Abstract
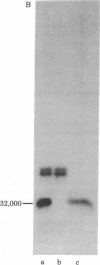
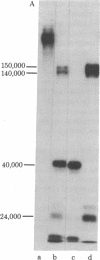
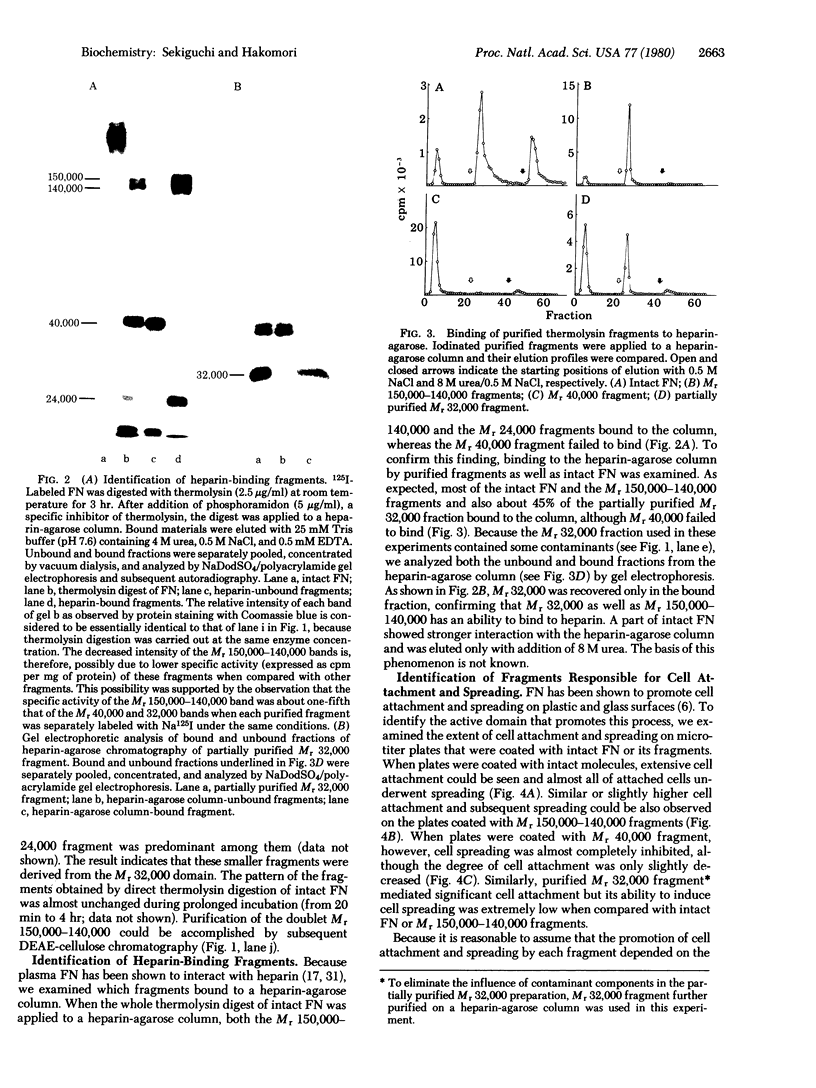
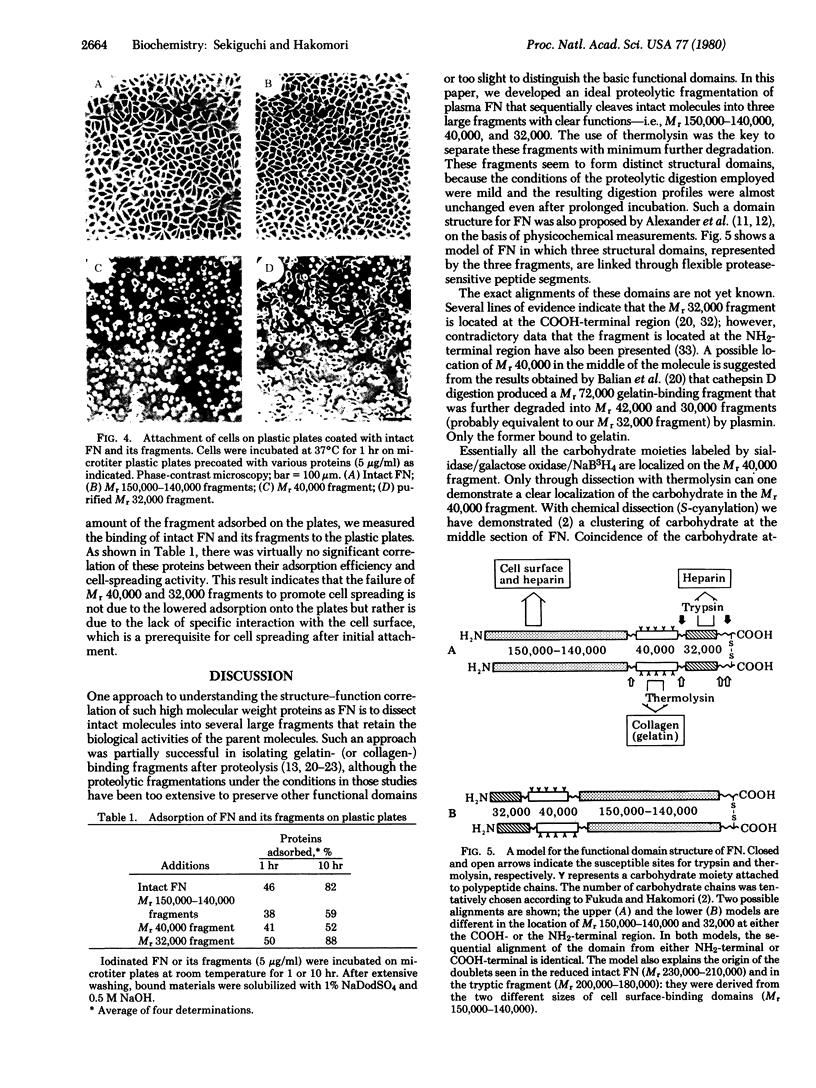
Structural domains of fibronectin (FN) and their ability to associate with cell surface components have been systematically investigated. Plasma FN was cleaved into three structural domains (Mr 150,000-140,000, 40,000, and 32,000) by sequential digestion with trypsin and thermolysin. A single digestion with thermolysin alone generated Mr 150,000-140,000, 40,000, and smaller fragments. With the inclusion of thermolysin, but not with other proteases, one can, with a high yield dissect FN simultaneously into three clearly distinctive functional domains. Of three major fragments only the Mr 40,000 fragment bound to a gelatin column; this fragment contained essentially all of the carbohydrates present in the original FN. In contrast, heparin-binding sites were localized on both the Mr 150,000-140,000 and 32,000 fragments but not on the Mr 40,000 fragment. Only the Mr 150,000-140,000 fragments and intact FN promoted cell spreading, whereas the Mr 40,000 and 32,000 fragments could induce cell attachment but failed to promote cell spreading. These results indicate that FN is composed of (at least) three structural domains that are functionally distinct from each other.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander S. S., Jr, Colonna G., Edelhoch H. The structure and stability of human plasma cold-insoluble globulin. J Biol Chem. 1979 Mar 10;254(5):1501–1505. [PubMed] [Google Scholar]
- Alexander S. S., Jr, Colonna G., Yamada K. M., Pastan I., Edelhoch H. Molecular properties of a major cell surface protein from chick embryo fibroblasts. J Biol Chem. 1978 Aug 25;253(16):5820–5824. [PubMed] [Google Scholar]
- Balian G., Click E. M., Crouch E., Davidson J. M., Bornstein P. Isolation of a collagen-binding fragment from fibronectin and cold-insoluble globulin. J Biol Chem. 1979 Mar 10;254(5):1429–1432. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Carter W. G., Hakomori S. I. A protease-resistant, transformation-sensitive membrane glycoprotein and an intermediate filament-forming protein of hamster embryo fibroblasts. J Biol Chem. 1978 Apr 25;253(8):2867–2874. [PubMed] [Google Scholar]
- Chen A. B., Amrani D. L., Mosesson M. W. Heterogeneity of the cold-insoluble globulin of human plasma (CIg), a circulating cell surface protein. Biochim Biophys Acta. 1977 Aug 23;493(2):310–322. doi: 10.1016/0005-2795(77)90187-8. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Hakomori S. Proteolytic and chemical fragmentation of galactoprotein a, a major transformation-sensitive glycoprotein released from hamster embryo fibroblasts. J Biol Chem. 1979 Jun 25;254(12):5442–5450. [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. Altered growth behavior of malignant cells associated with changes in externally labeled glycoprotein and glycolipid. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3329–3333. doi: 10.1073/pnas.70.12.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Grinnell F. Cellular adhesiveness and extracellular substrata. Int Rev Cytol. 1978;53:65–144. doi: 10.1016/s0074-7696(08)62241-x. [DOI] [PubMed] [Google Scholar]
- Hahn L. H., Yamada K. M. Identification and isolation of a collagen-binding fragment of the adhesive glycoprotein fibronectin. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1160–1163. doi: 10.1073/pnas.76.3.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn L. H., Yamada K. M. Isolation and biological characterization of active fragments of the adhesive glycoprotein fibronectin. Cell. 1979 Dec;18(4):1043–1051. doi: 10.1016/0092-8674(79)90217-4. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. Extensive disulfide bonding at the mammalian cell surface. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2855–2859. doi: 10.1073/pnas.74.7.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga S., Suzuki K., Hashimoto S. Bovine plasma cold-insoluble globulin: gross structure and function. Ann N Y Acad Sci. 1978 Jun 20;312:56–73. doi: 10.1111/j.1749-6632.1978.tb16793.x. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J., Mosher D. F., Vaheri A. Cross-linking of a major fibroblast surface-associated glycoprotein (fibronectin) catalyzed by blood coagulation factor XIII. Cell. 1976 Sep;9(1):29–35. doi: 10.1016/0092-8674(76)90049-0. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J., Mosher D. F., Vaheri A. Dimeric character of fibronectin, a major cell surface-associated glycoprotein. Biochem Biophys Res Commun. 1977 Jan 24;74(2):699–706. doi: 10.1016/0006-291x(77)90359-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mosher D. F. Cross-linking of cold-insoluble globulin by fibrin-stabilizing factor. J Biol Chem. 1975 Aug 25;250(16):6614–6621. [PubMed] [Google Scholar]
- Pearlstein E. Plasma membrane glycoprotein which mediates adhesion of fibroblasts to collagen. Nature. 1976 Aug 5;262(5568):497–500. doi: 10.1038/262497a0. [DOI] [PubMed] [Google Scholar]
- Perkins M. E., Ji T. H., Hynes R. O. Cross-linking of fibronectin to sulfated proteoglycans at the cell surface. Cell. 1979 Apr;16(4):941–952. doi: 10.1016/0092-8674(79)90109-0. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Kuusela P., Shively J. E., Engvall E. Isolation of a tryptic fragment containing the collagen-binding site of plasma fibronectin. J Biol Chem. 1979 Jul 10;254(13):6054–6059. [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G. Two active sites with different characteristics in fibronectin. FEBS Lett. 1979 Jan 15;97(2):221–224. doi: 10.1016/0014-5793(79)80088-5. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pekkala A., Engvall E. Effect of dextran sulfate on fibronectin-collagen interaction. FEBS Lett. 1979 Nov 1;107(1):51–54. doi: 10.1016/0014-5793(79)80461-5. [DOI] [PubMed] [Google Scholar]
- Stathakis N. E., Mosesson M. W. Interactions among heparin, cold-insoluble globulin, and fibrinogen in formation of the heparin-precipitable fraction of plasma. J Clin Invest. 1977 Oct;60(4):855–865. doi: 10.1172/JCI108840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Kurkinen M., Lehto V. P., Linder E., Timpl R. Codistribution of pericellular matrix proteins in cultured fibroblasts and loss in transformation: fibronectin and procollagen. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4944–4948. doi: 10.1073/pnas.75.10.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Mosher D. F. High molecular weight, cell surface-associated glycoprotein (fibronectin) lost in malignant transformation. Biochim Biophys Acta. 1978 Sep 18;516(1):1–25. doi: 10.1016/0304-419x(78)90002-1. [DOI] [PubMed] [Google Scholar]
- Wagner D. D., Hynes R. O. Domain structure of fibronectin and its relation to function. Disulfides and sulfhydryl groups. J Biol Chem. 1979 Jul 25;254(14):6746–6754. [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W. Fibroblast cellular and plasma fibronectins are similar but not identical. J Cell Biol. 1979 Feb;80(2):492–498. doi: 10.1083/jcb.80.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]