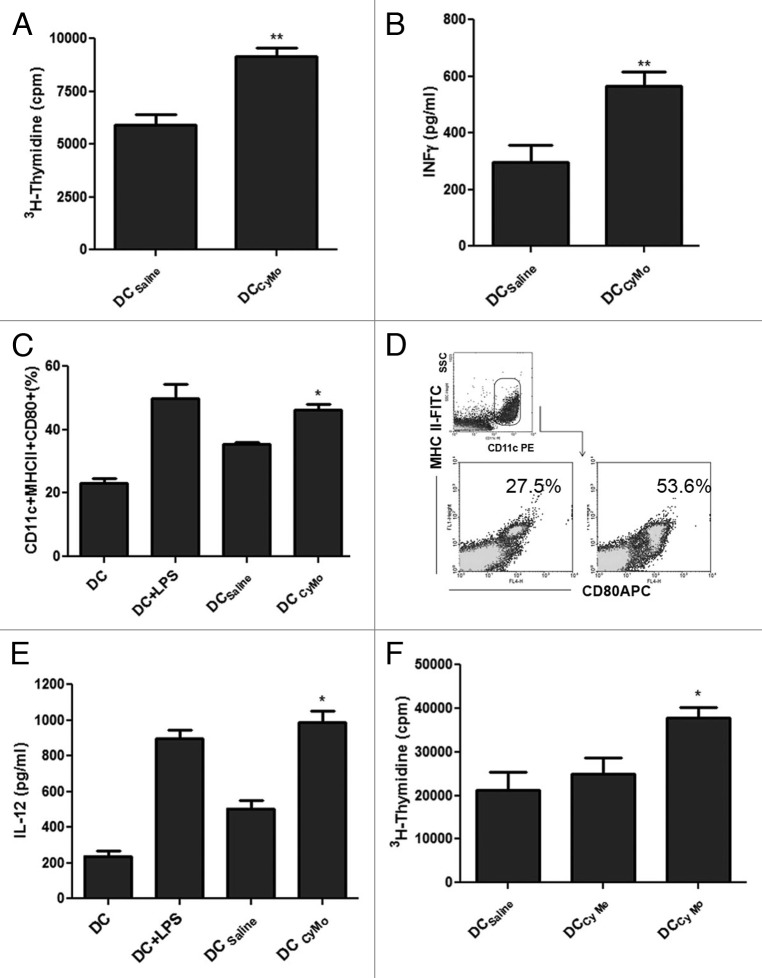
It has recently been reported that non-cytotoxic doses of some chemotherapeutic agents increase tumor immunogenicity, resulting in the generation of efficient DCs in vitro.12 In this context, we have previously demonstrated that in vivo Cy Mo was able to increase the activation of DCs.6 Here, we decided to assess whether Cy may increase tumor immunogenicity in our colorectal carcinoma (CRC) model. To this end, tumor extracts derived from CT26-bearing mice 4 d after the administration of Cy Mo were used to pulsed bone marrow-derived DCs. As shown in Figure 3A,B, treatment with a single low dose of Cy in vivo (DCCyMo), increased allogeneic splenocytes proliferation (p < 0.01) and IFNγ production (p < 0.01), as compared with no treatment (DCSaline). To determine whether these effects were due to changes in the maturation and/or the functional status of DCs, we measured expression levels of class II MHC molecules and CD80 as well as the production of IL-12 in CD11c+ cells derived from DCs pulsed with cancer cell lysates from tumor-bearing mice treated with Cy Mo (DCCyMo) or saline (DCSaline). Thus, DCCyMo showed a higher activation profile (p < 0.05; Figure 3C) and produced higher levels of IL-12 (p < 0.05; Figure 3D) than DCSaline. To explore whether Cy Me is also able to increase tumor immunogenicity, DCs were pulsed with tumor extracts obtained from CT26-bearing mice treated with Cy Me. As shown in Figure 3E, DCs loaded with tumor cell antigens from mice treated with Cy Mo induced a strong splenocytes proliferation rate (p < 0.05), while DCs loaded with tumor cell antigens from mice treated with Cy Me triggered a low proliferation rate, similar to that observed with DCsaline. These results may reflect a reduced capacity of Cy Me to modulate the tumor microenvironment toward immunogenicity .
Figure 3. (A) Single low-dose cyclophosphamide enhances allogeneic splenocyte proliferation. Splenocytes from C57BL/6 mice were stimulated in vitro with DCs pulsed with tumor extracts from CT26-bearing mice treated with single low-dose cyclophosphamide (Cy Mo) or saline during 5 d. Cell proliferation was evaluated by 3H-thymidine incorporation assay. ** p < 0.01: DCCyMo vs. DCsaline. (B) IFNγ quantification in supernatants from cell proliferation assay. ** p < 0.01: DCCyMo vs. DCsaline. (C) Percentage of MHC-II+CD80+ cells in CD11c+ gate from pulsed DCCyMo or DCsaline. * p < 0.05: DCCyMo vs. DCsaline. (D) IL-12 production from pulsed DCCyMo or DCsaline. * p < 0.05: DCCyMo vs. DCsaline (E) Allogeneic splenocytes proliferation induced by both treatment with single low-dose or metronomic cyclophosphamide. Splenocytes from C57BL/6 mice were stimulated in vitro with DCs pulsed with tumor extracts obtained from CT26-bearing mice treated with Cy Mo, metronomic cyclophosphamide (Cy Me) or saline during 5 d. Cell proliferation was evaluated as described above. * p < 0.05: DCCyMo vs. DCCyMe. Kruskal–Wallis and Dunn’s test were used to compare differences among groups.
Metronomic cyclophosphamide combined with AdIL-12 failed to generate specific antitumor CTL responses
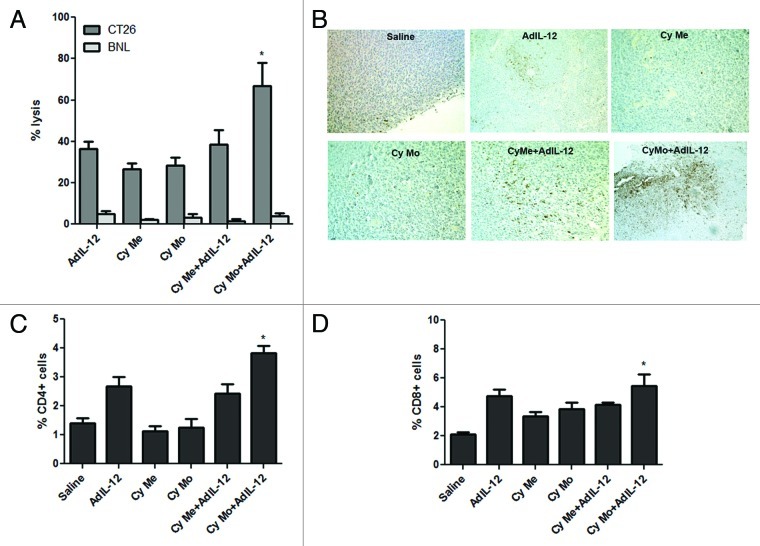
We evaluated the ability of Cy Mo + AdIL-12 and Cy Me + AdIL-12 to generate specific cytotoxic activity against CT26 CRC cells. As we previously demonstrated,6 splenocytes from tumor-bearing mice treated with Cy Mo + AdIL-12 displayed a significantly higher lytic activity against CT26 cells in comparison with either agent alone (p < 0.05) (Fig. 4A). On the contrary, the cytotoxic activity of splenocytes from animals treated with Cy Me + AdIL-12 was similar to that observed for each individual therapy. Importantly, the CTL activity generated by Cy Mo + AdIL-12 was significantly higher than that induced by Cy Me + AdIL-12 (p < 0.05). The specificity of CTLs was confirmed with the hepatocellular carcinoma BNL cell line.
Figure 4. (A) Single low-dose cyclophosphamide is superior to metronomic cyclophosphamide in inducing specific CTL activity. Splenocytes from different experimental groups were stimulated in vitro with mitomycin C–treated CT26 cells for 5 d. Specificity of CTL cytotoxicity was evaluated using BNL cells. The percentage of specific cytotoxicity was calculated according to the following formula: [(abs 492 nm experimental - abs 492 nm background)/(abs 492 nm maximum - abs 492 nm background)] × 100. Data represent the mean of cuatriplicate cultures. * p < 0.05: single low-dose cyclophosphamide (Cy Mo) + AdIL-12 vs. Cy Mo, AdIL-12 or metronomic cyclophosphamide (Cy Me) + AdIL-12. One way ANOVA and Tukey’s multiple comparison post-test was used to compare differences among groups. (B) Tumor-infiltrating T cells labeled with anti-CD3 antibodies were analyzed by immunohistochemistry (200X). (C) Percentage of intratumoral CD4+ and (D) CD8+ cells, as analyzed by flow cytometry. *p < 0.05: Cy Mo + AdIL-12 vs. Cy Me + AdIL-12.
Furthermore, we analyzed if the CTL activity induced by Cy Mo + AdIL-12 correlates with tumor-infiltrating T cells. Evaluation of tumor sections by immunohistochemistry revealed that control tumors (treated with saline) were poorly infiltrated by CD3+ cells (Fig. 4B) and similar results were obtained when CD4+ and CD8+ T cells were analyzed by flow cytometry (Fig. 4C, D). While both Cy Mo or Cy Me did not induce a significant infiltration of CD3+ T lymphocytes into the tumor, the administration of AdIL-12 was able to promote tumor infiltration by T cells. This CD3+ cell infiltration was greatly enhanced when AdIL-12 was combined with Cy Mo, but not Cy Me. This correlated with the amount of CD4+ and CD8+ cells detected by flow cytometry in tumor nodules (p < 0.05) (Fig. 4C, D).
Discussion
Cyclophosphamide is an alkylating agent that mediates DNA crosslinking and is widely used to treat a variety of diseases, including hematological and solid malignancies as well as autoimmune disorders. Moreover, cyclophosphamide is largely utilized as a conditioning regimen for bone marrow transplantation and stem-cell mobilization. High doses are required for direct effects on tumor cells, which invariably results in immunosuppression. In contrast, low doses of cyclophosphamide, alone or in combination with other immunotherapies such as cancer vaccines, adoptive T-cell transfer, monoclonal antibodies, exosomes or cytokines, have been shown to improve anticancer immune responses in preclinical models as well as in the clinic.9,13-15 We previously reported that a single low dose of cyclophosphamide in combination with suboptimal doses of AdIL-12 can overcome the tolerogenic microenvironment induced by CRC and promote a potent antitumor immune response in mice.6,7
More recent studies highlighted the immunostimulatory and/or antiangiogenic effects of the frequent and continuous administration of cyclophosphamide at low doses (metronomic regimen), opening up novel positive associations with complementary therapeutic strategies in the field of cancer immunotherapy.16-18 In this regard, several researchers have tested metronomic cyclophosphamide in combination with other agents to increase the therapeutic efficacy.19 In this work, we decided to investigate whether a metronomic schedule of cyclophosphamide (Cy Me) could be more effective than a single low-dose (Cy Mo) in the induction of antitumor immune responses against mouse CRC, when they are combined with an adenovirus expressing IL-12.
Although we show that of the combination of AdIL-12 and Cy Me inhibited CRC growth, this effect was clearly less potent than the response observed when AdIL-12 and Cy Mo were combined. While the Cy Mo + AdIL-12 combination resulted in synergistic effects and induced complete tumor regression in more than 50% of mice, combination of Cy Me + AdIL-12 was less than additive and exerted a mild antitumor effect (2 out of 18 complete tumor regression).
It has been widely reported that CD4+FOXP3+ Tregs facilitate tumor evasion20 and may hamper the success of immunotherapeutic strategies.21 Systemic accumulation of Tregs has been observed in a number of tumor models and in patients with poor prognosis.22-24 Taking into account our previous results, demonstrating the presence of active immunosuppression in the CT26 tumor model, which was potently inhibited by the combination of AdIL-12 and Cy Mo,7 we decided to assess whether these tolerogenic factors were still present upon the administration of AdIL-12 + Cy Me. We found that both combined therapies were able to decrease the presence of Tregs in the peripheral blood and spleens. In agreement with our results, Ghiringhelli et al. reported that metronomic cyclophosphamide leads to a selective reduction of circulating Tregs in patients with hormone-refractory metastatic prostate cancer.25 On the other hand, we observed that Cy Me is able to decrease the levels of serum IL-10, a type Th2 cytokine involved in tumor immune escape.26,27 These results are in agreement with our previous observations regarding the ability of Cy to reduce IL-10 production in different tumor models.6,28
It is evident that both schedules of cyclophosphamide administration (Mo or Me) in combination with AdIL-12 are able to reduce the prevalence of relevant immunosuppressive cells as well as the systemic levels of IL-10. Therefore, the dissimilar antitumoral effect exerted on CRC by these combined therapies cannot be explained by a different modulation of such immunosuppressive factors.
It has been observed in patients and in mice11,33 that advanced tumor disease is associated with increased MDSCs levels.32 Therefore, tumor burden might affect accumulation of these cells. MDSCs are key players of cancer-induced immune tolerance and a major obstacle for cancer immunotherapy.29 Of note, some chemotherapeutic agents including gemcitabine and 5-fluorouracil have been shown to constitute effective strategies for the reduction of MDSCs.30,31 In line with these observations, in the present study we demonstrated that, initially, the metronomic administration of Cy Me + AdIL-12 decreases the frequency of MDSCs similar than Cy Mo + AdIL-12.
However, we found that - after several weeks of administration - Cy Me + AdIL-12 is not as efficient as Cy Mo + AdIL-12 in eliminating MDSCs. These data correlated with the progressive increase of tumor burden observed in Cy Me + AdIL-12-treated mice, being one of the factors limiting the effectiveness of Cy Me + AdIL-12-based immunotherapy in our model.
Failure of cancer immunotherapy can be due to cancer-induced immunosuppressive mechanisms but also to the lack of specific T-cell responses. Since one of the main goals of immunotherapy is to stimulate a tumor-specific immune response, we evaluated whether cyclophosphamide might contribute to increase the immunogenicity of tumor cells and to induce tumor-specific CTL activity. DCs are the most potent antigen-presenting cells and have the ability to elicit specific immune responses in vivo.34 It has recently been reported that low doses of chemotherapeutic agents can block the ability of tumor cells to suppress the functional activation of DCs,12 resulting in the generation of potent and specific CTL responses.35 Similar results have been reported for cyclophosphamide. In this sense, a single dose of cyclophosphamide given to EG7 lymphoma-bearing mice induced the pre-apoptotic translocation of calreticulin on the surface of tumor cells, activating the DC antigen-processing machinery and inducing CD8+ T cell cross-priming.36 We evaluated whether a single low dose of cyclophosphamide might alter the immunogenicity of tumor cells in our CRC model. Using DCs pulsed with tumor cell extracts from CT26-bearing mice treated with cyclophosphamide, we found that single low-dose cyclophosphamide improves the maturation status of DCs, leading to an increased proliferation of splenocytes. On the contrary, metronomic cyclophosphamide failed to enhance the immunogenicity of tumor cells.
Increasing the frequency of circulating tumor-specific T cells is likely to be one important requirement for successful immunotherapies.37 Herein, we showed that a single dose of cyclophosphamide is superior than a metronomic schedule in generating a beneficial tumor microenvironment and improving tumor antigen presentation by DCs to obtain sufficient expansion of such lymphocytes. Importantly, we confirmed our results by demonstrating that Cy Mo + AdIL-12 induces a potent tumor-specific CTL activity while Cy Me + AdIL-12 fails to do so. In agreement with these observations, tumors derived from animals treated with Cy Mo + AdIL-12 showed an intense infiltration of CD4+ and CD8+ T cells, as opposed to the scarce infiltration detected in animals subjected to Cy Me + AdIL-12-based therapy. This phenomenon could be the key to achieve the therapeutic success observed after the use of Cy Mo + AdIL-12 and could explain, at least in part, the lack of response generated by the combination Cy Me + AdIL-12.
In summary, in this work we provide critical information to support the concept that Cy Mo + AdIL-12 therapy is superior to Cy Me +AdIL-12 in inducing immune responses against an animal model of CRC. Our results may have important implications in the design of cancer immunotherapy combinations using cyclophosphamide as an immunomodulatory agent.
Materials and Methods
Animals and cell lines
Six- to 8-week-old male BALB/c mice (syngeneic with CT26) were purchased from CNEA (National Atomic Energy Commission, Ezeiza, Argentina) and were maintained at our Animal Resources Facilities (School of Biomedical Sciences, Austral University) in accordance with the experimental ethical committee and the NIH guidelines on the ethical use of animals. CT26 cells (kindly provided by Prof. Prieto, University of Navarra, Spain) were maintained in DMEM 10% FCS, 2 mmol/L L-glutamine, 100 U/mL streptomycin, and 100 mg/mL penicillin and incubated at 37°C in a 5% CO2 humidified atmosphere.
Drugs
Cy (Filaxis) was dissolved in sterile water at a concentration of 20 mg/mL and injected intraperitoneally (i.p.) at the indicated dose.
Adenoviral vectors
Construction AdIL-12 was previously described elsewhere.38 Briefly, recombinant adenoviruses were isolated from a single plaque, expanded in 293 cells, and purified by double cesium chloride ultracentrifugation. Purified viruses were extensively dialyzed against 10 mmol/L Tris-1 mmol/L MgCl2 and stored in aliquots at -80°C, and were carefully titrated by plaque assay. The concentration of recombinant vectors was expressed as 50% tissue culture infectious doses (TCID50) per mL.6
In vivo experiments
We investigated the antitumoral effects of Cy, in different dose and schedules of administration, in combination with AdIL-12 in the CT26 colorectal carcinoma model. To perform in vivo experiments, CT26 cells were harvested and injected at a dose of 5x105 cells, subcutaneously, into the right flank of BALB/c mice (day 0). Tumors were allowed to reach approximately 85 mm3 in size before treatment was started. Animals were distributed in different treatment groups: saline (i.p.); single dose of Cy (Cy Mo; 50 mg/kg i.p., day 8); AdIL-12 (109 TCID50 i.t., day 9); Cy Mo (50 mg/kg i.p.) + AdIL-12 (109 TCID50 i.t.); metronomic dose of Cy (Cy Me; 25mg/kg i.p., 3 times/week, from day 8), or Cy Me (25mg/kg i.p., 3 times/week, from day 8)+ AdIL-12 (109 TCID50 i.t.). Tumor growth was assessed twice weekly by calliper and tumor volume (mm3) was calculated by the formula π/6 x larger diameter x (smaller diameter).2 Additional experiments were performed to obtain peripheral blood and spleen for Tregs and myeloid-derived suppressor cells quantification, and tumor samples for evaluation of CD3, CD4 and CD8 T lymphocytes infiltration.
Flow cytometry
Seven and 15 d after treatments were started, animals were anesthetized and peripheral blood was collected by cardiac puncture and anticoagulated with EDTA. The mice were then sacrificed, spleens and tumors were excised, and single cell suspensions were prepared by mechanical disruption in phosphate buffer solution (PBS). Cell suspensions were treated with red blood cell lysis buffer (0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM Na2–EDTA), and washed with PBS 1% BSA. Peripheral blood cells and splenocytes were stained with different conjugated-antibodies: anti-Foxp3PE (Cat.130–093–014; Miltenyi) and anti-CD4-PECy5 (Cat.553050; BD Biosciences), anti-CD11b PE(Cat.553312; BD Biosciences, anti-GR1 APC (Cat.551461 clone 1A8; BD Biosciences). Tumor cell suspensions were stained with anti-CD4 PECy5 and anti-CD8 Alexa Fluor 488 (Cat.557668 53–67; BD Biosciences). Bone marrow-derived dendritic cells (DCs) gwere stained with anti-CD11c PE (Cat.553802 clone HL3), anti-MHC class II FITC (Cat.553623 clone 269) and anti-CD80 APC (Cat. 560016 clone 16–10A1). Finally, cells were fixed with 1% paraformaldehyde and subjected to flow cytometry (FACSCalibur, BD Biosciences, San Diego, California, USA). Data were analyzed using WinMDI software.
Immunohistochemical analyses
Additional tumor samples from all experimental groups were obtained at the end of treatments and fixed in 10% phosphate-buffered formalin. Five-micrometer sections from paraffin-embedded tissues were stained with anti-CD3 (BD Biosciences) as published elsewhere.39
Cytokine quantification
IL-10, IL-12 or IFNγ concentrations were measured by specific ELISA kit (Cat.555252; 555256; 555138, respectively; OptEIA, BD Biosciences PharMingen). The assays were performed according to the instructions provided by the manufacturer. Standards and samples were assayed in duplicates.6
DC cultures and antigen presentation assays
Bone marrow-derived DCs from BALB/c mice were generated as described elsewhere,40 treated for 20 min with 50 mg/ml mitomycin C (Sigma Chemical), and washed three times with PBS. Then, DCs were incubated with CT26 tumor cell lysates from tumor-bearing mice treated with Cy, single dose or saline. As described above, DCs were incubated with tumor cell lysates from tumor-bearing mice treated with a single dose of Cy or saline. The next day, cell-free supernatants were collected for IL-12 quantification and DCs were used as stimulators in an allogeneic splenocytes proliferation assay. To this end, splenocytes (1 × 106) from C57BL/6 mice were co-cultured with DCs (1 × 105) in U-bottom 96-well plates and cell proliferation was evaluated after 4 d by [3H]thymidine incorporation assay as we describe previously.6 Also, IFNγ concentration was measured in cell-free supernatants as was described above. In a similar way, we investigated the effects of DCs pulsed with tumor cell lysates from tumor-bearing mice treated with Cy Me on allogeneic splenocyte proliferation. Each sample was assayed in cuatriplicate.
Cytotoxicity assays
Viable splenocytes (8 × 106) from all previously described experimental groups were stimulated in vitro with CT26 cells (previously treated with 150 μg/ml of mitomycin C for 30 min at 37°C; 8 × 105 cells/well in 24-well-plates) during 5 d. Then, cells were harvested and washed three times in serum-free medium, adjusted to 2.5 × 105/mL, and added to 96-well-plate (effector cells). To determine specific CTL cytotoxicity, CT26 cells were used as target cells at 5 × 104/ml. After incubation for 4 h at 37°C, plates were centrifuged and cell-free supernatants were obtained. Cytotoxicity was evaluated with the LDH Cytotoxicity Detection Kit (Cat 04744934001; Roche Diagnostics), following manufacturer instructions. The percentage of specific cytotoxicity was calculated according to the following formula: ([abs 492 nm experimental - abs 492 nm background])/[abs 492nm maximum- abs 492nm background]) × 100. Data represent means of cuatriplicate.
Statistical analysis
Mann-Whitney’s test, Student’s t-test, one-way ANOVA or Kaplan-Meier log rank test (InStat, GraphPad Software), were used to statistically examine the differences between groups. p values < 0.05 were considered as statistically significant.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
We would like to thank, Soledad Arregui, Guillermo Gastón and Marcos Cabrera for their technical assistance; Aizea Morales is acknowledged for her assistance with CD3 immunohistochemistry and Union for International Cancer Control (Yamagiwa-Yoshida Memorial international study grants for GM). This work was supported in part by Austral University (for GM T13–11), Agencia Nacional de Promoción Científica yTecnológica (ANPCyT) grants PICT-2007/00082 (MG, LA and GM); PICTO 2008/00115 (MG); PICT 2008/00123 (JA) PICTO 2008/00122 (JA);PICT 2010 2818 (MG and GM); PIP-CONICET 2009–2011 (PM).
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20684
References
- 1.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–51. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapira S, Lisiansky V, Arber N, Kraus S. Targeted immunotherapy for colorectal cancer: monoclonal antibodies and immunotoxins. Expert Opin Investig Drugs. 2010;19(Suppl 1):S67–77. doi: 10.1517/13543781003737668. [DOI] [PubMed] [Google Scholar]
- 3.Croci DO, Zacarías Fluck MF, Rico MJ, Matar P, Rabinovich GA, Scharovsky OG. Dynamic cross-talk between tumor and immune cells in orchestrating the immunosuppressive network at the tumor microenvironment. Cancer Immunol Immunother. 2007;56:1687–700. doi: 10.1007/s00262-007-0343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 5.Melero I, Mazzolini G, Narvaiza I, Qian C, Chen L, Prieto J. IL-12 gene therapy for cancer: in synergy with other immunotherapies. Trends Immunol. 2001;22:113–5. doi: 10.1016/S1471-4906(00)01824-X. [DOI] [PubMed] [Google Scholar]
- 6.Malvicini M, Rizzo M, Alaniz L, Piñero F, García M, Atorrasagasti C, et al. A novel synergistic combination of cyclophosphamide and gene transfer of interleukin-12 eradicates colorectal carcinoma in mice. Clin Cancer Res. 2009;15:7256–65. doi: 10.1158/1078-0432.CCR-09-1861. [DOI] [PubMed] [Google Scholar]
- 7.Malvicini M, Ingolotti M, Piccioni F, Garcia M, Bayo J, Atorrasagasti C, et al. Reversal of gastrointestinal carcinoma-induced immunosuppression and induction of antitumoural immunity by a combination of cyclophosphamide and gene transfer of IL-12. Mol Oncol. 2011;5:242–55. doi: 10.1016/j.molonc.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–36. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 9.Sistigu A, Viaud S, Chaput N, Bracci L, Proietti E, Zitvogel L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol. 2011;33:369–83. doi: 10.1007/s00281-011-0245-0. [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama Y, Dhanabal M, Griffioen AW, Sukhatme VP, Ramakrishnan S. Synergy between angiostatin and endostatin: inhibition of ovarian cancer growth. Cancer Res. 2000;60:2190–6. [PubMed] [Google Scholar]
- 11.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer J. 2010;16:348–53. doi: 10.1097/PPO.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- 12.Shurin GV, Tourkova IL, Kaneno R, Shurin MR. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol. 2009;183:137–44. doi: 10.4049/jimmunol.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matar P, Rozados VR, Gervasoni SI, Scharovsky GO. Th2/Th1 switch induced by a single low dose of cyclophosphamide in a rat metastatic lymphoma model. Cancer Immunol Immunother. 2002;50:588–96. doi: 10.1007/s00262-001-0237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taieb J, Chaput N, Schartz N, Roux S, Novault S, Ménard C, et al. Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J Immunol. 2006;176:2722–9. doi: 10.4049/jimmunol.176.5.2722. [DOI] [PubMed] [Google Scholar]
- 15.Perez EA, Suman VJ, Davidson NE, Gralow JR, Kaufman PA, Visscher DW, et al. Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer. J Clin Oncol. 2011;29:4491–7. doi: 10.1200/JCO.2011.36.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge Y, Domschke C, Stoiber N, Schott S, Heil J, Rom J, et al. Metronomic cyclophosphamide treatment in metastasized breast cancer patients: immunological effects and clinical outcome. Cancer Immunol Immunother. 2012;61:353–62. doi: 10.1007/s00262-011-1106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerullo V, Diaconu I, Kangasniemi L, Rajecki M, Escutenaire S, Koski A, et al. Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus. Mol Ther. 2011;19:1737–46. doi: 10.1038/mt.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rozados VR, Mainetti LE, Rico MJ, Zacarías Fluck MF, Matar P, Scharovsky OG. The immune response and the therapeutic effect of metronomic chemotherapy with cyclophosphamide. Oncol Res. 2010;18:601–5. doi: 10.3727/096504010X12777678141662. [DOI] [PubMed] [Google Scholar]
- 19.Penel N, Adenis A, Bocci G. Cyclophosphamide-based metronomic chemotherapy: after 10 years of experience, where do we stand and where are we going? Crit Rev Oncol Hematol. 2012;82:40–50. doi: 10.1016/j.critrevonc.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–67. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 21.Barnett BG, Rüter J, Kryczek I, Brumlik MJ, Cheng PJ, Daniel BJ, et al. Regulatory T cells: a new frontier in cancer immunotherapy. Adv Exp Med Biol. 2008;622:255–60. doi: 10.1007/978-0-387-68969-2_20. [DOI] [PubMed] [Google Scholar]
- 22.Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13:4345–54. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 23.Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6301–11. doi: 10.1158/1078-0432.CCR-07-1403. [DOI] [PubMed] [Google Scholar]
- 24.Yaqub S, Henjum K, Mahic M, Jahnsen FL, Aandahl EM, Bjørnbeth BA, et al. Regulatory T cells in colorectal cancer patients suppress anti-tumor immune activity in a COX-2 dependent manner. Cancer Immunol Immunother. 2008;57:813–21. doi: 10.1007/s00262-007-0417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wittke F, Hoffmann R, Buer J, Dallmann I, Oevermann K, Sel S, et al. Interleukin 10 (IL-10): an immunosuppressive factor and independent predictor in patients with metastatic renal cell carcinoma. Br J Cancer. 1999;79:1182–4. doi: 10.1038/sj.bjc.6690189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fortis C, Foppoli M, Gianotti L, Galli L, Citterio G, Consogno G, et al. Increased interleukin-10 serum levels in patients with solid tumours. Cancer Lett. 1996;104:1–5. doi: 10.1016/0304-3835(96)04213-9. [DOI] [PubMed] [Google Scholar]
- 28.Matar P, Rozados VR, Gervasoni SI, Scharovsky OG. Down regulation of T-cell-derived IL-10 production by low-dose cyclophosphamide treatment in tumor-bearing rats restores in vitro normal lymphoproliferative response. Int Immunopharmacol. 2001;1:307–19. doi: 10.1016/S1567-5769(00)00028-X. [DOI] [PubMed] [Google Scholar]
- 29.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–61. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–21. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 32.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–66. [PubMed] [Google Scholar]
- 33.Salvadori S, Martinelli G, Zier K. Resection of solid tumors reverses T cell defects and restores protective immunity. J Immunol. 2000;164:2214–20. doi: 10.4049/jimmunol.164.4.2214. [DOI] [PubMed] [Google Scholar]
- 34.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 35.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–63. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schiavoni G, Sistigu A, Valentini M, Mattei F, Sestili P, Spadaro F, et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res. 2011;71:768–78. doi: 10.1158/0008-5472.CAN-10-2788. [DOI] [PubMed] [Google Scholar]
- 37.van den Engel NK, Rüttinger D, Rusan M, Kammerer R, Zimmermann W, Hatz RA, et al. Combination immunotherapy and active-specific tumor cell vaccination augments anti-cancer immunity in a mouse model of gastric cancer. J Transl Med. 2011;9:140. doi: 10.1186/1479-5876-9-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazzolini G, Qian C, Xie X, Sun Y, Lasarte JJ, Drozdzik M, et al. Regression of colon cancer and induction of antitumor immunity by intratumoral injection of adenovirus expressing interleukin-12. Cancer Gene Ther. 1999;6:514–22. doi: 10.1038/sj.cgt.7700072. [DOI] [PubMed] [Google Scholar]
- 39.Melero I, Gabari I, Tirapu I, Arina A, Mazzolini G, Baixeras E, et al. Anti-ICAM-2 monoclonal antibody synergizes with intratumor gene transfer of interleukin-12 inhibiting activation-induced T-cell death. Clin Cancer Res. 2003;9:3546–54. [PubMed] [Google Scholar]
- 40.Alaniz L, Rizzo M, Malvicini M, Jaunarena J, Avella D, Atorrasagasti C, et al. Low molecular weight hyaluronan inhibits colorectal carcinoma growth by decreasing tumor cell proliferation and stimulating immune response. Cancer Lett. 2009;278:9–16. doi: 10.1016/j.canlet.2008.12.029. [DOI] [PubMed] [Google Scholar]