Abstract
Overexpression of HER-2 and VEGF plays a key role in the development and metastasis of several human cancers. Many FDA-approved therapies targeting both HER-2 (Trastuzumab, Herceptin) and VEGF (Bevacizumab, Avastin) are expensive, have unacceptable toxicities and are often associated with the development of resistance. Here, we evaluate the dual antitumor effects of combining designed particular HER-2 peptide vaccine with VEGF peptide mimics. In vitro, HER-2 phosphorylation and antibody-dependent cellular toxicity were used to validate whether combining HER-2- and VEGF-targeting therapies would be effective. Moreover, a two-pronged approach was tested in vivo: (1) active immunotherapy with conformational HER-2 B-cell epitope vaccines and (2) anti-angiogenic therapy with a peptide structured to mimic VEGF. A transplantable BALB/c mouse model challenged with TUBO cells was used to test the effects of the HER-2 peptide vaccine combined with VEGF peptide mimics. Tumor sections after treatment were stained for blood vessel density and actively dividing cells. Our results show that immunization with an HER-2 peptide epitope elicits high affinity HER-2 native antibodies that are effective in inhibiting tumor growth in vivo, an effect that is enhanced by VEGF peptide mimics. We demonstrate that the combination of HER-2 and VEGF peptides induces potent anti-tumor and anti-angiogenic responses.
Keywords: angiogenesis, antibodies, peptides, peptides/epitopes, peptidomimetic
Introduction
The oncoprotein human epidermal growth factor receptor 2 (HER-2) is an orphan member of the HER family of receptors,1 which includes HER-1, HER-3 and HER-4. The absence of a known HER-2 ligand makes it a preferential dimerization partner for other HERs. All members of the HER family have an extracellular domain, a single transmembrane domain and a cytoplasmic portion that contains a conserved tyrosine kinase domain flanked by a C-terminal tail with autophosphorylation sites.2 HER-2 is known to regulate the formation of neuromuscular synapses, and it is also important in muscle spindle development.3 High levels of HER-2 causes dysregulation of the HER network resulting in transformation, tumorigenesis and altered sensitivity to the cytotoxic effects of tumor necrosis factor α (TNFα).4 HER-2-overexpressing breast cancers are biologically different from other breast cancers, are resistant to hormonal agents, and have an increased ability to metastasize to other organs including the lung and brain.5 Amplification of ERBB2 (the gene encoding HER-2) has been observed in subsets of gastric, esophageal, ovarian, uterine, endometrial and lung cancers.6-9
HER-2 upregulation is always accompanied by upregulation of the vascular endothelial growth factor (VEGF), both at the RNA and protein level,10 and most drugs that target HER-2 are known to downregulate VEGF expression.11 This implies that the effects of HER-2 may partly be mediated by upregulation of VEGF. Tumor cells are known to upregulate the expression of VEGF and its receptors thereby stimulating angiogenesis.13,14 Targeting HER-2 alone might not be sufficient to kill tumor cells and interrupting with VEGF signaling is likely only to delay tumor growth, allowing for the activation of alternative pathway to angiogenesis.15 Immunization with both tumor and angiogenesis associated antigens has previously been shown to exert synergistic effects.12 These observations, the mechanistic links between HER-2 and VEGF and Dr Folkman’s hypothesis that tumor growth is angiogenesis-dependent led us to postulate that targeting both HER-2 and VEGF may exert synergistic anti-tumor effects.
Humanized monoclonal antibodies like trastuzumab and pertuzumab target two different sub-domains of the extracellular region of HER-216 and the former is currently being used in the clinic to treat breast cancer. Along similar lines, bevacizumab, which targets the C-termianal region of VEGF, is currently employed in the clinic against a spectrum of cancers.17 Despite some impressive clinical results with these compounds, monoclonal antibody-based therapies are very expensive and are associated with non-negligible side effects, including cardiotoxicity.
In order to circumvent these problems, we have proposed the use of active immunotherapy, whereby the body is trained to produce highly specific antibodies against tumor cells (as opposed to passive immunotherapy, whereby large amounts of antibodies and other immune cells are administered to the patient). During the past decade, our laboratory has focused on the development of B-cell vaccines targeting one HER-2 epitope. Our main hypothesis is that immunization with engineered HER-2 B-cell peptide epitopes as chimeric immunogens that encompass a promiscuous T-cell epitope elicits specific antibodies with high affinity for the native protein. More recently, we have engineered peptide mimics of VEGF to efficiently prevent the binding of endogenous VEGF to its major receptor (VEGFR2), resulting in anti-angiogenic and anti-tumor effects.
Based on the crystal structure of the extracellular domain of HER-2 complexed with pertuzumab, we have previously developed a HER-2 peptide (residues 266–296) that was able to elicit HER-2-specific antibodies. These antibodies inhibited the growth of a HER-2-dependent tumor cell line growth and showed superior anti-tumor effects in transgenic animals.18 We have also designed and synthesized a cyclic peptide (VEGF-P3-CYC) based on the binding of VEGF to VEGFR2. This engineered peptide mimicking VEGF demonstrated high affinity binding to VEGFR-, inhibited VEGFR2 phosphorylation, endothelial cell proliferation, migration and network formation and delayed tumor development in a transgenic model of VEGF+/−Neu2–5+/− cancer.19 The retro-inverso analog of the VEGF peptide (VEGF-P4) was designed and synthesized using D-amino acids, in order to circumvent the breakdown of the natural peptide by proteases, which could limit its efficacy in vivo. This peptide induced potent anti-angiogenic effects, both in vitro and in vivo.20
In this study, we explored the vaccination with the HER-2 peptide followed by the administration of the angiogenesis inhibitor VEGF-P3, as a means to improve the outcome of immunotherapeutic strategies. We used the MVF-HER-2 266–296 CYC peptide as the vaccine and the VEGF peptide mimics VEGF P3 and P4 as anti-angiogenic agents. We further validated the anti-angiogenic effects of our VEGF peptide mimics in two different assays, and here we report on the antitumor and anti-angiogenic effects of treatment with HER-2 vaccine followed by VEGF peptide mimics. Immunization with the HER-2 peptide epitope and treatment with a D-amino acid-based VEGF peptide mimic (RI-VEGF-P4CYC) produced superior anti-tumor and anti-angiogenic effects in vivo.
Results
Selection, design and characterization of peptides
The VEGF peptide mimic residues 102–122 (numbered as 76–96 in the crystal structure) correspond to the overlap between the binding sites for VEGFR2 and Avastin. Engineering of this peptide has been described in details elsewhere.19 The sequences of both the HER-2 and VEGF peptide mimics are shown in Table 1. The strategy to create a conformational peptide consisting of an anti-parallel β sheet is described elsewhere,19 Briefly, the sequence was modified in a way that the resulting non-cyclized (NC) peptide VEGF-P3 adopted a conformation very similar to the native structure. This required two artificial cysteines to be introduced between Gln79 and Gly92, and between Ile80 and Glu93. After synthesis and purification of VEGF-P3(NC) peptide, a disulfide bond was formed between these two cysteines by oxidation, enabling the formation of the twisted anti-parallel β-sheet structure in the cyclized (CYC) VEGF-P3. The retro-inverso (RI) peptide analog CYC RI-VEGF-P4 was synthesized using D-amino acids with the amino acid sequence in reverse order, such that the resulting peptide has a reversal of the peptide backbone but a topochemical equivalence to the parent peptide in terms of side-chain orientation. The rationale behind RI peptidomimetics is that they should present similar activity with the advantage of higher bioavailability.20
Table 1. Amino acid sequences and molecular weight of HER-2 and VEGF peptide mimics.
| Designation | Peptide | Sequence | M.Wt. (da) |
|---|---|---|---|
| MVF-HER-2-266-296(CYC) MVF-HER2 | 266-296 peptide with one disulfide bond. | MVF-LHCPA LVTYNTDTFESMPNPEGRYTFGASCV-COOH | 4927 |
| VEGF-P3(CYC)P3 | 76-96 peptide with one disulfide bond | CH3CONH-76-ITMQ-79-C-92-GIHQGQHPKIRMI-80-C-EMSF-96 | 2527 |
| RI-VEGF-P4(CYC)P4 | 96-76 peptide with D amino acids and one disulfide bond | CH3CONH-(D)-96-FSME-80-C-92-IMRIKPHQGQHIG-79-CQMTI-76 | 2527 |
Sequences of amino acids are represented from N to C-terminal except for the retro inverso peptide RI-VEGF-P4-CYC that was synthesized in the reverse order and using D-amino acids
HER-2–266–296 peptide (Table 1) was synthesized based on the crystal structure of the antigen-binding fragment of pertuzumab bound to the extracellular subdomain II of HER-2. The 266–333 region of HER-2 was selected for the design of the peptides with the objective of eliciting antibodies capable of inhibiting dimerization of HER-2 with other members of the HER family. The peptide can also be used to directly block dimerization due to its ability to bind the extracellular domain of HER-2.18 Peptides that were used for immunization, of both rabbits and mice, were collinearly synthesized with the promiscuous TH epitope MVF (MVF-HER-2), while those that were used for post- vaccination therapy were synthesized without any MVF (Table 1).
Antigenicity and immunogenicity of peptides
Earlier studies in our lab have shown that the MVF-HER-2–266–296 is highly immunogenic in both rabbits and mice.18 We also observed high antibody titers with the MVF-VEGF-P3 peptide19 and in our present study we showed that the D-amino acid-containing VEGF peptide CYC VEGF-P4 is also immunogenic, though not as its L- amino acid-based counterpart, probably due to the fact that D-amino acids are not natural so they are poorly recognized by the immune system. Additional boosters of up to six immunizations were required for the D-amino acid-based peptide before we could obtain higher antibody titers whereas only four immunizations were enough to produce higher titers for the L-amino acid-containing peptides.18 We used the antibodies raised against these peptides to test their effects on cancer cells in vitro.
Antiproliferative effects of anti-peptide antibodies
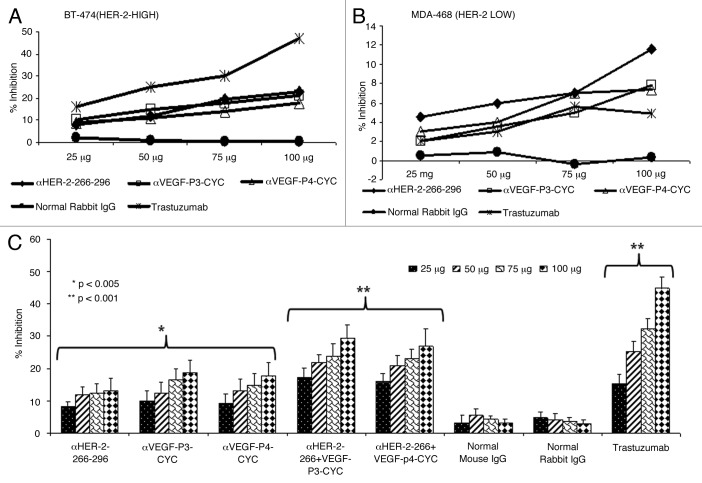
The antiproliferative effects of the antibodies raised against HER-2 and VEGF peptides in rabbits were tested using two different cell lines (BT-474, HER-2high and MDA-468, HER-2low (Fig. 1A,B) in the presence of heregulin (HRG) to activate the HER-3 receptor. Unlike trastuzumab, which is specific for HER-2 positive cells, pertuzumab is known by disrupting ligand-dependent receptor complexes independent of HER-2 expression.21 Cells were incubated with the anti-peptide antibodies followed by exposure to HRG. Results indicate that the antibodies raised against both the HER-2 peptides and VEGF peptides were able to inhibit tumor growth in a concentration-dependent manner (Fig. 1A,B). We used two different cell lines to show that the effects of the anti-peptide antibodies was dependent on HER-2 expression (Fig. 1A). We also tested the effects of combination treatment with both HER-2 and VEGF anti-peptide antibodies and the results showed an increase in the rate of inhibition when both anti-peptide antibodies were used as compared with single treatments (Fig. 1C). Single treatments showed a significant inhibition (*p < 0.005) while the combination regimen resulted in an even more significant effect (**p < 0.001). Control rabbit IgG did not show antiproliferative effects while trastuzumab (positive control) showed antiproliferative effects only on cells that express HER-2 (Fig. 1A).
Figure 1. Anti-proliferative effects of combination treatment with anti-HER-2 (266–296) and anti-VEGF-P3 antibodies. (A-C) Inhibition of proliferation with individual anti-HER-2 and anti-VEGF- antibodies in two different cell-lines. BT474 (A) and MDA-468 (B) cells were incubated with HER-2 peptide antibodies, VEGF peptide antibodies, Trastuzumab and unspecific rabbit IgG. Bioconversion of MTT was used to estimate the number of active tumor cells remaining after 3 d. Peptides were added at four different concentrations. The proliferation inhibition rate was calculated using the formula (OD normal Untreated - OD peptides or antibodies)/OD normal untreated x 100. Error bars represent SD. Inhibition of proliferation of combination treatment with anti-HER-2 and anti-VEGF antipeptide antibodies using B-T474 cell lines (C). BT-474 cells were treated in the same manner as above but with HER-2 peptide abs, VEGF peptide abs or combination of both. Trastuzumab and rabbit IgG were used as positive and negative controls. Rate of inhibition was calculated using the same formula above and all results represents the average of three different experiments. Error bars represent SD of the mean.
Effects of anti-peptide antibodies on HER-2 specific phosphorylation
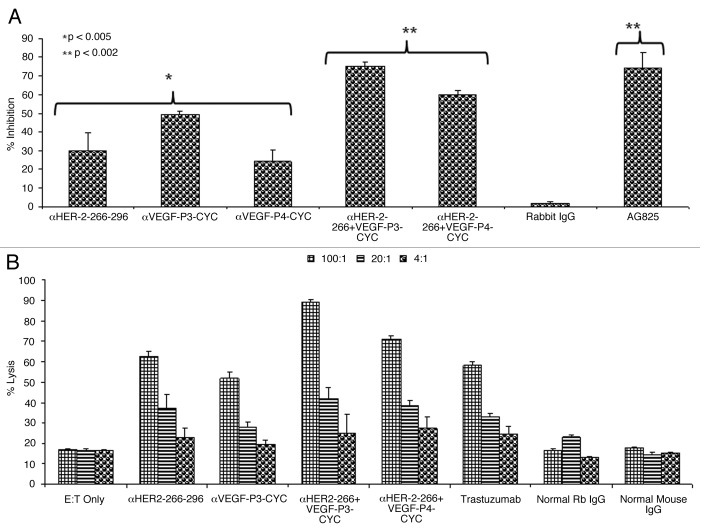
The main mode of action of pertuzumab is to inhibit the phosphorylation of HER-2. This is due to the fact that it sterically blocks the dimerization domain of HER-2, thereby preventing the formation of dimers with other HER receptors and thus interrupting downstream signaling. We have tested the effects of the anti-peptide antibodies on HER-2 phosphorylation, finding that anti-peptide antibodies were able to prevent phosphorylation of the HER-2 protein. Single treatment with the HER-2 anti-peptide antibody alone caused a 30% inhibition rate (*p < 0.005) while combination with the VEGF anti-peptide antibody increased inhibition from 30% to about 75% (**p < 0.002) (Fig. 2A). All treatments were compared with the positive control AG825 (Calbiochem), a HER-2-specific phosphorylation inhibitor. The negative control (unspecific rabbit IgG) showed no meaningful inhibitory effects on HER-2 phosphorylation.
Figure 2. Effects of combination treatment on HER-2 phosphorylation and Antibody dependent cellular cytotoxicity. (A) Inhibition of HER-2 phosphorylation by single and combination treatment with anti-HER-2 and anti-VEGF peptide antibodies. BT-474 cells were incubated with 100 µg/mL of anti-HER-2 266–296 and anti-VEGF-P3 antibodies or combination of both before being exposed to heregulin (HRG, an HER-3 activating ligand) for 10 min and lysed. Phosphorylated HER-2/neu was determined by indirect ELISA and percent inhibition was calculated as in (Fig. 1) above. AG825 (Calbiochem), a potent HER-2 phosphorylation inhibitor, was used as a positive control. (B) Anti-peptide antibodies raised in rabbits are capable of mediating antibody-dependent cell-mediated cytotoxicity (ADCC). The target cell line BT474 was coated with 50 μg of purified anti-peptide antibodies from rabbits, unspecific rabbit IgG, unspecific mouse IgG or trastuzumab and then cultured in the presence of human peripheral blood mononuclear effector cells to give an effector:target ratio of 100:1, 20:1, and 4:1 in triplicates. Error bars in panels (A) and (B) represent SD of the mean. Results represent average data from three different experiments with each treatment performed in triplicate.
Ability of anti-peptide antibodies to mediate antibody-dependent cellular cytotoxicity
It has been well documented that in vivo the Fc portions of antibodies can be of foremost importance for efficacy against tumor targets.22 When Fc binding is reduced or completely removed, trastuzumab virtually loses all in vivo efficacy.23 We measured the ability of rabbit antibodies raised against HER-2 and VEGF peptides to mediate antibody-dependent cellular cytotoxicity (ADCC) in vitro using a bioluminescence-based cytotoxicity assay (aCella-TOXTM). Our results show that combination treatments with anti-peptide antibodies induces a more potent response than individual treatments (Fig. 2B). Trastuzumab was used as a positive control while unspecific mouse and rabbit IgGs were used as negative controls. The effector cells were normal human peripheral blood mononuclear cells (PBMCs) from healthy donors while the target cells were BT-474 cells that overexpress HER-2.
Inhibition of microvascular-like outgrowth in the aortic ring angiogenesis assay by VEGF peptide mimics
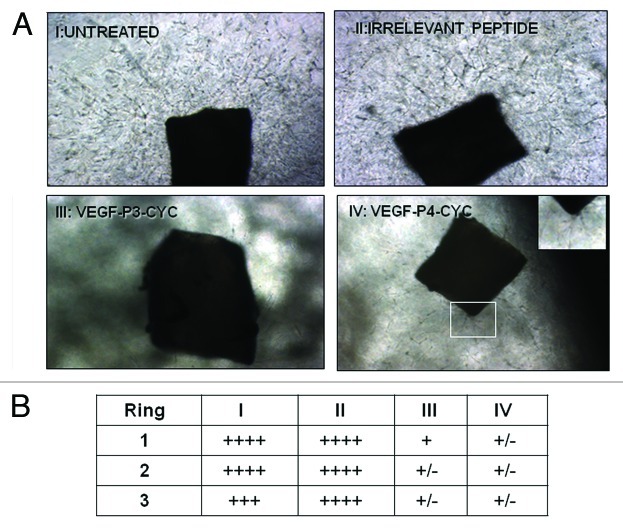
Angiogenesis was studied in vitro by culturing rings of mouse aorta in matrigel and using the peptide mimics as inhibitors. We observed a reduction in outgrowth induced by VEGF peptide mimics, while an irrelevant peptide (CD28 peptide mimic) had no effects (Fig. 3A). Outgrowth estimation using the brightfield microscope also showed increased growth in the untreated and control treatment group, and results were consistent for all three rings in each treatment group (Fig. 3B). These results strongly indicate that the VEGF peptides are able to prevent microvascular outgrowth from aortic rings, thus exerting anti-angiogenic effects.
Figure 3. Inhibition of microvascular-like outgrowth in the aortic ring angiogenesis assay by VEGF peptide mimics. Mouse aortic rings were incubated for 10 d with 100 µg/mL peptides or controls as in Figure 2A. Note the robust outgrowth in controls and the strong inhibition by the peptides. In IV, the insert is a higher magnification showing the limited amount of residual sprouting. Photos were taking using phase contrast microscopy with original magnification at 20X. In (B), outgrowth estimation was done using the brightfield microscope. + indicates presence of growth and table shows the number of rings per treatment group. I-IV are same as in (A).
VEGF peptide mimics decreases vascular endothelial cadherin expression in vivo
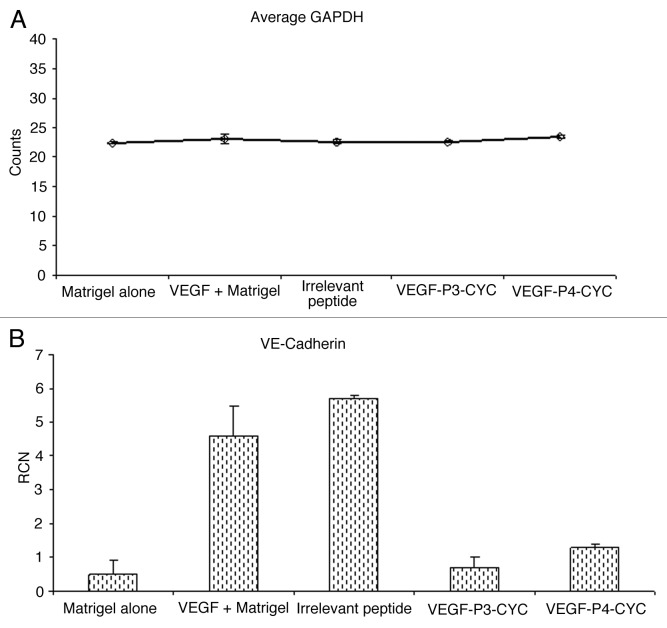
VEGF has been shown to play a major role in angiogenesis by upregulating VEGFR2 as well as vascular endothelial (VE)-cadherin, which forms a support network in endothelial junctions.24 To investigate VE-cadherin upregulation by VEGF and test the ability of our peptide mimics to interefere with this process, we injected mice with matrigel alone, matrigel with VEGF or matrigel with VEGF plus the different inhibitors. We observed that VEGF induces expression of VE-cadherin in vivo, a phenomenon that could b e inhibited by our VEGF peptide mimics (Fig. 4A). There was a marked reduction in VEGF-induced VE-cadherin expression in the presence of VEGF peptides while an irrelevant peptide had no effects. GADPH expression was measured as an endogenous control (Fig. 4B). These observations strongly suggest that our VEGF peptides are able to limit VEGF-induced VE-cadherin expression, further confirming their anti-angiogenic properties.
Figure 4. VEGF induces the expression of VE-cadherin mRNA and treatment with VEGF peptide mimics reverses this induction. (A,B) Total RNA was isolated from matrigel plugs after treatment with peptide inhibitors and used in RT-PCR to measure levels of VE-cadherin expression. GAPDH was measured as an endogenous control and the expression levels were similar in the different treatment groups (A). Addition of VEGF was able to induce VE-cadherin expression but treatment with VEGF peptide mimics inhibited this induction (B).
Transplantable tumor models
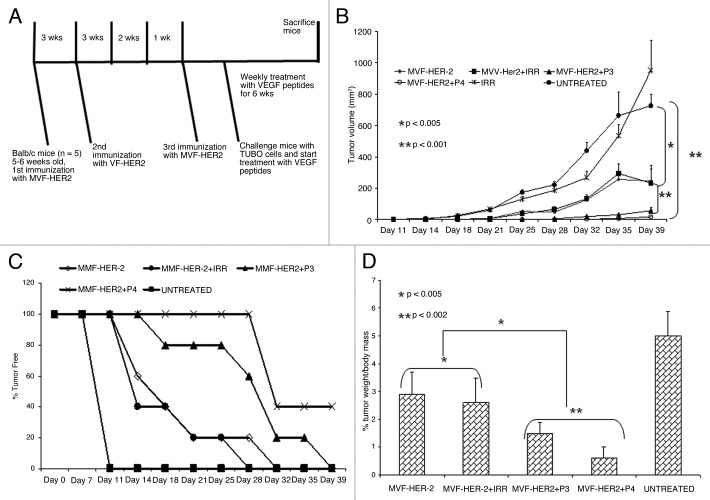
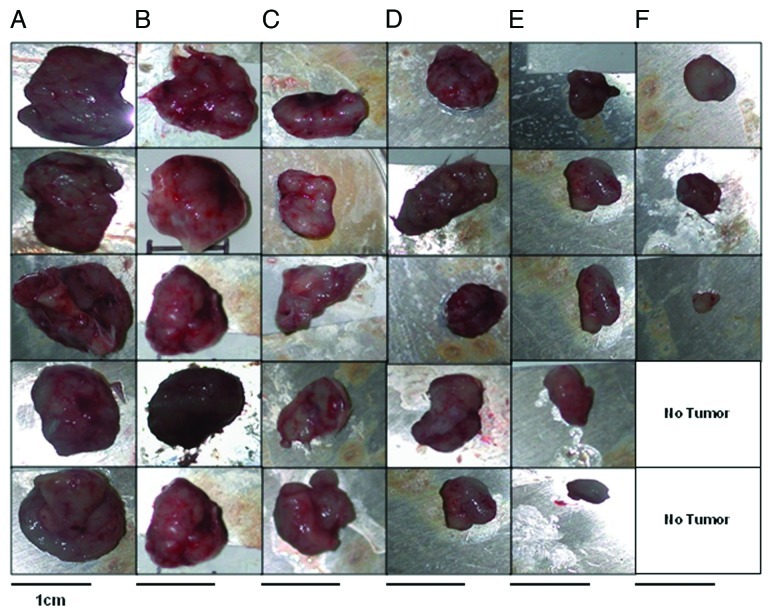
We used a rat neu-expressing tumor model (wild type BALB/c mice challenged with TUBO cells) to evaluate the anti-tumor effect of our HER-2 vaccine followed by therapy with VEGF peptides. The human HER-2 266–296 sequence has a 97% similarity with the corresponding rat neu sequence with only one different amino acid, and this model was previously validated by Allen et al.18 BALB/c mice were immunized with 100 μg of MVF-HER-2–266–296 peptide three times at three weeks intervals and - one week after the third immunization - mice were challenged with TUBO cells (derived from a spontaneous mammary carcinoma in BALB/c-neuT transgenic mice).18 Groups of mice (n = 5) were then treated with either VEGF peptides, the irrelevant peptide or left untreated (Fig. 5A). We found that immunization with MVF-HER-2 266–296 significantly (**p < 0.001) delays tumor development and growth (Fig. 5B) Most interestingly, there was a difference between immunization alone and immunization combined with VEGF peptides. In both cases, p values were < 0.001 but in the case of the D-amino acid-based VEGF-P4 peptide, the delay in tumor growth was even greater as compared with that induced by the L-amino acid-containing VEGF-P3 peptide (Fig. 5B). At the end of the experiment, some of the mice were tumor free and this was observed in 40% of the animals receiving the VEGF-P4 peptide upon immunization (Fig. 5C). We also weighed the tumors at the end of the experiment and calculated the % of tumor weight (weight of tumor alone / weight of tumor + weigth of mouse, all multiplied by 100). We observed a significant difference (95% confidence interval) between all treatments as compared with the control and the untreated groups. Immunization alone or combined with an irrelevant peptide resulted in approximately a 40% reduction in % tumor weight (*p < 0.005). The post-vaccination administration of both the VEGF-P3 and P4 peptides greatly enhanced this effect (**p < 0.002) (Fig. 5D). These results were fully corroborated by the visual inspection of tumors (Fig. 6). Taken together, these obsevrations suggest that the dual targeting of HER-2 and VEGF potently inhibits tumor development. In this sense, the RI D-amino acid-based peptide provided better results than its L-amino acid-based counterpart, both as as single agent and in combination regimens.
Figure 5. Effects of HER-2 Immunization and VEGF peptide treatment in a transplantable tumor model. (A) Immunization scheme for BALB/c mice. Mice were immunized subcutaneously with 100 μg of MVF-HER-2 three times at three weeks intervals. Two weeks after the third immunization, mice were challenged with TUBO cells and treated weekly with VEGF peptide mimics and irrelevant peptide for 6 weeks. (B) Effects of combination treatment on tumor growth. Wild type BALB/c mice (n = 5), at the age of 5–7 weeks were immunized subcutaneously three times at three weeks intervals with 100 μg of MVF-HER-2 emulsified in ISA720. After immunization, mice were challenged with TUBO cells and treated intravenously with VEGF peptide mimics or an irrelevant peptide. Tumor measurements were performed twice a week using calipers. The data are presented as the average tumor volume per group and are reported as mm3. Results show a statistical significant difference between the group immunized with MVF-HER-2 vs. the group treated with the VEGF peptide mimics (**p < 0.001). There was a significant difference between immunization plus irrelevant peptide vs. immunization plus treatment with VEGF peptide mimics (*p < 0.001). (C) Effects of immunization and treatment on tumor-free survival rates. Results show that immunization with MVF-HER-2 and treatment with VEGF-P4 produced the best results since 40% of the mice (2 out of 5) remained tumor-free at the end of the experiment. There was also a greater delay in onset of tumor development in the case of VEGF-P3 peptide as compared with the MVF-HER-2 immunization alone. (D) Effects of peptide treatment on % tumor weight per body mass. After treatment, tumors were removed and weighed and the results show a significant difference between treated and untreated groups. p values are reported.
Figure 6. Effects of combination treatment on tumor size. At the end of treatment, mice were euthanized and tumors extracted and pictures taken using a Nikon camera. After three immunizations BALB/c mice were challenged with TUBO cells and treated with VEGF-P3 and VEGF-P4. Representative photos from different treatment groups at day 39 after the inoculation of cancer cells are reported. (A) untreated; (B) irrelevant; (C) MVF-HER-2; (D) MVF-HER-2+IRRELEVANT; (E) MVF-HER2+VEGF-P3(CYC); (F) MVF-HER2+RI-VEGF-P4(CYC).
Combination treatment decreases the number of actively dividing cells and microvascular density
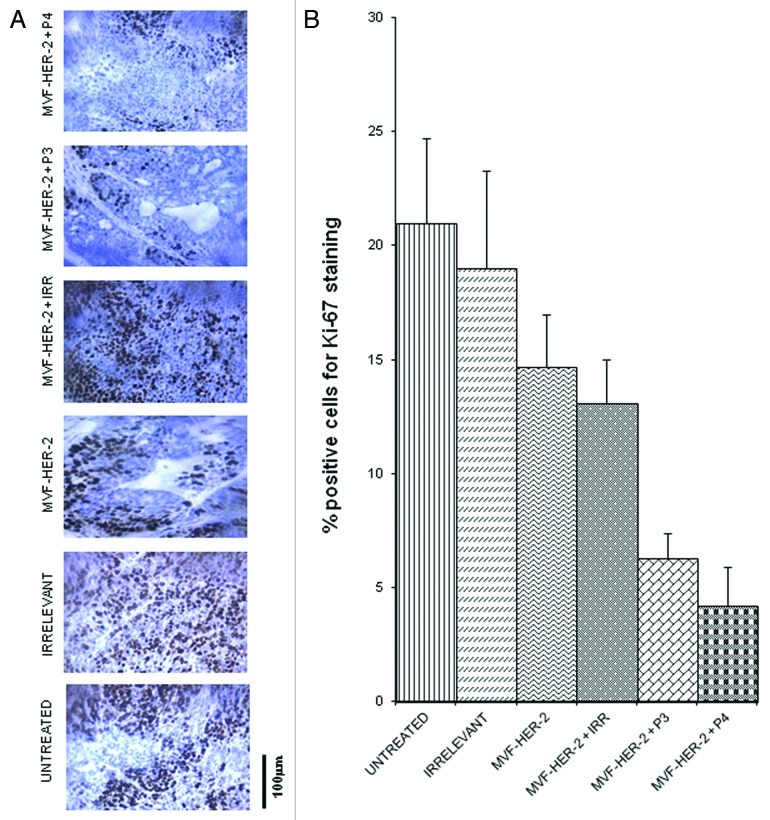
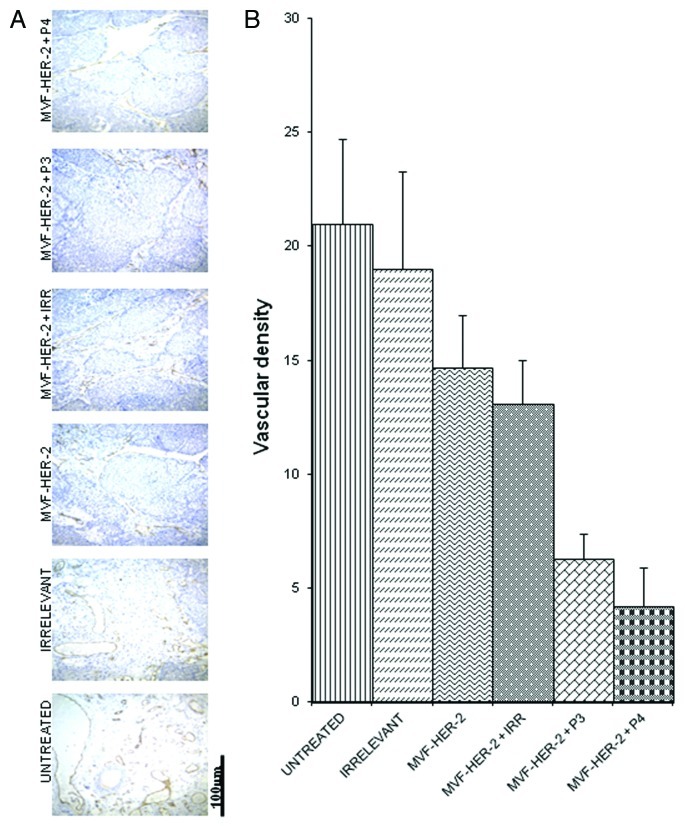
In order to evaluate how our peptides exert antit-tumor effects, we examined different treatments on the number of actively dividing cells. The amount of actively dividing cells was assessed in tumor section by immunohistochemistry for Ki67 and Image J-assisted quantification. The number of proliferating cells greatly decreased upon the administration of the HER-2 vaccine alone, and there was a further decrease given by the addition of either the VEGF-P3 or the VEGF-P4 peptide (Fig. 7A,B). To further clarify the mechanisms of action of these peptides, we examined the vessel density of the tumors by immunohistochemistry upon staining with an anti-CD31 antibody. We found that the number of CD31+ microvessels in the tumors treated with the HER-2 vaccine was reduced as compared with untreated tumors and tumors receiving an irrelevant peptide. As for actively proliferating cells, the inhibition given by vaccination was greatly enhanced by the administration of either VEGF-P3 or VEGF-P4 (Fig. 8A,B).
Figure 7. Combination treatment decreases the number of actively dividing cells in a transplantable mouse model of breast cancer. Quantification of the number of actively dividing cells in tumor sections using Ki-67 staining. (A) Tissue sections show the amount of positive cells. The stain is specific for cells that are actively dividing. (B) Quantification of the staining using Image J software. Data represent mean values from four different fields and error bars represent SD of the mean.
Figure 8. Combination treatment decreases microvascular density in tumors. Evaluation of vessel density in tumor sections. (A) Vascular staining using anti-CD31 antibody. (B) Effects of combination treatment on the tumor vessel density after quantification with the Image J software. Data represents mean values from four different fields and error bars represents SD of the mean.
Discussion
HER-2 is a member of the ErbB family of receptor tyrosine kinases (RTKs) associated with aggressive forms of several human cancers and a well-established target for both passive and active immunotherapy. VEGF is overexpressed in many different types of cancer and hence both VEGF and its receptors (VEGFR1 and VEGFR2) are prime targets for tumor-directed anti-angiogenic intervention. Agents targeting RTKs for cancer therapy include antibodies that block RTK ligands or the receptors themselves, as well as small-molecule inhibitors that inhibit the intracellular catalytic domain of RTKs. Many FDA-approved therapies targeting both HER-2 (Trastuzumab, Herceptin, Pertuzumab, Omnitarg) and VEGF (Bevacizumab, Avastin) have significant toxicities and are associated with the development of resistance. Clinical applications of monoclonal antibody-based therapy in general is limited by a number of concerns such as the frequency of treatments, associated costs, limited duration of action, undesired immunogenicity, and significant risks of carditoxicity. Similarly small-molecule RTK inhibitors such as sunitinib, which have entered clinical trials alone or in combination with radiotherapy or chemotherapy, show problems of efficacy, development of resistance and unacceptable safety profiles which altogether hamper their clinical progress.
Immunization or treatment with peptides offers the opportunity of stimulating the body’s immune response leading to immunological memory. Peptides are relatively safe, non toxic, cheaper than antibodies and highly specific. The only drawback associated with peptides is their relatively limited stability (owing to degradation by proteases). This can however be overcome by using D-amino acids, which cannot be recognized by proteases. Thus, peptides can be synthesized with a reversal of the peptide chirality and using D-amino acids, resulting to a topographical equivalent of the parent peptide.
The overexpression of HER-2 is associated with increased expression of VEGF at both the RNA and protein levels in human breast cancer cells, and exposure of HER-2 positive cells to trastuzumab significantly decreases VEGF.25 SHC, a downstream adaptor protein of the HER-2 signaling pathway, has been identified as a critical angiogenic switch for VEGF production.26 This suggests that the effects of HER-2 on tumor cell behavior may be mediated in part through the stimulation of angiogenesis. A two-pronged approach to target cancer cells by co-immunizing with defined tumor-associated antigens and angiogenesis-associated antigens has been shown to exert synergistic anti-tumor effects.27-29 Altogether, these observations indicate that combination therapy targeting both HER-2 and VEGF constitutes a superior strategy as compared with either monotherapy, since antiangiogenic therapy alone tends to only delay tumor growth15 while targeting HER-2 and VEGF will abrogate two distinct tumorigenic pathways.
We evaluated the antiproliferative effects of the antibodies raised by HER-2 and VEGF peptides, alone or combined, on different cell lines. Trastuzumab has been shown to act only on HER-2 positive cells and we made similar observations (Fig. 1A,B), whereby little inhibition was observed with the (HER-2 low) MDA-468 cell line as compared with the (HER-2 high) BT-474 cell line. The anti-peptide antibodies were effective in inhibiting both cancer cell lines. The HER-2–266–296 peptide antibody showed some inhibitory effects on the HER-2-low cell line (MDA-468) (Fig. 1B) and this is probably due to the fact that the peptide was synthesized using the pertuzumab epitope. Indeed, antibodies raised against this peptide should be able to function like pertuzumab, hence having inhibitory effects in cells independent of HER-2. We also evaluated the in vitro effects of the combination treatment with both HER-2 and VEGF anti-peptide antibodies on cell proliferation and viability, finding that the combination regimen produces greater anti-tumor effects than either treatment alone (Fig. 1C).
HER-2 is known to dimerize with its partner HER-1 and HER-3 leading to receptor phosphorylation and intracellular signaling. Pertuzumab mainly functions by sterically blocking HER-2 from binding to its partners and is therefore classified as a dimerization inhibitor.30,31 We therefore investigated the effects of antibodies raised by HER-2 and VEGF peptides on HER-2 phosphorylation, finding an amelioration in phosphorylation inhibition from less than 35% in the case of single treatments to about 75% in the case of the combination regimen (Fig. 2A). One of the main mode of action of antibodies is to cause ADCC, so we also evaluated the ability of our anti-peptide antibodies to cause ADCC against BT-474 cells. We observed that the anti-peptide antibodies were able to cause ADCC and their effects were comparable to that of the positive control trastuzumab (Fig. 2B). Also in the case of anti-HER-2 and anti-VEGF peptide antibodies in combination, there was an increase in ADCC as compared with single treatments as well as to trastuzumab. We have previously shown the anti-angiogenic effects of our VEGF peptide mimics in different angiogenesis assays32 and here we illustrated further their ability to inhibit microvascular outgrowth in the mouse aortic ring assay (Fig. 3). We also showed that the VEGF peptide mimics are able to prevent the VEGF-mediated induction of VE-cadherin in matrigel plugs that were implanted in mice (Fig. 4).
In order to evaluate the effects of peptide administration in vivo, we used a transplantable tumor mouse model. BALB/c mice were immunized with the HER-2 peptide before being challenged with TUBO cells and treated with VEGF peptides. We observed significant differences between treated and control groups a delay in tumor growth and development, and a decrease in tumor weight. Vaccination with MVF-HER-2 266–296 followed by the administration of VEGF-P4 produced the best results, and 40% of the mice in this group remained tumor-free at the end of the experiment (Fig. 5). The VEGF peptide treatment also appeared to cause a decrease in blood flow to the tumors, contributing to its anti-tumor effects (Fig. 6). Tumor sections stained for actively dividing cells and blood vessels showed a marked reduction in positively stained cells following the vaccination, which was exacerbated with VEGF peptides were administered post-treatment (Figs. Seven and 8). These results strongly suggest that tumor growth and development can be strongly inhibited by simultaneously targeting the tumors and their blood supply. This is also because while tumor cells are genetically unstable (thus constantly changing and hence baing prone to developing resistance), the tumor vasculature is genetically stable.33 Thus, active immunization with HER-2 peptide epitopes and treatment with VEGF peptide mimics is a better strategy than immunization alone. Also, the D-amino acid-based peptide produced greater inhibitory effects, probably due to its longer half-life in vivo.
In conclusion, our results show the potential synergy between immunotherapy with HER-2 peptide vaccines and antiangiogenic therapy with VEGF inhibitors that are able to prevent or delay tumor growth. This combination strategy targeting different aspects of the tumor microenvironment show enhanced efficacy as compared with indivual treatments. The simultaneous and sequential strategy to inhibit different signaling pathways shows great promise for the design and development of effective anti-tumor and anti-angiogenic therapies against cancer. Foremost in those strategies lies the intuitive and rational design of effective peptide molecules that mimic the corresponding native structure for high efficacy inhibition of both antigen:antibody and receptor ligand interactions. Such molecules are safe and non-toxic and might offer great advantages in the treatment and management of cancers.
Materials and Methods
Synthesis and characterization of conformational peptides
Peptide synthesis was performed on a Milligen/Biosearch 9600 peptide solid phase synthesizer (Bedford, MA) using Fmoc/t-But chemistry. Preloaded Fmoc-Val-clear acid resin (0.35 mmol/g) for the MVF-HER-2 266–296 and clear amide resin for the VEGF peptides (0.32 mmol/g) (Peptides International, Louisville, KY) were used for synthesis. The 266–296 cyclized epitope was collinearly synthesized with the promiscuous TH epitope MVF and assembled by choosing the regioselective side chain protector Trt on Cys residues 268 and 295, and in the VEGF peptides two cysteines were inserted between amino acid Gln79 and Gly92 and between Ile80 and Glu93. Peptides were cleaved from the resin using cleavage reagent B (trifluoroacetic acid: phenol: water: TIS, 90:4:4:2), and crude peptides purified by semi preparative reversed-phase-HPLC and characterized by electrospray ionization mass spectroscopy.34 Intramolecular disulphide bonds were formed using iodine oxidation as described35 and disulphide bridge formation was further confirmed by maleimide-PEO2-biotin reaction and subsequent analysis using electrospray ionization mass spectroscopy. Peptides that were used for immunization both in rabbits and mice were collinearly synthesized with the promiscuous TH epitope MVF (MVF-HER-2–266–296 CYC, MVF-VEGF-P3-CYC and MVF-RI VEGF-P4-CYC) while those that were used for intravenous treatment of mice after vaccination was synthesized without any MVF (VEGF-P3-CYC and RI-VEGF-P4-CYC) (Table 1).
Animals
Female New Zealand white outbred rabbits were purchased from Harlan (Indiana, IN). Female BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). Animal care and use was in accordance with institutional guidelines.
Cell lines and antibodies
All culture media, FBS, and supplements were purchased from Invitrogen Life Technologies (San Diego, CA). The human breast tumor cell lines BT-474 and MDA-468 were purchased from American Type Culture Collection (Rockville, MD) and maintained according to supplier’s guidelines. We also used TUBO cells, a cloned cell line established in vitro from a lobular carcinoma that arose spontaneously in BALB-neuT mouse.36 Humanized mouse mAb Trastuzumab was a gift from Dr William Carson.
Active immunization and antibody purification
Mice and rabbits were immunized subcutaneously at multiple sites with a total of 1 mg (rabbits) or 100 μg (mice) of peptide dissolved in ddH2O emulsified (1:1) in Montanide ISA720 vehicle (Seppic) with 100 μg of N-acetylglucosamine-3yl-acetyl-L-alanyl-D-isoglutamine (nor-MDP). Rabbits and mice were boosted with the respective doses at 3 week intervals. Rabbit blood was collected via the central auricular artery and sera tested for antibody titers. Anti-peptide antibodies were purified by affinity chromatography using a Protein A/G column (Pierce) from high titer antibody sera.
ELISA
Antibody titers were determined as previously described37 and is defined as the reciprocal of the highest serum dilution with an absorbance of 0.2 or greater after subtracting the background.
Proliferation assays
BT-474 and MDA-468 (1x104) were plated in 96-well flat-bottom plates overnight. Growth medium was replaced with low serum (1% FCS) medium and the cells were incubated overnight. Media were removed from the wells and replaced with low serum medium containing anti-HER-2 peptide and anti-VEGF mimic peptides antibodies at concentrations ranging from 25–100 μg/mL and plates were incubated an additional 1 h at 37°C before adding 10 ng/ml HRG in 1% medium. Plates were further incubated for 72 h at 37°C before adding MTT (5 mg/mL) to each well. Then, plates were incubated 2 h at 37°C, and 100 μL of extraction buffer (20% SDS, 50% dimethylformamide (pH 4.7) was added to each well. Finally plates were incubated overnight at 37°C and read on an ELISA reader at 570 nm with 655 nm background subtraction. Inhibition percentage was calculated as 100% x (Untreated cells – Peptide treated cells)/(Untreated cells).
Phosphorylation assays
One x 106 BT-474 cells were plated in each well of a six well plate and incubated overnight at 37°C. Culture medium was removed and the cell layer washed once with PBS low serum (1% FCS). Culture medium was then added to the wells and plates incubated overnight. Cells were washed and 50 µg of anti-peptide Abs and controls in binding buffer (0.2% w/v BSA, RPMI 1640 medium with 10 mM HEPES (pH 7.2) was added to the wells and incubated at room temperature for 1h. HRG (5 nM) was added and the incubation continued for 10 min. Binding buffer was removed and the cell layer washed once with PBS before adding 1ml of RIPA lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA). Plates were rocked at 4°C for 2 h. Lysates were removed, spun at 13000 X g and supernatants collected. Protein concentration of each sample was measured by Coomassie plus protein assay reagent kit and lysates were stored at -80°C. Phosphorylation was determined by Duoset IC for human phosphor-ErbB2 according to the manufacturer’s directions (R&D Systems).
Antibody-dependent cell-mediated cytotoxicity (ADCC)
We used the bioluminescence cytotoxicity assay (aCella-TOXTM Mountain View, CA) and all procedures were performed according to the manufacturer’s instructions. Briefly, The BT-474 target cells (1x 104/well) were plated on a 96 well plate and anti-peptide abs were added to the wells containing the target cells. The plate was incubated at 37°C for 15 min to allow opsonization of antibody to occur. Effectors cells (hPBMCs from red cross) were then added to the wells at three different E:T ratios (100:1, 20:1 and 4:1) and the plate incubated at 37°C for 3 h. The plate was then removed and equilibrated to room temperature for 15 min before adding 10 μL of lytic agent to the control wells for maximum lysis and incubated for 15 min at room temperature. 100 μL of the Enzyme Assay reagent containing G3P was then added to all wells followed by 50 μL of the detection reagent. The plate was immediately read using a luminometer.
Mouse aortic ring assays
Female BALB/c mice 6 weeks of age were sacrificed by cervical dislocation and thoraxic aortas were removed and immediately transferred to a culture dish containing MEM media. The peri-aortic fibroadipose tissue was carefully removed with fine microdissecting forceps and scissors without damaging the aortic wall. The aortic rings about 1mm long were sectioned and washed about five times in MEM media. 200 µL of matrigel was added to wells of a 96 well plates and the rings were placed on top of the gel and then covered with another 200 µL of matrigel. Cultue media containing VEGF peptides (500 µg/mL) and controls were then added to the wells and incubated at 37°C. Culture media was replaced daily with respective inhibitors. After 10 d, the culture was discarded and photos of the rings in the gel taken for observation of microvascular outgrowth in the rings.
In vivo matrigel assay and RNA purification from matrigel
Liquid matrigel (500 µL) was injected subcutaneously into the flanks of Balb/c mice. The matrigel (BD bioscience, CA) contained VEGF165 at a final concentration of 500 ng/mL to stimulate angiogenesis and VEGF peptides or irrelevant peptides were added at a concentration of 500 µg/mL. All treatment groups contained three mice and each mice had two plugs. After ten days, the mice were sacrificed and matrigel plugs were removed and stored in liquid nitrogen. For RNA isolation, the matrigel plugs were homogenized and the and treated with 600 µL of lysis buffer (Absolutely RNA RT-PCR Miniprep kit, Stratagene). Purification of total RNA was performed using the Miniprep kit and RNA concentration was measured using UV spectroscopy (GeneQuant, Amersham Pharmacia Biotech, Arlington Heights, IL). cDNA was synthesized from total matrigel RNA using the SuperScript First-Strand Synthesis System for RT-PCR (Life Technologies). Details of the experimental procedure are described in Eubank et al.38
VE-cadherin RT-PCR
Samples were pre-treated with RNase-free DNase (Promega) in order to avoid genomic DNA contamination. After treatment, two micrograms of total RNA from the different samples were reversed transcribed in a final volume of 50µl using Taqman Reverse Transcription Reagents (PE Applied Biosystems, Foster city, CA). Primers for mouse VE-cadherin were designed using the primer design software express (PE Applied Biosystems) The human glyceraldehyde phosphate dehydrogenase (GAPDH) (PE Applied Biosystems) was used as an endogenous control. All PCR reactions were performed using an ABI prism 7700 Sequence Detection System (PE Applied Biosystems). The expression levels were determined using comparative threshold cycle (CT) method as previously described.39
Peptide treatment in transplantable mouse model
BALB/c mice 5 to 6 weeks of age were immunized with 100 μg of MVF-HER-2–266 three times at three weeks intervals. Two weeks after the third immunization, the mice were challenged with 1 × 105 TUBO cells and after challenge, mice were treated intravenously with 100 μg of either VEGF-P3-CYC, RI-VEGF-P4-CYC or irrelevant peptide as inhibitors. Treatment was given weekly for six consecutive weeks. Mice were euthanized at week 10 and tumors removed. Tumors were measured twice a week using calipers and tumor volume was calculated using the formula (length × width2)/2.
Immunohistochemistry
In order to indentify the effects of treatment on the tumor microenvironment, tumors were formalin fixed, paraffin embedded, and stained immunohistochemically. Serial sections were cut and stained for Ki-67 staining for actively dividing cells, CD31 for blood vessels The immunohistochemical detection protocol was performed as published.40 Total staining was analyzed using the NIH Image J software and the staining index was calculated as the percentage area occupied by the positive cells to the total area occupied by all the cells. For CD31, vascular density was calculated as the relative area occupied by the blood vessels in each field with total area of field considered as 100. A total of four fields were observed for each treatment.
Statistical analysis
Tumor growth over time was analyzed using Stata’s XTGEE (cross-sectional generalized estimating equations) model which fits general linear models that allows to specify within animal correlation structure in data involving repeated measurements. For other experiments, Student’s t-tests were performed to observe the statistical relevancy in between different sets of experiments as well as the significant difference between treated and non-treated cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported in part by the National Institute of Health NCI Grant CA 84356 to P.T.P.K.
Glossary
Abbreviations:
- HER-2
human epidermal growth factor receptor 2
- VEGF
vascular endothelial growth factor
- MVF
measles virus fusion protein
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20708
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Barbacci EG, Guarino BC, Stroh JG, Singleton DH, Rosnack KJ, Moyer JD, et al. The structural basis for the specificity of epidermal growth factor and heregulin binding. J Biol Chem. 1995;270:9585–9. doi: 10.1074/jbc.270.16.9585. [DOI] [PubMed] [Google Scholar]
- 3.Leu M, Bellmunt E, Schwander M, Fariñas I, Brenner HR, Müller U. Erbb2 regulates neuromuscular synapse formation and is essential for muscle spindle development. Development. 2003;130:2291–301. doi: 10.1242/dev.00447. [DOI] [PubMed] [Google Scholar]
- 4.Hudziak RM, Schlessinger J, Ullrich A. Increased expression of the putative growth factor receptor p185HER2 causes transformation and tumorigenesis of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1987;84:7159–63. doi: 10.1073/pnas.84.20.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabos Z, Sinha R, Hanson J, Chauhan N, Hugh J, Mackey JR, et al. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol. 2006;24:5658–63. doi: 10.1200/JCO.2006.07.0250. [DOI] [PubMed] [Google Scholar]
- 6.Mimura K, Kono K, Hanawa M, Mitsui F, Sugai H, Miyagawa N, et al. Frequencies of HER-2/neu expression and gene amplification in patients with oesophageal squamous cell carcinoma. Br J Cancer. 2005;92:1253–60. doi: 10.1038/sj.bjc.6602499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison C, Zanagnolo V, Ramirez N, Cohn DE, Kelbick N, Copeland L, et al. HER-2 is an independent prognostic factor in endometrial cancer: association with outcome in a large cohort of surgically staged patients. J Clin Oncol. 2006;24:2376–85. doi: 10.1200/JCO.2005.03.4827. [DOI] [PubMed] [Google Scholar]
- 8.Yano T, Doi T, Ohtsu A, Boku N, Hashizume K, Nakanishi M, et al. Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncol Rep. 2006;15:65–71. [PubMed] [Google Scholar]
- 9.Cirisano FD, Karlan BY. The role of the HER-2/neu oncogene in gynecologic cancers. J Soc Gynecol Investig. 1996;3:99–105. doi: 10.1016/1071-5576(96)00001-9. [DOI] [PubMed] [Google Scholar]
- 10.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokoi A, McCrudden KW, Huang J, Kim ES, Soffer SZ, Frischer JS, et al. Blockade of her2/neu decreases VEGF expression but does not alter HIF-1 distribution in experimental Wilms tumor. Oncol Rep. 2003;10:1271–4. [PubMed] [Google Scholar]
- 12.Nair S, Boczkowski D, Moeller B, Dewhirst M, Vieweg J, Gilboa E. Synergy between tumor immunotherapy and antiangiogenic therapy. Blood. 2003;102:964–71. doi: 10.1182/blood-2002-12-3738. [DOI] [PubMed] [Google Scholar]
- 13.Grunstein J, Roberts WG, Mathieu-Costello O, Hanahan D, Johnson RS. Tumor-derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Res. 1999;59:1592–8. [PubMed] [Google Scholar]
- 14.Neufeld G, Tessler S, Gitay-Goren H, Cohen T, Levi BZ. Vascular endothelial growth factor and its receptors. Prog Growth Factor Res. 1994;5:89–97. doi: 10.1016/0955-2235(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 15.Kim DW, Huamani J, Fu A, Hallahan DE. Molecular strategies targeting the host component of cancer to enhance tumor response to radiation therapy. Int J Radiat Oncol Biol Phys. 2006;64:38–46. doi: 10.1016/j.ijrobp.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–28. doi: 10.1016/S1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 17.de Gramont A, Van Cutsem E. Investigating the potential of bevacizumab in other indications: metastatic renal cell, non-small cell lung, pancreatic and breast cancer. Oncology. 2005;69(Suppl 3):46–56. doi: 10.1159/000088483. [DOI] [PubMed] [Google Scholar]
- 18.Allen SD, Garrett JT, Rawale SV, Jones AL, Phillips G, Forni G, et al. Peptide vaccines of the HER-2/neu dimerization loop are effective in inhibiting mammary tumor growth in vivo. J Immunol. 2007;179:472–82. doi: 10.4049/jimmunol.179.1.472. [DOI] [PubMed] [Google Scholar]
- 19.Vicari D, Foy KC, Liotta EM, Kaumaya PT. Engineered conformation-dependent VEGF peptide mimics are effective in inhibiting VEGF signaling pathways. J Biol Chem. [DOI] [PMC free article] [PubMed]
- 20.Srinivasan M, Gienapp IE, Stuckman SS, Rogers CJ, Jewell SD, Kaumaya PTP, et al. Suppression of experimental autoimmune encephalomyelitis using peptide mimics of CD28. J Immunol. 2002;169:2180–8. doi: 10.4049/jimmunol.169.4.2180. [DOI] [PubMed] [Google Scholar]
- 21.Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–37. doi: 10.1016/S1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 22.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 23.Clynes R, Ravetch JV. Cytotoxic antibodies trigger inflammation through Fc receptors. Immunity. 1995;3:21–6. doi: 10.1016/1074-7613(95)90155-8. [DOI] [PubMed] [Google Scholar]
- 24.Wary KK, Thakker GD, Humtsoe JO, Yang J. Analysis of VEGF-responsive genes involved in the activation of endothelial cells. Mol Cancer. 2003;2:25. doi: 10.1186/1476-4598-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rugo HS. Bevacizumab in the treatment of breast cancer: rationale and current data. Oncologist. 2004;9(Suppl 1):43–9. doi: 10.1634/theoncologist.9-suppl_1-43. [DOI] [PubMed] [Google Scholar]
- 26.Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99:11393–8. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpenito C, Davis PD, Dougherty ST, Dougherty GJ. Exploiting the differential production of angiogenic factors within the tumor microenvironment in the design of a novel vascular-targeted gene therapy-based approach to the treatment of cancer. Int J Radiat Oncol Biol Phys. 2002;54:1473–8. doi: 10.1016/S0360-3016(02)03921-4. [DOI] [PubMed] [Google Scholar]
- 28.Casella I, Feccia T, Chelucci C, Samoggia P, Castelli G, Guerriero R, et al. Autocrine-paracrine VEGF loops potentiate the maturation of megakaryocytic precursors through Flt1 receptor. Blood. 2003;101:1316–23. doi: 10.1182/blood-2002-07-2184. [DOI] [PubMed] [Google Scholar]
- 29.Monsky WL, Mouta Carreira C, Tsuzuki Y, Gohongi T, Fukumura D, Jain RK. Role of host microenvironment in angiogenesis and microvascular functions in human breast cancer xenografts: mammary fat pad versus cranial tumors. Clin Cancer Res. 2002;8:1008–13. [PubMed] [Google Scholar]
- 30.Stern DF, Kamps MP. EGF-stimulated tyrosine phosphorylation of p185neu: a potential model for receptor interactions. EMBO J. 1988;7:995–1001. doi: 10.1002/j.1460-2075.1988.tb02906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wada T, Qian XL, Greene MI. Intermolecular association of the p185neu protein and EGF receptor modulates EGF receptor function. Cell. 1990;61:1339–47. doi: 10.1016/0092-8674(90)90697-D. [DOI] [PubMed] [Google Scholar]
- 32.Vicari D, Foy KC, Liotta EM, Kaumaya PT. Engineered conformation-dependent VEGF peptide mimics are effective in inhibiting VEGF signaling pathways. J Biol Chem. 2011;286:13612–25. doi: 10.1074/jbc.M110.216812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eskens FA. Angiogenesis inhibitors in clinical development; where are we now and where are we going? Br J Cancer. 2004;90:1–7. doi: 10.1038/sj.bjc.6601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sundaram R, Lynch MP, Rawale SV, Sun Y, Kazanji M, Kaumaya PT. De novo design of peptide immunogens that mimic the coiled coil region of human T-cell leukemia virus type-1 glycoprotein 21 transmembrane subunit for induction of native protein reactive neutralizing antibodies. J Biol Chem. 2004;279:24141–51. doi: 10.1074/jbc.M313210200. [DOI] [PubMed] [Google Scholar]
- 35.Söll R, Beck-Sickinger AG. On the synthesis of orexin A: a novel one-step procedure to obtain peptides with two intramolecular disulphide bonds. J Pept Sci. 2000;6:387–97. doi: 10.1002/1099-1387(200008)6:8<387::AID-PSC267>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 36.Rovero S, Amici A, Di Carlo E, Bei R, Nanni P, Quaglino E, et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol. 2000;165:5133–42. doi: 10.4049/jimmunol.165.9.5133. [DOI] [PubMed] [Google Scholar]
- 37.Dakappagari NK, Pyles J, Parihar R, Carson WE, Young DC, Kaumaya PT. A chimeric multi-human epidermal growth factor receptor-2 B cell epitope peptide vaccine mediates superior antitumor responses. J Immunol. 2003;170:4242–53. doi: 10.4049/jimmunol.170.8.4242. [DOI] [PubMed] [Google Scholar]
- 38.Eubank TD, Galloway M, Montague CM, Waldman WJ, Marsh CB. M-CSF induces vascular endothelial growth factor production and angiogenic activity from human monocytes. J Immunol. 2003;171:2637–43. doi: 10.4049/jimmunol.171.5.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu C, Schwartzbauer G, Sperling MA, Devaskar SU, Thamotharan S, Robbins PD, et al. Demonstration of direct effects of growth hormone on neonatal cardiomyocytes. J Biol Chem. 2001;276:22892–900. doi: 10.1074/jbc.M011647200. [DOI] [PubMed] [Google Scholar]
- 40.Chiesa-Vottero AG, Malpica A, Deavers MT, Broaddus R, Nuovo GJ, Silva EG. Immunohistochemical overexpression of p16 and p53 in uterine serous carcinoma and ovarian high-grade serous carcinoma. Int J Gynecol Pathol. 2007;26:328–33. doi: 10.1097/01.pgp.0000235065.31301.3e. [DOI] [PubMed] [Google Scholar]