Abstract
The versatility and plasticity of myeloid cell polarization/differentiation has turned out to be crucial in health and disease, and has become the subject of intense investigation during the last years. On one hand, myeloid cells provide a critical contribution to tissue homeostasis and repair. On the other hand, myeloid cells not only play an important role as first line defense against pathogens but also they are involved in a broad array of inflammation-related diseases such as cancer. Recent studies show that macrophages can exist in different activation states within the same tumor, underlining their plasticity and heterogeneity. In this review, we will discuss recent evidence on how the tumor microenvironment, as it evolves, shapes the recruitment, function, polarization and differentiation of the myeloid cell compartment, leading to the selection of myeloid cells with immunosuppressive and angiogenic functions that facilitate tumor progression and dissemination.
Keywords: ER stress, exosomes, hypoxia, myeloid cell plasticity, myeloid cells, myeloid-derived suppressor cells, tumor-associated dendritic cells, tumor-associated macrophages, tumor-associated neutrophils
Introduction
The tumor microenvironment consists of neoplastic cells and a heterogeneous group of untransformed cell populations, including fibroblasts, endothelial cells and leukocytes, as well as of soluble factors and the extracellular matrix. Such a complex microenvironment can support tumor growth, protect the tumor from host immunity, foster therapeutic resistance and provide niches for metastasis.1 However, the tumor microenvironment is not static: it is in constant evolution as a result of tissue remodeling, metabolic alterations in tumor cells and changes in the recruitment of stromal cells, including a diversity of immune cells.
Among the leukocytes that infiltrate the tumor microenvironment, myeloid cell populations are predominant. From an immunological point of view, it appears paradoxical that, despite their role as a first line of defense against pathogens, myeloid cells support primary tumor growth and progression,2 and play a crucial, non-redundant role in metastasis by preparing pre-metastatic niches.3 Cancer cell-myeloid cell interactions are very complex, but these cells can use common pathways/mediators that lead to immune regulation and proceed hand-to-hand with angiogenesis.4 This, together with emerging evidence on the plasticity of myeloid cell polarization, opens the door to therapeutic strategies.
In this review, we will give a brief overview on the type and function of tumor-associated myeloid cells and then focus on recent data on how the tumor microenvironment shapes the myeloid cells by instructing their recruitment, function, polarization and differentiation.
Myeloid Cells within the Tumor Microenvironment: A Brief Overview
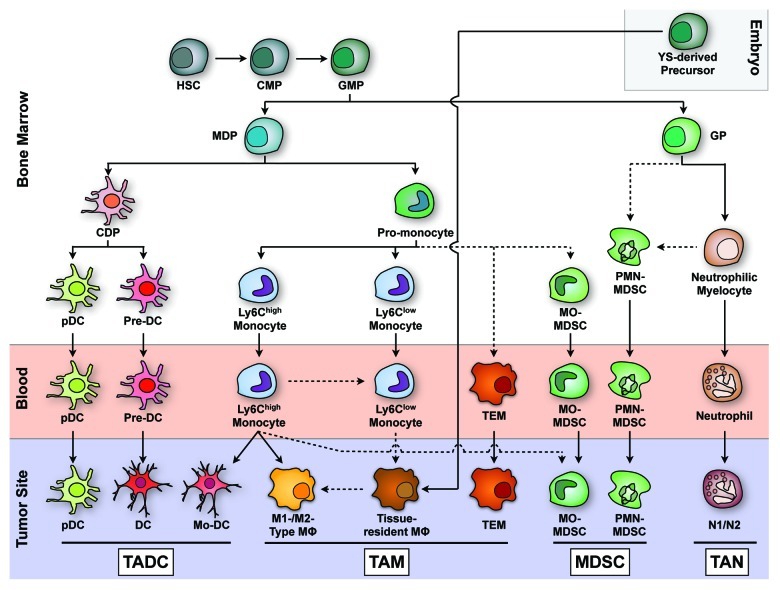
Myeloid cells are the most abundant immune cells within tumors. Intratumoral myeloid cells can be sub-divided in at least four different populations (Fig. 1):
Figure 1. Ontogeny and differentiation of tumor-associated myeloid cells (adapted from Geissmann et al., Science 2010, 327:656 and Fridlender et al., Cancer Cell 2009, 16:183). In the bone marrow, hematopoietic stem cells give rise to common lymphoid (not shown) and common myeloid precursors (CMPs), which in turn give rise to monocyte/macrophage and dendritic cell precursors (MDPs) and granulocyte precursors (GPs). Via a pro-monocyte stage, Ly6Chi and Ly6Clow monocytes are formed and leave the bone marrow to enter the blood. Highly monocyte-related cells include monocytic myeloid derived suppressor cells (MO-MDSCs) and Tie2-expressing monocytes (TEMs). Tissue-resident macrophages are primarily derived from yolk sac (YS) progenitors (Schulz et al., Science 2012, 236:86), although they may be derived from Ly6Clow monocytes when a replenishment of the population is needed. Under inflammatory conditions, Ly6Chi monocytes can become monocyte-derived DCs (Mo-DCs) and inflammatory macrophages, which include M1-like and M2-like cells depending on the tumor microenvironment. In addition, Ly6Chi monocytes may contribute to MO-MDSCs within tumors. Common dendritic cell precursors (CDPs) give rise to plasmacytoid dendritic cells (pDCs) and pre-DCs that become CD11b+-like and CD8+-like DCs in lymphoid tissues. The precise origin and type of TADCs requires further investigation. GPs give rise to neutrophils that in the tumor context can also polarize into an N1- and an N2-type phenotype. Granulocytic MDSCs (PMN-MDSCs) are immature precursors of mature neutrophils with a strong immunosuppressive capacity that, together with the immunosuppressive MO-MDSCs, accumulate in the periphery and in tumors. Dashed arrows represent possible differentiation pathways that require experimental confirmation.
Tumor-associated macrophages (TAMs)
TAMs are the predominant leukocytes infiltrating solid tumors and their presence is associated with poor prognosis.5 They promote angiogenesis and tissue remodeling through the production of multiple factors, including the vascular endothelial growth factor (VEGF), Bv8, and matrix metalloproteinase 9 (MMP9). TAMs also inhibit T-cell responses by the secretion of suppressive cytokines such as interleukin(IL)-10 and transforming growth factor β (TGFβ), high levels of arginase activity and the production of reactive oxygen/nitrogen intermediates.6 Importantly, macrophages are very plastic cells. Exposed to inflammatory cytokines and bacterial components, they become powerful inflammatory and cytotoxic cells, with anti-microbial and tissue-destructive properties (M1-type, classically activated macrophages). However, when exposed to immunosuppressive or Th2 cytokines, glucocorticoids or growth factors such as the colony-stimulating factor 1 (CSF-1), macrophages acquire an alternatively activated phenotype (M2-type) and promote tissue remodeling, repair and angiogenesis.7 This classification in a M1 and a M2 phenotype constitutes an oversimplification, as macrophages occur in a context-dependent continuum of polarization states. Thus, while in early phases of tumorigenesis, the production of M1 inflammatory mediators such as the tumor necrosis factor α (TNFα) and reactive oxygen species (ROS) may support neoplastic transformation,8 M2–type macrophages promote immune escape, tumor growth and malignancy in later stages of disease and an M2 profile correlates with poor prognosis in several carcinomas.9,10 Most recently, differentially activated TAM subsets were reported to co-exist in several transplantable mouse tumors, residing in different tumor regions and performing distinct functions.11,12 These TAM subsets may include another specialized population, Tie2-expressing macrophages (TEMs), which perform a non-redundant role in angiogenesis.13,14
Myeloid-derived suppressor cells (MDSCs)
MDSCs constitute a heterogeneous population of myeloid cells sharing an immature state and the ability to suppress T-cell responses. MDSCs have been abundantly observed in cancer, both in mice and in humans, accumulating within primary and metastatic tumors, as well as in the bone marrow, spleen and circulation.15,16 Two main MDSC subpopulations have been characterized in mice: monocytic MO-MDSCs (CD11b+Ly6G-Ly6Chi) and granulocytic PMN-MDSCs (CD11b+Ly6G+Ly6Clow).17,18 Their equivalents in humans have also been described.19 These two cell populations depend on different factors for their expansion/survival and exert immunosuppressive functions via different mechanisms. Thus, MO- but not PMN-MDSCs are expanded by the granulocyte macrophage colony-stimulating factor (GM-CSF).20,21 MO-MDSCs have been reported to be more immunosuppressive than PMN-MDSCs on a per cell basis,17,20 mainly owing to an elevated activity of the inducible NO synthase (iNOS)17 and via mechanisms that are contact-dependent but non-antigen-specific.22 In contrast, PMN-MDSCs suppress antigen-specific responses in a ROS-dependent manner.23 Finally, the distribution of MO- and PMN-MDSC within tumors and in the periphery is different; while PMN-MDSCs are most abundant in the blood, spleen and bone marrow, MO-MDSCs are enriched within the majority of tumors.18 This may be accounted for by differential recruitment mechanisms or intratumoral expansion, as discussed below.
Tumor-associated dendritic cells (TADCs)
Dendritic cells (DCs) are differentiated myeloid cells that specialize in antigen processing and presentation to naïve T cells. Human and mouse CD11c+ DC subsets can be organized into four broad subsets based on shared phenotypic markers and functional specialization and irrespective of their primary location in secondary lymphoid organs or in the parenchyma of non-lymphoid organs: (1) CD11b+ DC-like cells, (2) CD8α+ DC-like cells (3) CD11b+Ly6C+ monocyte-derived DCs and (4) SiglecH+ plasmacytoid DCs (pDCs).24 The main characteristic of DCs is their ability to mature in response to stimuli such as pathogen- or danger-associated molecular patterns. Like macrophages, classically activated DCs are immunogenic, owing to the upregulation of co-stimulatory molecules and cytokines such as IL-12. Conversely, “alternatively” activated or semi-mature DCs induce T-cell tolerance via deletion, anergy or induction of regulatory T cells (Tregs).25 Also this dichotomy appears as an oversimplification, and it is likely that TADCs exist in a multitude of functional states, and may be conditioned by the tumor to maintain immune tolerance or immunosuppression.26 Indeed, a recent study in a mouse model of breast cancer showed that DCs at the tumor margin present tumor antigens and stably engage tumor-specific T cells but do not support full activation.27 TADCs may play a pro-tumor role by inducing tumor-specific T-cell tolerance via the upregulation of inhibitory molecules such as B7-H1,28 or by the activation of arginase,29 oxygen-dependent pathways that downregulate CD330 or indoleamine 2,3-dioxygenase (IDO).31 However, infiltration of tumors by DCs has been associated with good prognosis in several cancers,32 in particular at early stages.33 It is likely that the pro- vs. anti-tumor role of TADCs depends on the stage of tumor growth.34
Tumor-associated neutrophils (TANs)
Neutrophils are the most abundant granulocyte type and are specialized in engulfing and destructing bacteria. Normally, they are only released from the bone marrow when fully mature but, during inflammation, immature precursors (myelocytes and promyelocytes) can also be detected in the circulation.35 Recent evidence has linked neutrophils to angiogenesis and metastasis36 and, again, the existence of anti-tumor N1-type and pro-tumor N2-type neutrophils has been proposed.37
Different myeloid cells such as MDSCs, DCs, neutrophils and macrophages possess both angiogenic and immunosuppressive capacities and play a crucial role in maintaining tissue homeostasis by eliminating dying cells and mediating tissue remodeling. It has therefore been proposed that tumors co-opt the homeostatic tissue repair program, promoting concurrent pathological angiogenesis and immunosuppression at the tumor site.4 The mechanisms underlying the immunosuppressive and angiogenic capacities of the different types of tumor-associated myeloid cells have been extensively described in recent reviews38-40 and will not be further discussed here.
How the Tumor Shapes Infiltrating Myeloid Cell Populations
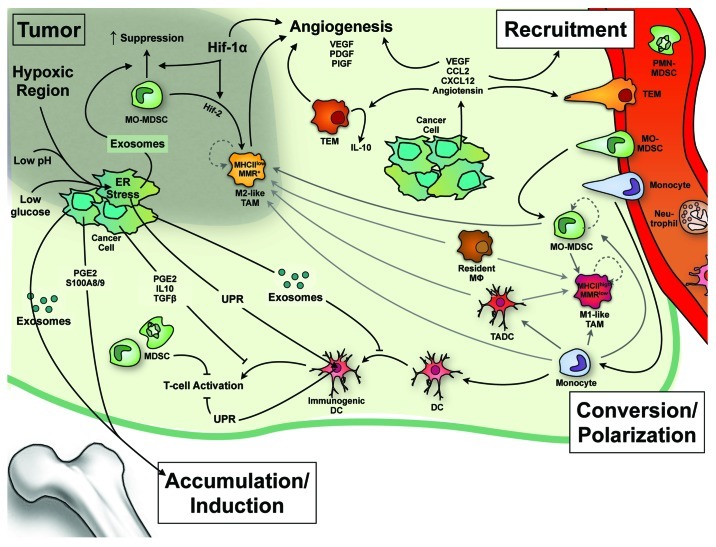
Both malignant and stromal components of tumors are influenced by the tumor microenvironment. Notably, cells within the tumor are often confronted to low levels of glucose, high levels of reductive metabolites, low pH and extreme hypoxia. This results in endoplasmic reticulum (ER) stress as well as in the release of multiple factors by cancer cells, in a vesicle-associated or soluble form. It is becoming clear that all these factors play a role in shaping the myeloid cell compartment and it is likely that during tumor progression there is a selection of specific phenotypes of tumor-associated myeloid cells that co-evolves with the tumor (Fig. 2).
Figure 2. Effects of the tumor on the induction, recruitment and differentiation of myeloid cells. Hypoxia, low pH and glucose deprivation induce an endoplasmic reticulum (ER) stress that leads to the unfolded protein response (UPR) and transcription of pro-inflammatory factors by neoplastic cells. An “infectious” UPR spreading to immune cells can alter their antigen-processing capacity and increase their production of inflammatory factors. Hypoxia also enhances angiogenesis via the induction of vascular endothelial growth factor (VEGF) and, together with tumor-derived exosomes, can increase the immunosuppressive activity of monocytic monocytic myeloid derived suppressor cells (MO-MDSCs), block their differentiation into dendritic cells (DCs) and enhance their conversion into MHC class IIlow MMRhigh M-2 type macrophages, which concentrate in hypoxic areas. Soluble and vesicle-associated factors produced by the neoplastic cell induce MDSC production/accumulation in bone marrow and periphery, and their recruitment to the tumor site. The possible interconversion between the different types of myeloid cells within the tumor, as well as their self-renewal capacity in situ (dashed arrows) are crucial questions that require further investigation and are thus depicted in gray. MMR, macrophage mannose receptor; PDGF, platelet growth factor; PIGF, placental growth factor; PGE2, prostaglandin E2.
Hypoxia
Hypoxia (0.1–3% O2) is a prominent feature of solid tumors as a result of defective vascularization and intense metabolic activity. Cells adapt to O2 shortage by upregulating and stabilizing the transcription factor hypoxia-inducible factor 1α (HIF-1α). HIF-1α transactivates a broad array of genes, including genes that are involved in diverting aerobic to anaerobic ATP production as well as pro-angiogenic genes such as VEGF.41
Hypoxic cancer cells may secrete CXCL12 and IL-8, which have been shown to induce the recruitment of myeloid cells to glioblastomas42 and to disorient DC migration.43 The IL-8-mediated recruitment of neutrophils to hypoxic regions results in inhibition of constitutive neutrophil apoptosis,44 and their clearance by macrophages. Importantly, macrophages exposed to levels of hypoxia similar to those found in tumors upregulate HIF-1α, in turn enhancing the expression of tumor-promoting genes like IL-1β, IL-8, CXCR4 and angiopoietin-2, thus inducing an M2-like phenotype.45,46 In line with these observations, our group has shown in several transplantable mouse carcinomas that TAMs associated with hypoxic regions are more M2-like and express higher levels of the macrophage mannose receptor (MMR) and monocyte chemoattractants (CCL2, CCL8), while TAMs with an M1-like phenotype are localized in normoxic tumor areas.11,12 In view of these data, it is interesting to note that HIF-2α, another hypoxia-inducible factor that upregulates arginase 1 and is restricted to certain cell types including macrophages, appears to be induced upon M2 polarization.47 Furthermore, immunohistological studies demonstrate high levels of HIF-2 expression by TAMs in different human cancers48 and myeloid HIF-2-deficient mice display reduced TAM infiltration and tumor progression in dextran sulfate sodium (DSS)-induced colon carcinomas,49 suggesting the existence of a hypoxia-HIF-2α-M2 polarization axis. Taken together, these data suggest that a collaboration between HIF-1 and HIF-2 might maximize the hypoxic response in TAMs.
Similar to TAMs, MDSC functions are altered in hypoxic conditions. In MDSCs, hypoxia upregulates RGS2, a factor that has been shown to promote CCL2 production and angiogenesis in distinct transplantable mouse tumor models.50 Moreover, HIF-1α stimulates the suppressive potential of MDSCs in the tumor microenvironment.51
Finally, the effects of hypoxia on DCs remain less characterized, although low O2 levels have been shown to modulate immune regulatory receptors, to promote the secretion of high levels of inflammatory cytokines and to reduce migration to draining lymph nodes.52
Tumor ER stress
The pathophysiological conditions unique to the tumor microenvironment, such as hypoxia, low extracellular pH and glucose deprivation, initiate stress signals that converge to the ER, resulting in the accumulation of un/misfolded proteins in the ER lumen and hence in an ER stress. This activates intracellular signaling pathways that are collectively known as the unfolded protein response (UPR), which facilitates the cellular adaptation to ER stress.53 The UPR activates pro-inflammatory cascades via NFκΒ and JNK-AP1 activation, resulting in the production of potentially tumorigenic cytokines such as IL-6, IL-23 and TNFα. The functional link between the UPR and tumorigenesis was suggested by experiments in which the silencing of Grp-78, a chaperone involved in the UPR, in fibrosarcoma cells inhibited tumor growth in vivo.54 The tumor UPR may also affect host immune function in a cell-extrinsic manner. Indeed, culturing macrophages in conditioned medium from mouse carcinoma cell lines experiencing ER stress resulted in the upregulation of the UPR signaling by macrophages and the production of pro-inflammatory molecules.55 In addition, antigen-presenting cells (APCs) experiencing an ER stress undergo a remodeling in the antigen processing machinery, resulting in decreased presentation of immunodominant peptides and hence decreased priming of tumor-specific T cells.53 Although the precise factors responsible for this “transmissible” ER stress have yet to be identified, this effect has been shown to partly depend on Toll-like receptor 4 (TLR4) signaling.55 Importantly, the cross-talk between the UPR and inflammatory pathways is bidirectional, as it has been observed that inflammatory cytokines and reactive cell metabolites, such as ROS, can themselves induce the UPR.56
Exosomes
Exosomes are nanometer-sized membranous vesicles that develop from the exophytic budding of the cellular membrane and contain proteins, peptides, microRNAs, mRNAs and lipids.57 Tumor-derived exosomes (TDEs) can be isolated from tumors and body fluids from cancer patients and are believed to play an important role not only in the removal of therapeutic drugs but also in autocrine and paracrine signaling.58 In particular, growing evidence shows that molecules associated with TDEs, such as FASL, PD1, MMPs, can modulate the immune system. Thus, TDEs not only mediate signal transduction without need for direct cell-cell contact but also can fuse with target cells, leading to the acquisition of novel molecules and the delivery of bioactive mRNA and miRNA.59 With respect to myeloid cells, it has been shown that blood-derived exosomes from melanoma patients promote the generation of MDSCs and impair differentiation of DCs from peripheral blood monocytes60 or the bone marrow, resulting in an accumulation of immature myeloid precursors in the spleen.61 Hence, injection of breast carcinoma-derived exosomes in mice promotes the generation of MDSCs, which is dependent on exosome-associated prostaglandin E2 (PGE2) and TGFβ.62 Furthermore, exosomes from several independent mouse tumor cell lines trigger MDSC suppressive function in an HSP72/TLR2 dependent manner.63 At the level of macrophages, exosome-associated fibronectin induces IL-1β production64 and microvesicles released by late stage mouse melanoma cells were found to exert a dose-dependent suppression of MHC Class II expression in these cells.65 Recently, it has been proposed that miRNAs from tumor exosomes may also play a role in mobilizing myeloid cells to pre-metastatic niches.3 Conversely, fibrosarcoma-derived exosomes may contribute to the efficient DC-mediated priming of anti-tumor T-cell responses in vivo.66
Tumor-derived soluble factors
Multiple factors secreted by neoplastic cells have been shown to be involved in the generation, recruitment and/or activation of myeloid cells, notably MDSCs. Thus, VEGF, GM-CSF, Bv8, CXCL12 and S100A8/9 all can increase the burden of intratumoral MDSCs.16 S100A8/9 can also promote MDSC accumulation indirectly, by impairing DC maturation and enhancing inflammation.67 Moreover, it has been reported that DCs exposed to tumor-derived factors polarize into regulatory DCs that suppress the proliferation of pre-activated T cells and are different from immature DCs.68
PGE2, a key factor secreted by many tumor cells, has multiple effects on the recruitment and polarization of myeloid cells: it blocks the expression of CCR5 in macrophages, suppresses tumor production of CCL5 and other pro-inflammatory chemokines, and enhances the secretion of CXCL12 and CCL22, which recruit MDSCs and Tregs, respectively.69 PGE2 also upregulates IL-1070 and arginase 171 production by macrophages and participates in the induction of HIF-1α in MDSCs.51 Importantly, PGE2 has been shown to inhibit the early stages of DC development, and DC matured in the presence of PGE2 display a defect in the priming of cytotoxic T lymphocytes (CTLs), natural killer (NK) cells and Th1 immunity while promoting Th2 responses.69
Obviously, other tumor-derived soluble factors may also play a role in myeloid cell polarization. Known examples are immunosuppressive cytokines such as TGFβ72 and IL-10.73 Additionally, necrotic mouse hepatocarcinoma cells have been shown to release hitherto uncharacterized TLR4 ligands that stimulate Gr-1+CD11b+F4/80+ cells to induce the apoptotic demise of activated T cells via the induction of high levels of arginase 1 and IL-10.74 Angiopoietin 2, which is produced by endothelial cells in the tumor, can also stimulate the production of IL-10 by TEMs, leading to enhanced T-cell suppression and Treg induction.75 As a matter of fact, TEMs express enhanced levels of M2-type genes as compared with Tie2neg TAMs, in line with a healing/angiogenic type of activation/differentiation.76 Finally, IL-4 produced by PyMT mouse mammary carcinoma-infiltrating CD4+ T cells may also promote the pro-tumor properties of macrophages,77 perhaps by enhancing cathepsin activity.78 Hence, it is clear that factors from distinct sources in the tumor microenvironment, cancer cells, endothelial cells, tumor-infiltrating lymphocytes, contribute to shaping the TAM phenotype.
The Dynamics of Myeloid Cell Populations in the Tumor: Open Questions On Their Recruitment and Their Plasticity
It is clear that a constantly active interaction between cancer cells and myeloid cells exists. However, the precise nature and dynamics of this interaction is not completely understood, and we would like to discuss two open questions concerning the effect of the tumor microenvironment on the evolution of the myeloid compartment throughout tumor progression. Addressing these questions will help to develop future strategies to block tumor progression and metastasis.
Selective recruitment vs. in situ expansion
In established solid tumors of various origin, myeloid cells can account for a significant percentage of infiltrating cells (the exact % depends on tumor type and stage), where they play a crucial role in suppressing anti-tumor immune responses and promoting angiogenesis. One obvious question arises from this observation: why are there so many myeloid cells in the tumor - are they selectively recruited, and/or do they expand locally?
Recruitment of myeloid cells to the tumor
Myeloid cells are actively recruited to the tumor microenvironment from the bloodstream, and the process starts early after the initiation of the transforming program.79 Tumor-derived angiogenic factors such as VEGF, plateled-derived growth factor (PDGF) and CSF-1, as well as pro-inflammatory molecules, like S100A8/9 proteins play a role in monocyte recruitment (Box 1).16 Angiopoietin 2 has been reported to mediate TEM infiltration in several mouse tumors,75 although its role might be more to orient TEMs near the blood vessels rather than recruiting them to the tumor site.80 However, the main players in myeloid cell recruitment to the tumor are chemokines, produced by both cancer and stromal cells. Of note, oncogenic changes can lead to the de novo production of inflammatory chemokines that attract myeloid cells.81 A variety of chemokines and chemokine receptors have been reported to attract neutrophils (mainly CXCL8) and DCs, including CCL20, CCL17, CCR5 and CCR6.82 However, most of the available information concerns the mediators of monocyte/macrophage infiltration into tumors. CCL2 induces the migration and activation of monocytes through its only reported receptor, CCR2.83 CCL2 is involved in the recruitment of macrophages and their expression of MMP-9 in several tumor types84 and, as recently shown, in the recruitment of inflammatory monocytes to pulmonary metastasis formed by PyMT mouse breast carcinomas (Box 2).85 Remarkably, CCL2 may differentially influence the mobilization and accumulation of distinct MDSC subsets, since CCR2 deficiency caused a significant loss of CD11b+Gr-1loLy-6Chi MO-MDSCs with a preponderance of infiltrating CD11b+Gr-1hiLy-6Cint PMN-MDSCs in Lewis Lung carcinoma (LLC) primary lesions.86 This would explain why, in a CCR2-deficient mouse model of cervical carcinogenesis, tumor progression was barely impaired despite lower monocyte/macrophage infiltration, due to a rise in infiltrating MMP-9+ neutrophils.87 Importantly, a recent study using the PyMT model of spontaneous breast cancer has shown that chemotherapy leads to stromal CCL2 expression and acute recruitment of CCR2-expressing monocytic cells to regions of necrotic cell death, which contribute to tumor re-growth after treatment.88
Box 1. Tumor-associated factors involved in the recruitment of myeloid cells.
• CCL2: recruitment of monocytic MDSCs or monocytes to primary tumors11,86,87and pulmonary metastases85
• VEGF: recruitment of monocytes to primary tumors110
• Angiopoietin-2: recruitment of Tie2-expressing monocytes75
• CXCL12: recruitment of MDSCs111
• S100A8 and S100A9: recruitment and activation of MDSCs112
• Complement byproduct C5a: recruitment of granulocytic MDSCs113
• CSF-1: recruitment of MO-MDSCs and TAMs114
• PGE2 and LTB4 (two eicosanoids): recruitment of DCs, macrophages and neutrophils115
Box 2. Monocyte subset recruitment to tumors: model-specific differences.
It should be realized—given the complexity and heterogeneity of cancer as a disease, and given the limitations inherent to any mouse tumor model—that model-specific and tumor type-specific mechanistic differences are to be expected. One example is the recruitment of distinct monocyte subsets to the primary tumor, and the involvement of the CCL2-CCR2 axis.
Genetic models of carcinogenesis and tumor progression
• MMTV-PyMT transgenic model of mammary carcinogenesis: preferential recruitment of Ly6Clow monocytes to the primary tumor, preferential CCR2-dependent recruitment of Ly6Chigh monocytes to pulmonary metastases.85
• K14-HPV/E2 transgenic model of cervical carcinogenesis: CCR2-dependent recruitment of monocytes to the primary tumor.87
• KrasLSL/G12D/+/Tp53fl/fl conditional genetic mouse model of lung adenocarcinoma: CCR2-dependent recruitment of monocytes from the spleen to the primary tumor.116
Transplantable tumor models
• TS/A mammary carcinoma: preferential recruitment of Ly6Chigh monocytes to the primary tumor.11
• Lewis Lung Carcinoma (LLC): CCR2-dependent recruitment of monocytes to the primary tumor.12,86
• ID8 ovarian carcinoma: early dominant CCR2-dependent recruitment of Ly6Chigh CX3CR1low monocytes to the primary tumor, followed by a dominance of Ly6Clow CX3CR1high cells.117
• TRAMP-C1 prostate cancer: CCR2-dependent recruitment of CX3CR1high cells (Ly6Clow monocytes?) to the primary tumor.118
CXCR4 and its ligand, CXCL12, also play a role in the recruitment of myeloid cells to the primary tumor. For example, in ascites from ovarian cancer patients, tumor-derived PGE2 induces CXCL12 production in the tumor microenvironment and CXCR4 expression by MDSCs, resulting in MDSC accumulation.89 HIF-1α contributes to the production of CXCL12 by glioblastoma cells, which in turn promotes tumor progression by recruiting MMP9+ bone marrow cells.42
Besides the type and level of chemokines at the tumor site, additional mechanisms could account for the predominance of suppressive myeloid cells in the tumor. For example, it has recently been demonstrated that the intratumoral production of reactive nitrogen species (RNS) induces nitration/nitrosylation of CCL2 in different human and mouse cancers. As a result, modified CCL2 can no longer attract tumor-specific CTLs, but still is able to recruit suppressive myeloid cells to the tumor.90 Selective myeloid cell trafficking could also be explained by the induction of adhesion molecules such as CD31 or CD99.91
Expansion in situ
Although most myeloid lineage cells are bone marrow (BM)-derived under steady-state conditions, extramedullary myelopoiesis occurs under chronic inflammatory conditions, including cancer.92 Indeed, MDSCs, and in particular MO-MDSCs, have been shown to actively proliferate in the spleen of tumor-bearing mice.93 Furthermore, tumor-derived GM-CSF is critical for the expansion and suppressive function of MO-MDSC, although this expansion occurs primarily in the bone marrow.21 Interestingly, recent data have demonstrated the proliferation of terminally differentiated macrophages in tissues under conditions of sustained IL-4 stimulation.94 Self-renewal of mature macrophages can be triggered by a deficiency in the MafB and c-Maf transcription factors.95 It is therefore tempting to speculate that IL-4 or other M2-inducing stimuli may affect MafB/c-Maf function. However, data on MafB/c-Maf activity in TAMs are lacking and only a few studies have addressed the proliferation of myeloid cells within tumors. One such study, using in vivo BrdU labeling experiments, revealed low proliferation rates for CD11b+ cells in the periphery of LCC-bearing mice, as compared with the bone marrow.86 Interestingly, parabiotic experiments in the same model demonstrated that the tumor microenvironment may support monocyte/macrophage survival, rather than their proliferation.86 Employing a transplantable breast carcinoma model, we also found no evidence for TAM proliferation upon cell cycle analysis.11 However, future studies should generate more complete data sets in multiple tumor types, especially in those tumors where the TAM phenotype is regulated by IL-4.77,78
Plasticity and gradient of polarization/maturation/differentiation of myeloid cell populations in the same tumor
It is possible that tumor-derived factors recruit immature myeloid cells and precursors from the periphery, which in the tumor microenvironment become immunosuppressive and pro-angiogenic. Alternatively, considering the plasticity of myeloid cells, it may be equally probable that mature differentiated myeloid cells, either tissue-recruited or tissue-resident, are “edited” by the tumor to acquire a suppressive and pro-angiogenic phenotype. In this respect, distinct tumor regions, for example, hypoxic vs. normoxic areas, may imprint different characteristics onto resident myeloid cells, generating a sizeable myeloid cell heterogeneity within the same tumor. In any case, the possibility that the pro-tumor phenotype of the different tumor-associated myeloid cells reflects a reversible functional state, rather than a terminal and irreversible differentiation, is worth addressing, since this would open the door to pharmacological manipulation strategies. Consequently, much attention is directed toward understanding the mechanisms and molecules driving the pro-tumor polarization of myeloid cells within the tumor itself. However, the main driving forces behind TAM heterogeneity and the possibility of interconversion between different myeloid cell populations at the tumor site remain an open question.
TAM heterogeneity
Within the same tumor, different populations of macrophages that evolve along with tumor progression can be found at different locations, and have been predicted to perform different functions.96 Using MHC Class II and MMR as discriminative markers between distinct TAM subsets, we found that MHC Class IIlowMMRhigh TAMs are more M2-like, tend to associate with hypoxic regions and are consequently more pro-angiogenic in several transplantable tumor models.11,12 However, thus far, the main environmental stimuli driving this TAM heterogeneity are unknown. Importantly, the clinical significance of these findings is evidenced by the presence of MHC Class IIhigh and MHC Class IIlow TAM subsets in different regions of human hepatocellular carcinomas, the latter of which are IL-10+, suggestive of a more M2-like phenotype.97 Moreover, in a murine hepatocellular carcinoma model, a MHC Class IIhigh TAM population appeared during the early phases of tumor growth and was associated with tumor suppression, whereas MHC IIlow TAMs became predominant as tumor progressed.98
Besides MHC Class II expression, the migratory capacity has been used as parameter that discriminates TAM subsets. In the PyMT model of mammary carcinoma, CD68+MMR- monocytes/macrophages are migratory in the tumor-stroma border but not deeper within the tumor mass, further highlighting the existence of distinct intratumoral regions and their modulatory effect on myeloid cells.99 In addition, TAMs that co-migrate with cancer cells in an in vivo migration assay have been shown to express lower levels of M2-associated markers than their sessile counterparts.100
Hence, TAM heterogeneity seems to be supported by recent experimental data, but a main future challenge will be to understand how this heterogeneity is regulated at the molecular level and how it can be exploited therapeutically. In this respect, a better understanding of temporal (e.g., cycling tumor hypoxia)101 and regional differences in the tumor microenvironment will be mandatory.
MΟ-MDSC-to-TAM conversion
Ly6ChiCCR2+ monocytes were reported to be the most prominent tumor-infiltrating monocyte population in several transplantable tumor models, giving rise to mature TAM subsets in situ.11,12 Accordingly, CCR2-deficiency resulted in a severely reduced presence of macrophages in transplantable tumors and in a transgenic model of cervical carcinogenesis,86,87 suggestive of a CCR2+ monocyte precursor. However, primary mammary tumors in transgenic PyMT mice contain more resident than inflammatory monocytes, while CCR2-mediated recruitment of inflammatory monocytes mainly occurs within lung metastases.85 Of note, the surface marker profile of inflammatory monocytes strongly resembles that of MO-MDSC (Ly6ChiCCR2+), and discriminating between these cells is difficult.
Splenic MO-MDSCs can give rise to strongly immunosuppressive and M2-oriented macrophages in vitro,102,103 and within the tumor MO-MDSCs rapidly differentiate into macrophages when exposed to hypoxia, in a HIF-1α-dependent way.51 Tumor-derived factors such as HSP27, a heat shock protein that is highly expressed in human breast cancer cells, can cause the differentiation of monocytes, and probably MO-MDSCs, to macrophages that are strongly pro-angiogenic and display an immunotolerizing phenotype that induces severe unresponsiveness in T cells.104 However, MO-MDSCs can also differentiate into macrophages exerting tumoricidal activity and secreting Th1 cytokines when stimulated via TLR9 with CpG ODN,105 illustrating the versatility of these cells.
TADC-to-TAM conversion
Both DC precursors and monocytes infiltrate tumors and can potentially give rise to DC subsets. However, the tumor microenvironment can inhibit monocyte differentiation into immunogenic DCs by a variety of mechanisms. For example, interaction between Mac-2BP (on colorectal carcinoma cells) and DC-SIGN (on DCs) significantly inhibited DC functional maturation.106 Moreover, tumor-associated cytokines such as IL-10 and IL-6 were shown to inhibit the differentiation of monocytes to DC, while promoting their maturation to macrophages.107,108
As with macrophages, DCs may also exist in different functional states that evolve with tumor progression. Thus, in an ovarian cancer model, DCs within the tumor were shown to evolve from being immunogenic at early stages to being immunosuppressive (MHC IIlow CD40low PDL1high) later on, a switch that depended on tumor-derived TGFβ and PGE2.34 Intriguingly, immunostimulatory DCs can generate a progeny with morphologic, phenotypic and functional characteristics of regulatory macrophages that suppress T-cell responses through NO, arginase and IL-10.109 This conversion seems to occur preferentially in the tumor microenvironment (at least in LLC and B16 tumors) and is mediated by a relative lack in GM-CSF.
Hence, at the tumor site, potent microenvironmental conditions seem to exist that strongly favor macrophage differentiation from distinct precursors, including DCs. These data may explain the dominance of TAMs within the tumor-associated myeloid compartment and further corroborate the importance of TAMs for tumor progression.
Concluding Remarks
The crosstalk between cancer cells and myeloid cells is complex and dynamic. However, common mechanisms, cellular players and factors underlie angiogenesis and immune suppression, thereby opening the door to therapeutic intervention. Further efforts are needed to fully understand the plasticity of tumor-associated myeloid cells, not only in terms of their activation and differentiation state, but also in terms of interconversion. This emerging field holds the promise of identifying novel strategies aimed at manipulating the phenotype of these tumor-promoting cells.
Glossary
Abbreviations:
- cDC
common DC precursor
- CMP
common myeloid precursor
- DC
dendritic cell
- GMP
granulocyte-monocyte precursor
- GP
granulocyte precursor
- HSC
hematopoietic stem cell
- Mϕ
macrophage
- MDP
monocyte/macrophage and DC precursor
- MDSC
myeloid-derived suppressor cell
- Mo-DC
monocytic DC
- pDC
plasmacytoid DC
- TADC
tumor-associated DC
- TAM
tumor-associated macrophage
- TAN
tumor-associated neutrophil
- TEM
Tie2-expressing macrophages
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21566
References
- 1.Swartz MA, Iida N, Roberts EW, Sangaletti S, Wong MH, Yull FE, et al. Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res. 2012;72:2473–80. doi: 10.1158/0008-5472.CAN-12-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–7. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. 2011;21:139–46. doi: 10.1016/j.semcancer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011;11:702–11. doi: 10.1038/nri3064. [DOI] [PubMed] [Google Scholar]
- 5.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–65. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 6.Laoui D, Van Overmeire E, Movahedi K, Van den Bossche J, Schouppe E, Mommer C, et al. Mononuclear phagocyte heterogeneity in cancer: different subsets and activation states reaching out at the tumor site. Immunobiology. 2011;216:1192–202. doi: 10.1016/j.imbio.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–66. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 10.Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, et al. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res. 2011;167:e211–9. doi: 10.1016/j.jss.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–39. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 12.Movahedi K, Schoonooghe S, Laoui D, Houbracken I, Waelput W, Breckpot K, et al. Nanobody-based targeting of the Macrophage Mannose Receptor for effective in vivo imaging of tumor-associated macrophages. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-11-2994. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Coffelt SB, Tal AO, Scholz A, De Palma M, Patel S, Urbich C, et al. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res. 2010;70:5270–80. doi: 10.1158/0008-5472.CAN-10-0012. [DOI] [PubMed] [Google Scholar]
- 14.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–26. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–44. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 18.Youn J-I, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filipazzi P, Huber V, Rivoltini L. Phenotype, function and clinical implications of myeloid-derived suppressor cells in cancer patients. Cancer Immunol Immunother. 2012;61:255–63. doi: 10.1007/s00262-011-1161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 21.Lesokhin AM, Hohl TM, Kitano S, Cortez C, Hirschhorn-Cymerman D, Avogadri F, et al. Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res. 2012;72:876–86. doi: 10.1158/0008-5472.CAN-11-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solito S, Bronte V, Mandruzzato S. Antigen specificity of immune suppression by myeloid-derived suppressor cells. J Leukoc Biol. 2011;90:31–6. doi: 10.1189/jlb.0111021. [DOI] [PubMed] [Google Scholar]
- 23.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–35. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guilliams M, Henri S, Tamoutounour S, Ardouin L, Schwartz-Cornil I, Dalod M, et al. From skin dendritic cells to a simplified classification of human and mouse dendritic cell subsets. Eur J Immunol. 2010;40:2089–94. doi: 10.1002/eji.201040498. [DOI] [PubMed] [Google Scholar]
- 25.Joffre O, Nolte MA, Spörri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–47. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 26.Ma Y, Aymeric L, Locher C, Kroemer G, Zitvogel L. The dendritic cell-tumor cross-talk in cancer. Curr Opin Immunol. 2011;23:146–52. doi: 10.1016/j.coi.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Engelhardt JJ, Boldajipour B, Beemiller P, Pandurangi P, Sorensen C, Werb Z, et al. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell. 2012;21:402–17. doi: 10.1016/j.ccr.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–7. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 29.Liu Q, Zhang C, Sun A, Zheng Y, Wang L, Cao X. Tumor-educated CD11bhighIalow regulatory dendritic cells suppress T cell response through arginase I. J Immunol. 2009;182:6207–16. doi: 10.4049/jimmunol.0803926. [DOI] [PubMed] [Google Scholar]
- 30.Kuang D-M, Zhao Q, Xu J, Yun JP, Wu C, Zheng L. Tumor-educated tolerogenic dendritic cells induce CD3epsilon down-regulation and apoptosis of T cells through oxygen-dependent pathways. J Immunol. 2008;181:3089–98. doi: 10.4049/jimmunol.181.5.3089. [DOI] [PubMed] [Google Scholar]
- 31.Muller AJ, Sharma MD, Chandler PR, Duhadaway JB, Everhart ME, Johnson BA, 3rd, et al. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci U S A. 2008;105:17073–8. doi: 10.1073/pnas.0806173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fridman W-H, Galon J, Pagès F, Tartour E, Sautès-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011;71:5601–5. doi: 10.1158/0008-5472.CAN-11-1316. [DOI] [PubMed] [Google Scholar]
- 33.Hiraoka N, Yamazaki-Itoh R, Ino Y, Mizuguchi Y, Yamada T, Hirohashi S, et al. CXCL17 and ICAM2 are associated with a potential anti-tumor immune response in early intraepithelial stages of human pancreatic carcinogenesis. Gastroenterology. 2011;140:310–21. doi: 10.1053/j.gastro.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Scarlett UK, Rutkowski MR, Rauwerdink AM, Fields J, Escovar-Fadul X, Baird J, et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med. 2012;209:495–506. doi: 10.1084/jem.20111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–24. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71:2411–6. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 37.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sica A, Porta C, Morlacchi S, et al. Origin and Functions of Tumor-Associated Myeloid Cells (TAMCs) Cancer Microenviron. 2012;5:133–49. doi: 10.1007/s12307-011-0091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–31. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 41.Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151–62. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- 42.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegué E, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–20. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alfaro C, Suárez N, Martínez-Forero I, Palazón A, Rouzaut A, Solano S, et al. Carcinoma-derived interleukin-8 disorients dendritic cell migration without impairing T-cell stimulation. PLoS One. 2011;6:e17922. doi: 10.1371/journal.pone.0017922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hannah S, Mecklenburgh K, Rahman I, Bellingan GJ, Greening A, Haslett C, et al. Hypoxia prolongs neutrophil survival in vitro. FEBS Lett. 1995;372:233–7. doi: 10.1016/0014-5793(95)00986-J. [DOI] [PubMed] [Google Scholar]
- 45.Fang H-Y, Hughes R, Murdoch C, Coffelt SB, Biswas SK, Harris AL, et al. Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood. 2009;114:844–59. doi: 10.1182/blood-2008-12-195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burke B, Giannoudis A, Corke KP, Gill D, Wells M, Ziegler-Heitbrock L, et al. Hypoxia-induced gene expression in human macrophages: implications for ischemic tissues and hypoxia-regulated gene therapy. Am J Pathol. 2003;163:1233–43. doi: 10.1016/S0002-9440(10)63483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeda N, O’Dea EL, Doedens A, Kim JW, Weidemann A, Stockmann C, et al. Differential activation and antagonistic function of HIF-alpha isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010;24:491–501. doi: 10.1101/gad.1881410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, et al. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157:411–21. doi: 10.1016/S0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, et al. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest. 2010;120:2699–714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boelte KC, Gordy LE, Joyce S, Thompson MA, Yang L, Lin PC. Rgs2 mediates pro-angiogenic function of myeloid derived suppressor cells in the tumor microenvironment via upregulation of MCP-1. PLoS One. 2011;6:e18534. doi: 10.1371/journal.pone.0018534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–53. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mancino A, Schioppa T, Larghi P, Pasqualini F, Nebuloni M, Chen IH, et al. Divergent effects of hypoxia on dendritic cell functions. Blood. 2008;112:3723–34. doi: 10.1182/blood-2008-02-142091. [DOI] [PubMed] [Google Scholar]
- 53.Mahadevan NR, Zanetti M. Tumor stress inside out: cell-extrinsic effects of the unfolded protein response in tumor cells modulate the immunological landscape of the tumor microenvironment. J Immunol. 2011;187:4403–9. doi: 10.4049/jimmunol.1101531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jamora C, Dennert G, Lee AS. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc Natl Acad Sci U S A. 1996;93:7690–4. doi: 10.1073/pnas.93.15.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahadevan NR, Rodvold J, Sepulveda H, Rossi S, Drew AF, Zanetti M. Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc Natl Acad Sci U S A. 2011;108:6561–6. doi: 10.1073/pnas.1008942108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–99. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 57.Théry C, Boussac M, Véron P, Ricciardi-Castagnoli P, Raposo G, Garin J, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–18. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 58.Zhang HG, Grizzle WE. Exosomes and cancer: a newly described pathway of immune suppression. Clin Cancer Res. 2011;17:959–64. doi: 10.1158/1078-0432.CCR-10-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin Immunopathol. 2011;33:441–54. doi: 10.1007/s00281-010-0234-8. [DOI] [PubMed] [Google Scholar]
- 60.Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, et al. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66:9290–8. doi: 10.1158/0008-5472.CAN-06-1819. [DOI] [PubMed] [Google Scholar]
- 61.Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178:6867–75. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- 62.Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124:2621–33. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–71. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takizawa T, Nishinarita S, Kitamura N, Hayakawa J, Kang H, Tomita Y, et al. Interaction of the cell-binding domain of fibronectin with VLA-5 integrin induces monokine production in cultured human monocytes. Clin Exp Immunol. 1995;101:376–82. doi: 10.1111/j.1365-2249.1995.tb08367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poutsiaka DD, Schroder EW, Taylor DD, Levy EM, Black PH. Membrane vesicles shed by murine melanoma cells selectively inhibit the expression of Ia antigen by macrophages. J Immunol. 1985;134:138–44. [PubMed] [Google Scholar]
- 66.Zeelenberg IS, Ostrowski M, Krumeich S, Bobrie A, Jancic C, Boissonnas A, et al. Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res. 2008;68:1228–35. doi: 10.1158/0008-5472.CAN-07-3163. [DOI] [PubMed] [Google Scholar]
- 67.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–49. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shurin GV, Ouellette CE, Shurin MR. Regulatory dendritic cells in the tumor immunoenvironment. Cancer Immunol Immunother. 2012;61:223–30. doi: 10.1007/s00262-011-1138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–8. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang M, Stolina M, Sharma S, Mao JT, Zhu L, Miller PW, et al. Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res. 1998;58:1208–16. [PubMed] [Google Scholar]
- 71.Rodríguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–9. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554–67. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sato T, Terai M, Tamura Y, Alexeev V, Mastrangelo MJ, Selvan SR. Interleukin 10 in the tumor microenvironment: a target for anticancer immunotherapy. Immunol Res. 2011;51:170–82. doi: 10.1007/s12026-011-8262-6. [DOI] [PubMed] [Google Scholar]
- 74.Liu YY, Sun LC, Wei JJ, Li D, Yuan Y, Yan B, et al. Tumor cell-released TLR4 ligands stimulate Gr-1+CD11b+F4/80+ cells to induce apoptosis of activated T cells. J Immunol. 2010;185:2773–82. doi: 10.4049/jimmunol.1000772. [DOI] [PubMed] [Google Scholar]
- 75.Coffelt SB, Chen Y-Y, Muthana M, Welford AF, Tal AO, Scholz A, et al. Angiopoietin 2 stimulates TIE2-expressing monocytes to suppress T cell activation and to promote regulatory T cell expansion. J Immunol. 2011;186:4183–90. doi: 10.4049/jimmunol.1002802. [DOI] [PubMed] [Google Scholar]
- 76.Pucci F, Venneri MA, Biziato D, Nonis A, Moi D, Sica A, et al. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114:901–14. doi: 10.1182/blood-2009-01-200931. [DOI] [PubMed] [Google Scholar]
- 77.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gocheva V, Wang H-W, Gadea BB, Shree T, Hunter KE, Garfall AL, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24:241–55. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fukuda A, Wang SC, Morris JP, 4th, Folias AE, Liou A, Kim GE, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–55. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512–26. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 81.Borrello MG, Alberti L, Fischer A, Degl’innocenti D, Ferrario C, Gariboldi M, et al. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci U S A. 2005;102:14825–30. doi: 10.1073/pnas.0503039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Viola A, Sarukhan A, Bronte V, Molon B. The pros and cons of chemokines in tumor immunology. Trends Immunol. 2012 doi: 10.1016/j.it.2012.05.007. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 83.Bottazzi B, Polentarutti N, Acero R, Balsari A, Boraschi D, Ghezzi P, et al. Regulation of the macrophage content of neoplasms by chemoattractants. Science. 1983;220:210–2. doi: 10.1126/science.6828888. [DOI] [PubMed] [Google Scholar]
- 84.Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6:3282–9. [PubMed] [Google Scholar]
- 85.Qian B-Z, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J, et al. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood. 2008;111:5457–66. doi: 10.1182/blood-2008-01-136895. [DOI] [PubMed] [Google Scholar]
- 87.Pahler JC, Tazzyman S, Erez N, Chen YY, Murdoch C, Nozawa H, et al. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. 2008;10:329–40. doi: 10.1593/neo.07871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakasone ES, Askautrud HA, Kees T, Park JH, Plaks V, Ewald AJ, et al. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell. 2012;21:488–503. doi: 10.1016/j.ccr.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 2011;71:7463–70. doi: 10.1158/0008-5472.CAN-11-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–62. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3:143–50. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- 92.Ueha S, Shand FHW, Matsushima K. Myeloid cell population dynamics in healthy and tumor-bearing mice. Int Immunopharmacol. 2011;11:783–8. doi: 10.1016/j.intimp.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 93.Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD, et al. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70:3526–36. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–8. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aziz A, Soucie E, Sarrazin S, Sieweke MH. MafB/c-Maf deficiency enables self-renewal of differentiated functional macrophages. Science. 2009;326:867–71. doi: 10.1126/science.1176056. [DOI] [PubMed] [Google Scholar]
- 96.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 97.Kuang D-M, Wu Y, Chen N, Cheng J, Zhuang SM, Zheng L. Tumor-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood. 2007;110:587–95. doi: 10.1182/blood-2007-01-068031. [DOI] [PubMed] [Google Scholar]
- 98.Wang B, Li Q, Qin L, Zhao S, Wang J, Chen X. Transition of tumor-associated macrophages from MHC class II(hi) to MHC class II(low) mediates tumor progression in mice. BMC Immunol. 2011;12:43. doi: 10.1186/1471-2172-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Egeblad M, Ewald AJ, Askautrud HA, Truitt ML, Welm BE, Bainbridge E, et al. Visualizing stromal cell dynamics in different tumor microenvironments by spinning disk confocal microscopy. Dis Model Mech. 2008;1:155–67, discussion 165. doi: 10.1242/dmm.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ojalvo LS, Whittaker CA, Condeelis JS, Pollard JW. Gene expression analysis of macrophages that facilitate tumor invasion supports a role for Wnt-signaling in mediating their activity in primary mammary tumors. J Immunol. 2010;184:702–12. doi: 10.4049/jimmunol.0902360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matsumoto S, Yasui H, Mitchell JB, Krishna MC. Imaging cycling tumor hypoxia. Cancer Res. 2010;70:10019–23. doi: 10.1158/0008-5472.CAN-10-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu Y, Van Ginderachter JA, Brys L, De Baetselier P, Raes G, Geldhof AB. Nitric oxide-independent CTL suppression during tumor progression: association with arginase-producing (M2) myeloid cells. J Immunol. 2003;170:5064–74. doi: 10.4049/jimmunol.170.10.5064. [DOI] [PubMed] [Google Scholar]
- 103.Van Ginderachter JA, Meerschaut S, Liu Y, Brys L, De Groeve K, Hassanzadeh Ghassabeh G, et al. Peroxisome proliferator-activated receptor gamma (PPARgamma) ligands reverse CTL suppression by alternatively activated (M2) macrophages in cancer. Blood. 2006;108:525–35. doi: 10.1182/blood-2005-09-3777. [DOI] [PubMed] [Google Scholar]
- 104.Banerjee S, Lin C-FL, Skinner KA, Schiffhauer LM, Peacock J, Hicks DG, et al. Heat shock protein 27 differentiates tolerogenic macrophages that may support human breast cancer progression. Cancer Res. 2011;71:318–27. doi: 10.1158/0008-5472.CAN-10-1778. [DOI] [PubMed] [Google Scholar]
- 105.Shirota Y, Shirota H, Klinman DM. Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol. 2012;188:1592–9. doi: 10.4049/jimmunol.1101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nonaka M, Ma BY, Imaeda H, Kawabe K, Kawasaki N, Hodohara K, et al. Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN) recognizes a novel ligand, Mac-2-binding protein, characteristically expressed on human colorectal carcinomas. J Biol Chem. 2011;286:22403–13. doi: 10.1074/jbc.M110.215301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Allavena P, Piemonti L, Longoni D, Bernasconi S, Stoppacciaro A, Ruco L, et al. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur J Immunol. 1998;28:359–69. doi: 10.1002/(SICI)1521-4141(199801)28:01<359::AID-IMMU359>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 108.Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, et al. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–91. [PubMed] [Google Scholar]
- 109.Diao J, Mikhailova A, Tang M, Gu H, Zhao J, Cattral MS. Immunostimulatory conventional dendritic cells evolve into regulatory macrophage-like cells. Blood. 2012;119:4919–27. doi: 10.1182/blood-2011-11-392894. [DOI] [PubMed] [Google Scholar]
- 110.Linde N, Lederle W, Depner S, van Rooijen N, Gutschalk CM, Mueller MM. Vascular endothelial growth factor-induced skin carcinogenesis depends on recruitment and alternative activation of macrophages. J Pathol. 2012;227:17–28. doi: 10.1002/path.3989. [DOI] [PubMed] [Google Scholar]
- 111.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, et al. Abrogation of TGF β signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gebhardt C, Németh J, Angel P, Hess J. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol. 2006;72:1622–31. doi: 10.1016/j.bcp.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 113.Markiewski MM, Lambris JD. Unwelcome complement. Cancer Res. 2009;69:6367–70. doi: 10.1158/0008-5472.CAN-09-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, Moughon DL, et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115:1461–71. doi: 10.1182/blood-2009-08-237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–93. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cortez-Retamozo V, Etzrodt M, Newton A, Rauch PJ, Chudnovskiy A, Berger C, et al. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci U S A. 2012;109:2491–6. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hart KM, Bak SP, Alonso A, Berwin B. Phenotypic and functional delineation of murine CX(3)CR1 monocyte-derived cells in ovarian cancer. Neoplasia. 2009;11:564–73, 1, 573. doi: 10.1593/neo.09228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Izhak L, Wildbaum G, Jung S, Stein A, Shaked Y, Karin N. Dissecting the autocrine and paracrine roles of the CCR2-CCL2 axis in tumor survival and angiogenesis. PLoS One. 2012;7:e28305. doi: 10.1371/journal.pone.0028305. [DOI] [PMC free article] [PubMed] [Google Scholar]