Abstract
An incomplete understanding on the effect of surgery on tumor-specific immunity continues to hamper efforts to combine surgery with immunotherapy in the clinic. Herein, we describe the impact of tumor resection on the tumor-specific T-cell response, showing that complete tumor resection is associated with (1) a decline in the amount of cross-presented tumor antigens, (2) a decline of cytolytic tumor-specific CD8+ T cell activity, and (3) the development of systemic CD8+ T cell-mediated protective immunity. Our findings are consistent with a model whereby tumor resection releases antitumor CD8+ T cells from chronic antigen exposure, allowing a gradual differentiation toward functional antitumor memory T cells. This process depends on sentinel lymph nodes, as their removal at the time of surgery was associated with a strong negative effect on survival. We conclude that complete tumor resection provides a unique environment that boosts protective immunological memory and might provide a powerful platform for immunotherapy. Our findings also carry important implications for the design and timing of post-surgery immunotherapeutic regimens.
Keywords: CD8+ T cell, cross-presentation, cross-priming, immunotherapy, surgery
Introduction
There is an urgent need for novel treatment modalities for solid tumors, and in this context antitumor immunotherapy (especially tumor vaccination) has received considerable attention. To date, with a few notable exceptions, immune treatments have been disappointing in the clinic, with response rates usually < 20%.1-6 Nevertheless, increasing evidences indicates that immunotherapy can be effective when combined with surgery.7-16 Indeed, surgical resection of a primary cancer might provide a unique opportunity for effective immunomodulation, since it may disrupt the tumor-associated immunosuppressive network. Possible mechanisms include a reduction in tumor associated soluble factors such as interleukin (IL)-10, IL-4 and the vascular endothelial growth factor (VEGF),17-19 a reduction in myeloid-derived suppressor cell (MDSC) levels,20,21 a shift in the CD4+ T cell memory phenotype,22 a debulking in the pool of immunosuppressive regulatory T cells (Tregs),23 and a decline in the effector requirements for tumor eradication.17
In order to exploit the post-surgery environment, it is important to understand how surgery affects the antitumor immune response. A number of critical questions remain to be answered. What happens to tumor antigen presentation after complete resection of the primary tumor? Can effective antitumor T cell responses develop after tumor debulking? What is the role of sentinel lymph nodes in these processes?
The effects of surgery on soluble tumor antigens are well characterized in the literature, including declines in the circulating levels of CEA24 and PSA25 after resection of colorectal carcinoma and prostate cancer, respectively. However it is less clear how this relates to tumor-specific immunity, and there is a paucity of information on other forms of tumor antigens (cytosolic, membrane bound etc).
To address these issues, we used a well characterized murine model of malignant mesothelioma (AB1-HA) induced by the prototypic carcinogen crocidolite asbestos, which strongly resembles its human counterpart. In order to track the antitumor immune response, we used a model antigen that mimics strong tumor neo-antigens as produced by mutations,26 rather than the weak self tumor antigens that are produced by overexpression of wild type self proteins. Expression of HA as a neo-tumor antigen allowed us to track the antitumor immune response using TCR-transgenic CD4+ and CD8+ T cells, MHC Class I pentamer staining and in vivo analysis of tumor (HA)-specific cytotoxic activity. Importantly, HA acts as an ideal marker antigen as anti-HA responses can be followed but the HA itself is not a rejection antigen in any of our studies.27-29 We have previously reported that HA is constitutively cross-presented to CD8+ T cells in this model.30 In addition, we have shown that the AB1-HA murine tumor serves as a useful model for analyzing the immune effects of surgery.8 However, in these experiments we observed very high rates (75%) of tumor recurrence after complete tumor resection, most likely resulting from inadequate surgical margins. In the current study, complete resection was attained, such that post-surgery immune responses could be analyzed in the absence of tumor recurrence. This allowed us to interrogate the post-surgery immune environment for the durability of tumor antigen presentation, the evolution of effector CD8+ T cell responses and the induction of protective memory.
Results
AB1-HA in wild type and immunodeficient mice
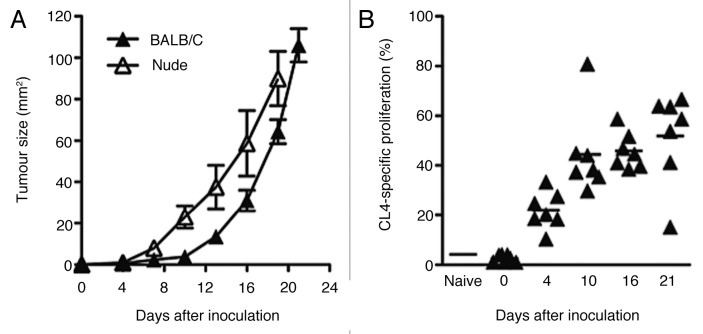
AB1-HA tumor growth rates were compared in wild type and immunodeficient mice, to evaluate the role of antitumor T cell responses. Subcutaneous inoculation of 1 × 106 AB1HA cells into wild type BALB/c and BALB/cnu/nu mice resulted in the growth of solid and vascularized tumors. AB1-HA growth rates were slightly slower in wild type BALB/c mice relative to their immunodeficient counterparts (p < 0.05, two-way ANOVA) (Fig. 1A) suggesting that tumor growth is controlled by T cells, at least to some extent, and implying that AB1-HA tumors are capable of inducing an immune response. However, this immune response was not protective because all mice eventually succumbed to tumor growth (data not shown).
Figure 1. Growth kinetics of subcutaneous AB1HA in wild type and nude mice, and kinetics of HA-specific presentation. (A) AB1HA cells were grown subcutaneously in BALB/c and congenic BALB/cnu/nu. Mean tumor diameter ± SEM shown for each cohort. Data are shown from a single experiment (n = 10 animals for each group). (B) HA specific CD8+ proliferation in DLN assessed throughout AB1HA tumor growth. Each data point depicts proportion of proliferating CFSE+ CL4 T cells detected in pooled inguinal and axillary DLN from an individual mouse, mean proliferation for each time point depicted by the bar.
HA is constitutively cross-presented in the tumor-draining (sentinel) lymph nodes
AB1-HA tumor antigens are constitutively cross-presented to CD8+ T cells.30 To further evaluate the localization of cross-presented antigen, we measured proliferation of TCR-transgenic HA-specific CD8+ T cells (CL4 T cells) using CFSE dye dilution31 in lymph nodes adjacent to the tumor (ipsilateral axillary and inguinal), more distant nodes (contralateral inguinal, contralateral axillary, brachial, cervical, iliac, caudal, para-aortic, celiac, mesenteric, mediastinal) and spleens. Proliferation of CFSE-labeled CL4 CD8+ T cells was analyzed at days 4, 10, 16 and 21 after tumor-cell inoculation. Statistically significant proliferation was observed in the tumor-draining inguinal and axillary lymph nodes (p < 0.01, two-tailed Student’s t-test, relative to non-tumor bearing mice) as early as day 4 after tumor cell inoculation, that is when tumors had an average diameter of just 0.85 ± 0.11 mm. Tumor antigen cross-presentation increased with increasing tumor sizes, reaching 51.96 ± 6.93% by day 21 after inoculation (Fig. 1B). No cross-presentation was detectable in any of the other lymph nodes nor in the spleen at any timepoint (data not shown).
Complete resection of AB1-HA tumors can be curative
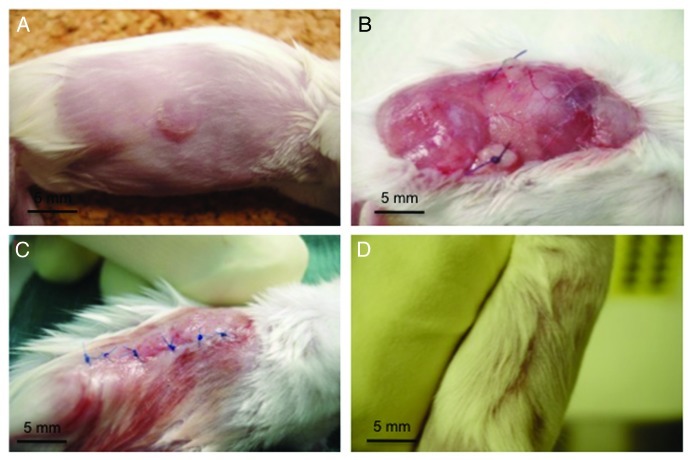
Surgical resection of the tumor provides an excellent opportunity to study the persistence of antigen cross-presentation. Previously described resections of AB1-HA tumors were associated with high recurrence rates (75%),8 possibly as a consequence of inadequate surgical resection. To determine the persistence of cross-presented antigen in the absence of remaining tumor cells, it was necessary to accurately control the vascular pedicles of the tumor, and perform all resections with a meticulous 5 mm tissue margin on both the lateral and deep aspects to the wound (Fig. 2). Surgery was quality-controlled for completeness by three methods. First, surgical margins and resection bed biopsies were assayed for AB1-HA cells using PCR and found to be negative (n = 6). Second, surgical experiments were repeated in syngenic BALB/cnu/nu mice with all animals remaining free of recurrence for > 60 d after surgery. Finally, histological analysis revealed no tumor at the resection margins. From this, we concluded that optimized surgical technique resulted in complete tumor resection.
Figure 2. Method for complete subcutaneous tumor resection. (A) Typical presentation of day 16 subcutaneous AB1HA tumors prior to surgery. (B) Extensive elliptical incision with ligated pellicles, retracted surrounding fascia, and central extraperitoneal fascia that is clear of tumor. (C) Typical closure of wound with suture and minimal impact on hind leg mobility. (D) Recovered flank at 7 d post surgery.
HA-specific cross-presentation declines after surgery
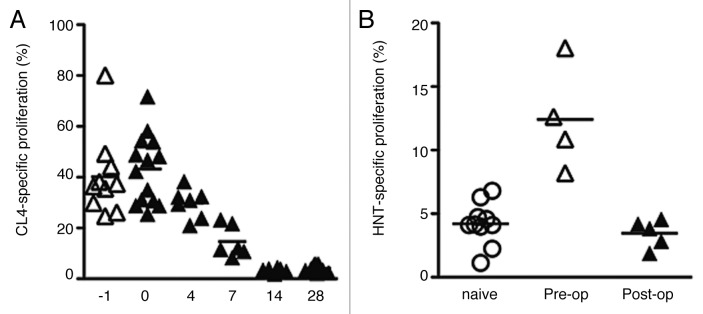
To evaluate the effect of complete tumor resection on antigen cross-presentation, proliferation of CSFE-labeled CL4 transgenic CD8+ T cells was analyzed at various time points before and after surgery. Antigen cross-presentation was not seen in the non-draining lymph nodes or in the spleen at any timepoint (data not shown). In the draining lymph nodes, robust proliferation was observed at the day of surgery but then declined steadily thereafter, reaching non-detectable levels by day 14 after surgery (Fig. 3A). This data shows that antigen cross-presentation was sustained for at least 7 but no longer than 14 d after surgical resection of the tumor. Antigen presentation through Class II MHC molecules was analyzed using TCR-transgenic HA-specific CD4+ T cells (HNT cells) and also declined after resection (Fig. 3B).
Figure 3. HA-specific CD8+ and CD4+ T-cell proliferation in the draining nodes pre- and post-complete AB1HA tumor resection. (A) CL4 CD8+ T cells were transferred at day 16 of tumor growth (day -1), at the time of surgery (day 0), or on post-operative days: 4,7,14, and 28. Each data points depicts proportion of proliferating CFSE+ CL4 T cells detected in pooled inguinal and axillary DLN from an individual mouse. Mean proliferation of group indicated by bars. (B) HNT proliferation was assayed in BALB/c with established (day 16) AB1HA tumors, and two weeks after surgery. Experiment was performed once, with five animals in the pre-operative and 2 week post-operative groups (n = 10 for naïve group). Each data points depicts proportion of proliferating CFSE+ HNT T cells detected in pooled inguinal and axillary DLN from an individual mouse.
Emerging T cell-mediated antitumor immunity after surgery
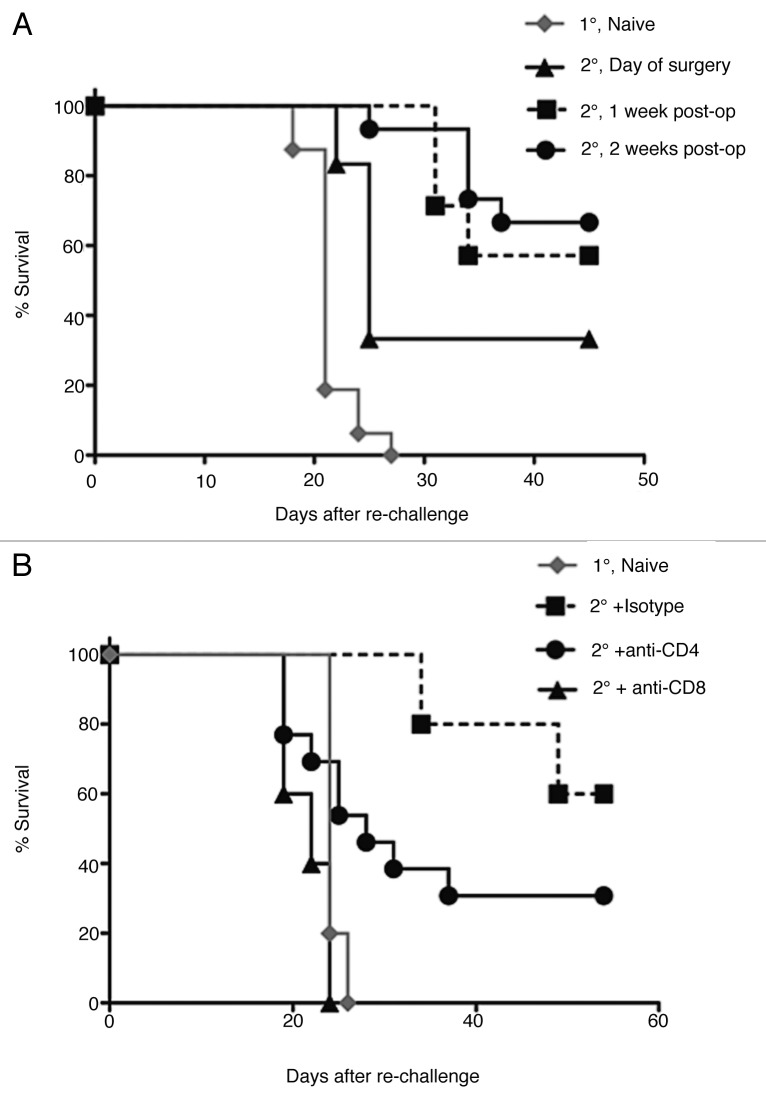
We next wanted to determine how the decreasing levels of antigen presentation affected the development of protective antitumor immunity. To address this, BALB/c mice were curatively resected of primary AB1-HA tumors and then re-challenged with 1 × 106 cells of AB1HA into the opposite flank. Re-challenge inocula were administered either on, either one week after or two weeks after surgery. These time points were chosen based on the levels of tumor antigen cross-presentation: high levels at the day or surgery, low but detectable levels at one week and non-detectable levels at two weeks. Naïve mice were used as a control (Fig. 4A). Significant levels of protection against tumor re-challenge were observed in resected mice at all time points after surgery (p < 0.005 for day of surgery challenge and p < 0.001 for delayed challenge). Interestingly, protective immunity was more pronounced when mice were challenged at 2 weeks after surgery than challenge at the day of surgery (p = 0.05). This suggests that protective parameter(s) increase with time after surgery, coinciding with a decrease in antigen cross-presentation.
Figure 4. Impact of complete tumor resection on antitumor immunity. (A) Post-operative tumor resistance was assessed by re-challenge with AB1HA (2°) into the healthy flank of primary tumor-bearing BALB/c mice (1°) on either the day of their surgery, 1 week post operatively, or 2 weeks after surgery. This corresponded with 3 distinct levels of antigen-presentation (respectively: high, detectable, and absent). Primary tumor growth in naive mice from the same inoculum was used as a control. Kaplan-Meier survival curves shown are generated from multiple experiments, with a minimum of 10 animals in each group. (B) The T-cell dependence of post-surgical antitumor immunity was assessed 14 d post surgery by re-challenge with AB1HA (2°) into the healthy flank in combination with either CD4+ or CD8+ depleting antibodies (starting 1 d prior to re-challenge). Co-treatment with corresponding antibody isotype or challenge in naïve mice (1°) served as controls. Data show Kaplan-Meier survival curves from a single experiment, with 10 animals per group.
To determine the cellular requirements for protection at two weeks post surgery, tumor re-challenge experiments were performed in CD4+ or CD8+ T cell-depleted mice. YTS 169 and GK1.5 mAbs were used to selectively deplete mice of CD8+ and CD4+ cells, respectively. A cohort of control animals was treated with rat IgG2a isotype antibodies. In each instance, monoclonal antibodies were administered one day prior to tumor re-challenge (i.e., 13 d post surgery) and continued for two weeks with doses every 2 d. This treatment resulted in depletion of > 95% of the relevant T-cell subset, as verified by flow cytometric analysis on whole blood samples. Post-operative control mice treated with the isotype control antibodies had resisted tumor re-challenge (60 ± 20.91% cure), consistent with preserved sinecomitant immunity (Fig. 4B). However, CD8+ T-cell depletion led to a complete ablation of protective immunity (Fig. 4B) (p < 0.005 compared with isotype control). In contrast, CD4+ T-cell depletion resulted in a trend toward reduced survival compared with isotype control (p = 0.15) but this was clearly less marked than what was observed in CD8+ T cell-depleted mice. We concluded that CD8+ T cell responses are predominantly responsible for protection after surgery and these responses improve over time.
CD8+ T cell responses after tumor resection: loss of effector phenotype
Given that antitumor CD8+ T cell-mediated immunity improved after surgery, we evaluated the changes in functional tumor-specific CD8+ T cell responses after surgery using an in vivo assay to measure cytotoxic T cell (CTL) activity. For this purpose, HA-peptide-pulsed and non-pulsed CFSE-labeled target cells were injected intravenously, using target cells pulsed with the Kd-restricted epitope from HA expressed by the tumor (Fig. 5A). 2–3% background killing was observed in naive control mice, whereas 40% HA-specific target cell lysis was routinely measured in positive control CL4 TCR transgenic mice (Fig. 5A). In vivo CTL activity was measured before surgery (day 16 tumors), or 24 h, 1 week, or 2 weeks after surgery. Inguinal, axillary, non-draining (e.g.. contralateral inguinal/axillary) and spleens were assessed in each animal. At day 16 post tumor-cell inoculation, robust HA-specific target cell lysis (23.77 ± 2.45%) was observed in the axillary (draining) lymph nodes of tumor bearing mice (Fig. 5B). Consistent with previously reported data,32 only modest endogenous responses (< 10% killing) were seen in other tissues of tumor bearing mice. In vivo CTL activity at the day of surgery was higher in the inguinal nodes (24.00 ± 4.56% post-surgery vs. 6.13 ± 0.35% post-surgery, p = 0.0039) (< 10%) and lower in the axillary nodes (p = 0.0294). At 1 and 2 weeks post surgery, we observed a gradual decrease of in vivo cytolytic function in all analyzed tissues. Thus, increased antitumor memory after surgery is associated with declining levels of in vivo CTL activity and antigen cross-presentation.
Figure 5. Impact of surgery on tumor-specific effector function (in vivo CTL). (A) Gating of target and reference populations for in vivo CTL assay (upper left panel). Remaining panels show typical histogram profiles of in vivo CTL lysis from pre-injection, naïve, and CL4 mice. (B) HA-specific in vivo CTL function in lymphoreticular tissues in tumor-bearing mice before and after surgery. Mean ± SEM percentage killing of HA-pulsed targets shown for each tissue and timepoint (n = 5–10 mice per group). p values derived from student’s t test at the 95% level of significance.
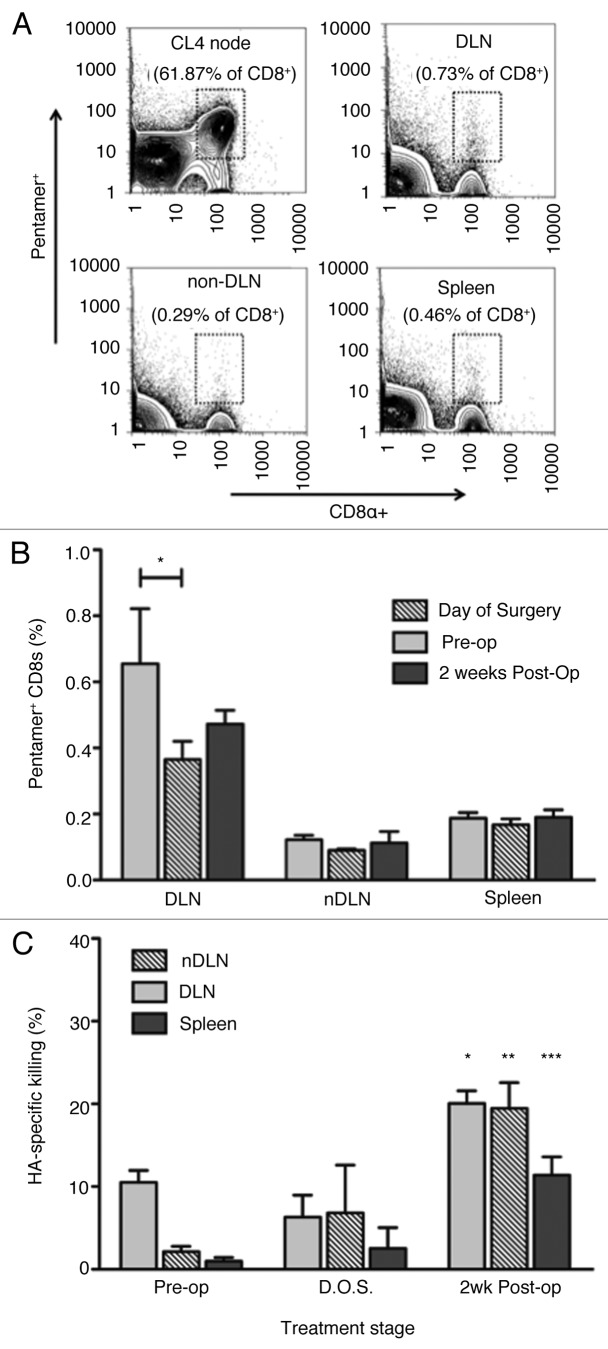
Class I MHC pentamer staining was used to compare in vivo CTL activity with the actual frequencies of HA-specific CD8+ T cells (Fig. 6A). HA-specific CD8+ T cells, detected by pentamer staining, were predominantly present in the tumor draining lymph nodes (Fig. 6B). Interestingly, the frequencies of pentamer+ HA-specific CD8+ T cells were maintained after surgery in the draining lymph nodes, although there was a small but significant drop in their frequency within the draining lymph nodes (Fig. 6B). Low frequencies (< 0.2%) of HA-specific CD8+ T cells were detected in non-draining lymph nodes and in the spleen and no changes were observed after surgery (Fig. 6B).
Figure 6. Effect of surgery on the distribution of tumor-specific T cells and their responsiveness to tumor re-challenge. (A) HA-specific CD8+ cells were identified using IYSTVASSL-MHC pentameric complexes (Pro5® MHC Pentamer, ProImmune). Data show representative (1 of 4 animals) flow cytometry plots from CL4 nodes, axillary nodes, non-draining nodes, and spleen. (B) Proportion (%) of CD8+ cells expressing IYSTVASSL-specific TCR in lymphatic tissues pre and post surgery. Mean ± SEM (n = 4) are shown. *p < 0.05 (student’s t test) comparing levels in pre-op DLN to post-op DLNs. (C) In vivo CTL response in post-surgical animals, four days after re-challenge with AB1-HA. Draining nodes, non-draining nodes, and spleens were processed separately. Experiment was performed once, with a minimum of ten animals per group. Mean HA-specific lysis ± SEM shown for each group. Cohorts compared by two sample student’s t test at the 95% level of significance. *p = 0.0011, **p = 0.0079, ***p = 0.0162.
Tumor recurrence is associated with renewed systemic tumor-specific CTL activity
To address the impact of tumor recurrence (i.e., renewed exposure to tumor antigens) after surgical resection on tumor-specific cytolytic activity, in vivo CTL activity was assessed after tumor re-challenge into the healthy flank. This experiment assesses the capacity of tumor-specific T cells to respond to cognate antigens in vivo and to acquire CTL effector function. It can therefore be interpreted as a surrogate test for immunological memory. Tumor re-challenge was performed at day 16 of primary tumor growth, at the day of surgery and at two weeks after surgery. CTL activity following re-challenge was measured. Four days after tumor re-challenge, HA-peptide-pulsed and non-pulsed CFSE-labeled target cells were administered intravenously to all animals and CTL responses were measured in draining and non-draining lymph nodes as well as in the spleen. Prior to surgery, in vivo CTL activity was mostly detectable in the tumor draining nodes (Fig. 6C). No significant changes (p = 0.14) were observed in mice that were re-challenged immediately after surgery (day of surgery). However, when re-challenge was administered two weeks after surgery, increased levels of HA-specific killing were observed in the draining nodes (p = 0.0011), suggesting that memory T cells were effectively activated. Potent CTL activity (12- 19% killing) was also observed in non-draining nodes and in the spleens of these mice, suggesting that antitumor immunity was systemic at this time point.
Immediate, but not delayed, sentinel node removal impedes tumor resistance after surgery
Given that antitumor protective immunity seemed to increase systemically after surgery whereas initial effector CD8+ T cell responses (in vivo CTL) were constrained to the draining lymph nodes, we hypothesized that the draining or sentinel lymph node could be the staging ground for the development of protective memory responses. As such, it was postulated that sentinel node excision could impair post-operative antitumor immunity. To determine the effect of sentinel node removal on sinecomitant immunity, mice that underwent tumor excision and sentinel node removal, were re-challenged with AB1-HA at two weeks post surgery (i.e., the optimal time point for protection). Under these circumstances, sentinel lymph node removal on the day of tumor surgery led to a dramatic decrease in survival from re-challenge, as compared with mice with intact sentinel nodes (Fig. 7A). Based on the assumption that a systemic tumor-specific CD8+ T cell memory pool had been generated within the two weeks after surgery, we reasoned that resecting the sentinel lymph nodes at 2 weeks post surgery should not impede antitumor immunity. Indeed, survival from re-challenge in mice that underwent sentinel node excision at two weeks after surgery was not significantly different from mice with intact sentinel nodes (Fig. 7B).
Figure 7. Removal of draining lymph nodes at the time of surgery ablates antitumor immunity, while delayed removal preserves it. Survival from re-challenge into the healthy (opposite) flank at two weeks after tumor resection ± sentinel lymph node (LN) removal. BALB/c underwent tumor resection on day 16 after AB1HA inoculation, and re-challenge into the healthy flank at two weeks following tumor removal. Tumor sizes were matched across cohorts and surgical impost was similar. Data were pooled from two separate experiments. At least 10 animals were present in each group. Kaplan-Meier survival shown for each cohort. Significance was tested using the Log Ranks test; *p < 0.05. (A) Survival from re-challenge into the healthy flank after sentinel node removal (axillary and inguinal lymphadenectomy) at the time of tumor resection. (B) Survival from re-challenge into the healthy flank, when sentinel nodes (inguinal and axillary) were removed at two weeks post tumor resection.
Discussion
It is increasingly clear that tumor progression is associated with an active process of immunosuppression and immune evasion. This is clearly illustrated by ongoing immunoediting of the tumor under immune pressure and the demonstrated immunosuppressive roles of Tregs and MDSCa. Current thinking postulates that suppression of antitumor immune responses is driven by the tumor cells themselves and the tumor stroma. Thus, it can be envisaged that tumor resection releases antitumor immune responses from suppression and that these responses could be mobilized by immunotherapy or vaccination. Many questions remain to be answered before such an approach can be translated into the clinic. Two of the most pressing among these questions are: what is the impact of partial vs. complete resection, and, second, what is the impact of surgical removal of the sentinel lymph nodes?
So far, it has been shown that in situ tumor ablation creates a source of antigens and an immune response that is enhanced by immunotherapy.33 Our own previous work using the AB1-HA model of mesothelioma has shown that partial resection promoted antitumor immune memory when surgery was followed by combination chemo/immunotherapy.8 These results were interpreted to indicate that remaining tumor cells after surgery provide a source of antigen after undergoing chemotherapy-induced apoptosis. However, this work left unresolved the question of how the antitumor immune response develops after complete resection, due to a high rate of tumor recurrence after complete resection (75%). In the current study we were able to achieve 0% rates of local recurrence by heeding the tenets of wide local excision and confirmed negative pathologic margins. This enabled us to emulate the clinical setting of complete local resection and examine the host anticancer response in that context.
Consistent with previous work,30 we identified that the HA tumor neo-antigen is constitutively cross-presented during tumor growth. Given that dendritic cells (DCs) are responsible for the cross-presentation of HA neo-antigens in this model,34 the observed decline in tumor antigen presentation was understandable. Presumably, the complete removal of the tumor removed the source of antigens and the duration of post-surgery antigen presentation was then limited by the half-life of remaining antigen-bearing DCs. Moreover, the fact that antigen cross-presentation was not immediately ablated by tumor resection, but instead waned gradually over ~10 d, highlighted the importance of extra-tumor antigen presentation (cross-presentation in the draining nodes). This initial persistence of cross-presentation in the draining nodes after primary resection suggests a possible window of opportunity for locally delivered immune adjuvant therapy into the resection bed.
In addition to the findings on cross-presentation, surgery had a profound impact on endogenous tumor-specific CTL function. While in vivo CTL declined in parallel with cross-presentation after complete resection, recurrent tumors provided a fresh source of antigens that evoked a powerful and systemic CTL response. This correlated with an evolution of protective antitumor immunity after primary resection. We hypothesize that declining antigen levels may facilitate the differentiation of HA-specific effector CD8+ T cells (i.e., the cells responsible for in vivo killing) into memory CD8+ T cells. These cells do not possess direct cytolytic functions and will therefore score negatively in the in vivo CTL assay, explaining the decline of in vivo CTL activity. However, the differentiation of these tumor-specific CD8+ T cells into memory cells leads to increased resistance to tumor re-challenge. In short, the decline in antigen cross-presentation and in vivo CTL activity are both aspects of the same phenomenon: the gradual differentiation of antitumor effector cells into memory cells when the original source of persistent antigen (and suppression) is removed.
Our results suggest a central role for sentinel lymph nodes in this process. Removal of sentinel lymph nodes at the time of surgery had a clear negative impact on the subsequent development of protective memory, whereas delayed lymph node resection did not affect survival. It therefore seems likely that the differentiation from effector into memory cells depends on local lymph nodes. As shown previously and confirmed here, both antigen cross-presentation and in vivo CTL activity are confined to the draining lymph nodes, indicating that these are the likely site of memory differentiation once antigen levels are declining. Thus, surgical removal of the lymph nodes at this early time point would prevent memory differentiation. However, memory CD8+ T cells can exit from the local lymph nodes, and so node excision at the later time point has less impact on the animals ability to resist re-challenge.
One possible theory to explain the present results is that the interruption of tumor-antigen persistence by surgical resection enables the transition of CD8+ T cells to effector or central memory cells, and that this is a requirement to generate antitumor immunity. Evidence that either effector or central memory CD8+ cells give rise to more effective/persistent antitumor responses under therapeutic conditions or after adoptive transfer has been provided.35,36 Why such a transition does not effectively occur during tumor development is not clear. One possibility is that T cells succumb to a form of antigen-induced paralysis, whereby the continuous presence of antigen induces the upregulation of PD-1, as reported during chronic viral infection.37 Another possibility is that the tumor tethers cytotoxic cells to the draining lymph nodes by virtue of a constant antigen stream and tumor-associated chemokine milieu.18 Perhaps this somehow prevents CTL cells from developing into fully competent effector cells. Future experiments tracking specific memory populations under physiologically relevant conditions (by transfer of small numbers of congenically distinct CD8+ cells) are warranted to test these hypotheses.
Primary resection is associated with a wane in tumor antigen cross-presentation over a duration restricted by the DC lifespan. Antitumor immunity emerges reciprocally with the decline in tumor antigen cross-presentation and in vivo CTL function after surgery, and this process is absolutely dependent upon CD8+ lymphocytes and the sentinel lymph nodes. Our findings would be consistent with a release of antitumor CD8+ T cells from chronic antigen exposure, allowing a gradual differentiation toward functional antitumor memory T cells. We conclude that complete primary resection has the potential to boost protective memory and provide a powerful platform for post-operative immuneotherapies. While human tumors feature prolonged antigen exposure and a more established immune suppressive network than their murine counterparts, cancer eradication might be possible in the clinic, with contributions from conventional treatments and multiple modalities of immunotherapy. We anticipate the use of a number of reagents to achieve this goal, including anti-PD1 antibodies and CTL-licensing therapies (such as agonistic anti-CD40 antibodies).
Materials and methods
Animals
BALB/c (H-2d) wild-type and nude mice were purchased from the Animal Resources Centre (Canning Vale, Western Australia) and maintained under specific pathogen free conditions. TCR transgenic mice (CL4 mice) which express a TCR specific for the H-2d-restricted peptide IYSTVASSL (residues 518–526) of A/PR8/8/34 (H1N1) influenza virus hemagglutinin (HA) were generated and screened as described previously.32 All animal experiments used female mice between 6 to 8 weeks of age and was performed in compliance with University of Western Australia Animal Ethics Committee approvals 03/100/434 and 04/100/024.
Tumor cell culture and inoculation
Generation of the BALB/c-derived mouse mesothelioma cell line AB1 and transfection with the gene encoding influenza HA (AB1-HA) has been previously described.32 Cell lines were maintained in RPMI 1640 (Life Technologies, Gaithersburg, MD) supplemented with 20 mM HEPES, 0.05 mM 2-ME, 60 µg/mL penicillin (CSL, Melbourne, Australia), 50 µg/mL gentamicin (West, Bentley, Australia), and 5% FCS (Life Technologies). AB1-HA transfectants were selected by culture in medium containing a neomycin analog (geneticin, Life Technologies) at a final concentration of 400 µg/mL. The level of HA expression on transfected cells was measured by FACS analysis, using the biotinylated HA-specific mAb H18, originally obtained from Dr. Walter Gerhard (The Wistar Institute, Philadelphia, PA). Tumor cells (1x106 in 100 μL of PBS) were injected subcutaneously into the shaven right flank of recipient mice on day 0 and subsequent tumor growth monitored by taking two perpendicular diameter measurements using microcalipers. Surgical debulking of tumors was performed (as outlined below) on day 16 after implantation (when tumors where typically 16 mm2). For re-challenge experiments, mice were inoculated with 1 × 106 tumor cells in the contralateral flank on days 0, 7 and 14 post-surgery and euthanized when tumors reached 100 mm2, which we used as the basis for calculated survival times.
Surgical resection of tumors
BALB/c mice were anesthetized with intraperitoneal 3.6% chloral hydrate (Orion Laboratories, Balcatta Australia) 10 mL/Kg and inhaled methoxyflurane (Medical Developments International Limited, Springvale Australia) as necessary. Resections were performed via elliptical incisions, centered over the subcutaneous tumors. The incisions spanned three times the length of the lesion, with average lateral margins of 3 mm. Skin flaps were elevated to expose the adherent tumor. Once tumors were dissected clear of adjacent fascia, the pedicles were tied with 5/0 vicryl ties (polyglactin 910, Ethicon, North Ryde Australia). Wounds were closed primarily using 5/0 vicryl interrupted sutures (Ethicon). Mice received intraperitoneal 0.05 mg/kg buprenorphine (Reckitt and Coleman, Chiswick, UK) four times daily for postoperative analgesia, as required.
Sentinel/draining lymph node resections
Primary resections were performed via elliptical incisions, centered over the subcutaneous tumors. Adjacent inguinal lymph nodes were formally identified and included in the resection margin (if sentinel lymphadenectomy was to be undertaken concurrently), or preserved in situ (if sentinel nodes were to remain intact). In some instances, sentinel node biopsy was undertaken as a separate procedure. With this technique, inguinal nodes could be exposed by a 10mm longitudinal incision in the caudal flank. Inguinal nodes were sited at the confluence of the inferior epigastric and lateral thoracic veins, and were excised without vascular disruption. Axillary nodes were approached via 5mm mid-axillary oblique incisions. Murine pectoral muscles were retracted, and the axillary vessels were freed from adjacent fat and fascia. Each axillary node was delicately teased from its adherent vessels and delivered. All wounds were closed primarily using 5/0 vicryl (polyglactin 910, Ethicon Australia) interrupted sutures. Mice received intraperitoneal 0.05 mg/Kg buprenorphine (Temgesic, Reckitt and Coleman Chiswick United Kingdom) four times daily as postoperative analgesia, as required.
Assessment of surgical margins using HA-specific RT-PCR
Quantitative RT-PCR (reverse transcriptase PCR) was based on a previously established RT-PCR procedure.30 Completeness of resection was assessed by this technique. Biopsies were removed from three randomly selected cohorts of post-operative animals: on the day of surgery, 24h post-operatively, and 2 weeks after surgery. Biopsies were depleted of protein and lysed in Trizol. Purified RNA was subjected to reverse transcription, followed by amplification using HA-specific primers (5′-CAATTGGGGAAATGTAACATCGCCC-3′ and 5′-AGCTTTGGGTATGAGCCCTCCTTC-3′). For quantitative real-time PCR, PCR Master Mix (Qiagen, Doncaster Australia) and the iCycler RTPCR machine (Bio-Rad) was used. Fluorescence and melt curves from the subject animals were compared with positive (HA tumor) and negative (healthy tissue, sham surgery, water) controls.
Cell depletion studies
CD4+ and CD8+ T cell depletion was performed using purified GK1.5 and YTS.169 monoclonal antibodies, respectively (prepared by Dr Kathy Davern, Monoclonal Antibody Facility, Western Australian Institute for Medical Research). Mice received an initial dose of 200 μg i.v., one day prior to tumor re-challenge (day 13 post-surgery), followed by a second dose of 150 μg administered i.p. on the day of re-challenge (day14) and then 150 μg i.p every second day thereafter for a total of 12 doses. CD4+ and CD8+ depletion (> 95%) was verified during treatment by FACS analysis of peripheral blood using antibodies specific for TCRβ, CD4+, and CD8α.
In vivo antigen presentation
In vivo presentation of the Kd-restricted IYSTVASSL epitope from the HA protein was assessed by measuring proliferation of adoptively transferred HA-specific TCR-transgenic CD8+ T cells through CFSE dye dilution.38 Lymph node cells from T cell receptor transgenic (CL4) mice were labeled with CFSE (Molecular Probes, Eugene USA) and 2x107 cells were injected intravenously into control, tumor bearing, pre-operative or post-operative mice. Animals were assayed at numerous time points: pre-operative days 0, 4, 10, 16, 21 and post-operative days 0, 4, 7, 10, 14. Tumors, draining lymph nodes, non-draining lymph nodes, and spleens were harvested from recipient mice at three days after adoptive transfer. Erythrocytes were lysed with buffered 0.83% NH4Cl. Samples were stained with rat anti-mouse CD8α-PE.Cy5.5 antibody (Caltag, Burlgingame USA) or rat anti-mouse IgG2b- PE.Cy5.5 (Caltag) isotype control. Antigen presentation in each tissue sample was quantified by flow cytometry and expressed as a percentage of CFSE positive cells that had less than 50% of their original dye concentration.
In vivo CTL assay
Detection of HA-specific in vivo CTL activity was performed as described previously.39 Syngeneic splenocytes were divided into two populations, one of which was pulsed with 1 µg/mL of CL4 peptide for 90 min at 37°C. Cells were then labeled with CFSE (10’, RT) using a concentration of 5 µM CFSE for peptide-pulsed cells (CFSE high) and 0.5 µM for unpulsed cells (CFSE low). CFSE high and low cells were washed with FCS and PBS before being pooled in equal proportions and injected i.v. into recipients. After 18 h, draining and non-draining lymph nodes, tumors, and spleens were harvested as above and analyzed by FACS for recovery of CFSE high and low cells. The percentage kill was determined as follows: 100 × [1 – (CFSE high events / CFSE low events)].
Pentamer staining
Endogenous HA-specific CD8+ T cells were identified by fluorescently labeled IYSTVASSL-MHC I pentameric complexes (Pro5® MHC Pentamer, ProImmune, Oxford United Kingdom) in conjunction with CD8+ staining. Samples of axillary, inguinal and non-draining (e.g., mediastinal, mesenteric) lymph nodes and spleens were harvested from naïve, tumor bearing, post-operative, and post-sham surgery BALB/c mice of various time points. Single cell suspensions were stained with the pentamer (10’ RT), washed and surface stained with anti-CD8α-PECy5 (BioLegend), anti-CD44-FITC (BioLegend) and anti-CD127-APC (eBioscience) (20’).
Statistical calculations
The power calculation for all experiments was based on 5 mice per group. The significance level was set at α 0.05 and the calculated difference to generate a power of 0.9 (i.e., 90%) was 24.7%. Statistical analysis was performed using Prism 3 (GraphPad Software, San Diego CA). The Student’s t-test was used to compare data groups and differences were considered significant when p value were < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by funding from the National Health and Medical Research Council of Australia
Footnotes
These authors contributed equally to this manuscript.
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20924
References
- 1.Boutin C, Nussbaum E, Monnet I, Bignon J, Vanderschueren R, Guerin JC, et al. Intrapleural treatment with recombinant gamma-interferon in early stage malignant pleural mesothelioma. Cancer. 1994;74:2460–7. doi: 10.1002/1097-0142(19941101)74:9<2460::AID-CNCR2820740912>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 2.Robinson BW, Mukherjee SA, Davidson A, Morey S, Musk AW, Ramshaw I, et al. Cytokine gene therapy or infusion as treatment for solid human cancer. J Immunother. 1998;21:211–7. doi: 10.1097/00002371-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Powell A, Creaney J, Broomfield S, Van Bruggen I, Robinson B. Recombinant GM-CSF plus autologous tumor cells as a vaccine for patients with mesothelioma. Lung Cancer. 2006;52:189–97. doi: 10.1016/j.lungcan.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Curti BD. Renal cell carcinoma. JAMA. 2004;292:97–100. doi: 10.1001/jama.292.1.97. [DOI] [PubMed] [Google Scholar]
- 5.Fosså SD. Interferon in metastatic renal cell carcinoma. Semin Oncol. 2000;27:187–93. [PubMed] [Google Scholar]
- 6.Figlin RA, Thompson JA, Bukowski RM, Vogelzang NJ, Novick AC, Lange P, et al. Multicenter, randomized, phase III trial of CD8(+) tumor-infiltrating lymphocytes in combination with recombinant interleukin-2 in metastatic renal cell carcinoma. J Clin Oncol. 1999;17:2521–9. doi: 10.1200/JCO.1999.17.8.2521. [DOI] [PubMed] [Google Scholar]
- 7.Mukherjee S, Nelson D, Loh S, van Bruggen I, Palmer LJ, Leong C, et al. The immune anti-tumor effects of GM-CSF and B7-1 gene transfection are enhanced by surgical debulking of tumor. Cancer Gene Ther. 2001;8:580–8. doi: 10.1038/sj.cgt.7700347. [DOI] [PubMed] [Google Scholar]
- 8.Broomfield S, Currie A, van der Most RG, Brown M, van Bruggen I, Robinson BW, et al. Partial, but not complete, tumor-debulking surgery promotes protective antitumor memory when combined with chemotherapy and adjuvant immunotherapy. Cancer Res. 2005;65:7580–4. doi: 10.1158/0008-5472.CAN-05-0328. [DOI] [PubMed] [Google Scholar]
- 9.Repmann R, Goldschmidt AJ, Richter A. Adjuvant therapy of renal cell carcinoma patients with an autologous tumor cell lysate vaccine: a 5-year follow-up analysis. Anticancer Res. 2003;23(2A):969–74. [PubMed] [Google Scholar]
- 10.Repmann R, Wagner S, Richter A. Adjuvant therapy of renal cell carcinoma with active-specific-immunotherapy (ASI) using autologous tumor vaccine. Anticancer Res. 1997;17(4B):2879–82. [PubMed] [Google Scholar]
- 11.Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004;171:1071–6. doi: 10.1097/01.ju.0000110610.61545.ae. [DOI] [PubMed] [Google Scholar]
- 12.Bromwich E, Hendry D, Aitchison M. Cytoreductive nephrectomy: is it a realistic option in patients with renal cancer? BJU Int. 2002;89:523–5. doi: 10.1046/j.1464-410X.2002.02631.x. [DOI] [PubMed] [Google Scholar]
- 13.Flanigan R, Salmon M, Blumenstein B, Bearman S, Roy V, McGrath P, et al. Nephrectomy followed by interferon alfa-2b alone for metastatic renal cell cancer. N Engl J Med. 2001;345:1655–9. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 14.Fallick ML, McDermott DF, LaRock D, Long JP, Atkins MB. Nephrectomy before interleukin-2 therapy for patients with metastatic renal cell carcinoma. J Urol. 1997;158:1691–5. doi: 10.1016/S0022-5347(01)64097-7. [DOI] [PubMed] [Google Scholar]
- 15.Walther M, Yang J, Pass H, Linehan W, Rosenberg S. Cytoreductive surgery before high dose Il-2 based therapy in patients with metastatic renal cancer. J Urol. 1997;158:1675–8. doi: 10.1016/S0022-5347(01)64091-6. [DOI] [PubMed] [Google Scholar]
- 16.Weigel BJ, Rodeberg DA, Krieg AM, Blazar BR. CpG oligodeoxynucleotides potentiate the antitumor effects of chemotherapy or tumor resection in an orthotopic murine model of rhabdomyosarcoma. Clin Cancer Res. 2003;9:3105–14. [PubMed] [Google Scholar]
- 17.Ben-Eliyahu S. The promotion of tumor metastasis by surgery and stress: immunological basis and implications for psychoneuroimmunology. Brain Behav Immun. 2003;17(Suppl 1):S27–36. doi: 10.1016/S0889-1591(02)00063-6. [DOI] [PubMed] [Google Scholar]
- 18.Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66:5527–36. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 19.Morton DL, Ollila DW, Hsueh EC, Essner R, Gupta RK. Cytoreductive surgery and adjuvant immunotherapy: a new management paradigm for metastatic melanoma. CA Cancer J Clin 1999; 49:101-16, 65. [DOI] [PubMed]
- 20.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–45. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 21.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–66. [PubMed] [Google Scholar]
- 22.Benigni F, Zimmermann VS, Hugues S, Caserta S, Basso V, Rivino L, et al. Phenotype and homing of CD4 tumor-specific T cells is modulated by tumor bulk. J Immunol. 2005;175:739–48. doi: 10.4049/jimmunol.175.2.739. [DOI] [PubMed] [Google Scholar]
- 23.Betts G, Godkin A, Gallimore A. Reply: Forkhead box P3-positive regulatory T cells in immune surveillance and cancer. Br J Cancer. 2007;97:1017–8. doi: 10.1038/sj.bjc.6603939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wichmann MW, Lau-Werner U, Müller C, Hornung HM, Stieber P, Schildberg FW, Colorectal Cancer Study Group Carcinoembryonic antigen for the detection of recurrent disease following curative resection of colorectal cancer. Anticancer Res. 2000;20(6D):4953–5. [PubMed] [Google Scholar]
- 25.Lange PH, Ercole CJ, Lightner DJ, Fraley EE, Vessella R. The value of serum prostate specific antigen determinations before and after radical prostatectomy. J Urol. 1989;141:873–9. doi: 10.1016/s0022-5347(17)41037-8. [DOI] [PubMed] [Google Scholar]
- 26.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 27.Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–13. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 28.Stumbles PA, Himbeck R, Frelinger JA, Collins EJ, Lake RA, Robinson BWS. Cutting edge: tumor-specific CTL are constitutively cross-armed in draining lymph nodes and transiently disseminate to mediate tumor regression following systemic CD40 activation. J Immunol. 2004;173:5923–8. doi: 10.4049/jimmunol.173.10.5923. [DOI] [PubMed] [Google Scholar]
- 29.van der Most RG, Currie AJ, Cleaver AL, Salmons J, Nowak AK, Mahendran S, et al. Cyclophosphamide chemotherapy sensitizes tumor cells to TRAIL-dependent CD8 T cell-mediated immune attack resulting in suppression of tumor growth. PLoS One. 2009;4:e6982. doi: 10.1371/journal.pone.0006982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marzo AL, Lake RA, Lo D, Sherman L, McWilliam A, Nelson D, et al. Tumor antigens are constitutively presented in the draining lymph nodes. J Immunol. 1999;162:5838–45. [PubMed] [Google Scholar]
- 31.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–7. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 32.Marzo AL, Lake RA, Robinson BW, Scott B. T-cell receptor transgenic analysis of tumor-specific CD8 and CD4 responses in the eradication of solid tumors. Cancer Res. 1999;59:1071–9. [PubMed] [Google Scholar]
- 33.den Brok MH, Sutmuller RP, van der Voort R, Bennink EJ, Figdor CG, Ruers TJ, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64:4024–9. doi: 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- 34.McDonnell AM, Robinson BW, Currie AJ. Tumor antigen cross-presentation and the dendritic cell: where it all begins? Clin Dev Immunol. 2010;2010:539519. doi: 10.1155/2010/539519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kilinc MO, Rowswell-Turner RB, Gu T, Virtuoso LP, Egilmez NK. Activated CD8+ T-effector/memory cells eliminate CD4+ CD25+ Foxp3+ T-suppressor cells from tumors via FasL mediated apoptosis. J Immunol. 2009;183:7656–60. doi: 10.4049/jimmunol.0902625. [DOI] [PubMed] [Google Scholar]
- 36.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 38.Weston SA, Parish CR. New fluorescent dyes for lymphocyte migration studies. Analysis by flow cytometry and fluorescence microscopy. J Immunol Methods. 1990;133:87–97. doi: 10.1016/0022-1759(90)90322-M. [DOI] [PubMed] [Google Scholar]
- 39.Oehen S, Brduscha-Riem K. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J Immunol. 1998;161:5338–46. [PubMed] [Google Scholar]