Abstract
Intravesical bacillus Calmette-Guérin (BCG) immunotherapy results in neutrophil recruitment and subsequent secretion of cytokines to eliminate non-muscle invasive bladder cancer cells. However, bladder cancer cells often resist BCG immunotherapy. Thus, understanding the mechanism of action of BCG, and designing appropriate combination therapies might help to overcome BCG resistance and redirect neutrophils against bladder cancer cells.
Keywords: BCG, FasL, Smac mimetics, TNFα, TRAIL, bladder cancer, neutrophils
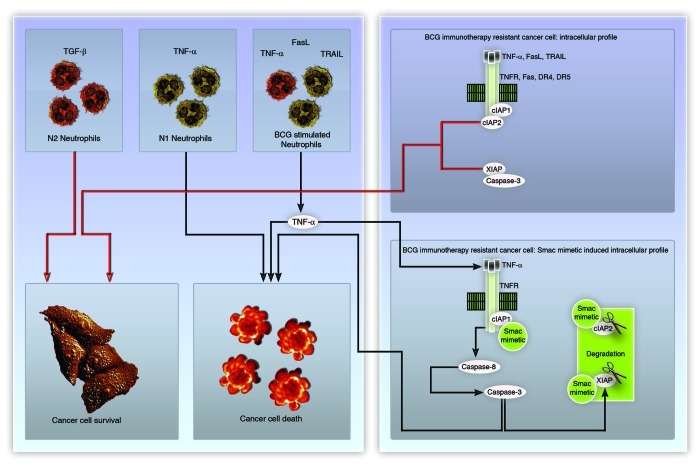
The immune system is an important player in the treatment of non-muscle invasive bladder cancer, as evidenced by the fact that intravesical bacillus Calmette-Guérin (BCG) therapy has been the gold standard therapy for this type of disease for more than 3 decades. In many tumor types, studies have reported on varying association between tumor cells and immune cells such as tumor associated macrophages (TAMs), tumor-associated neutrophils (TANs), tumor-associated T cells (TATs). All these immune cells behave in a context-specific manner, and can indeed exert both pro-tumor and anti-tumor functions. It has been postulated that normal circulating neutrophils are of the N1 type (capable of attacking cancer cells), whereas in the tumor microenvironment neutrophils acquire an N2 phenotype (i.e., they are educated not to attack cancer cells, but to provide cytokines that exert pro-tumor functions) (Fig. 1).1 It is believed that the switch from the N1 to the N2 phenotype (and vice versa) is mainly controlled by cytokines and chemokines.1
Figure 1. Schematic representation of neutrophil phenotypes and the mechanism of action of BCG-stimulated neutrophils plus Smac mimetics. Although BCG-stimulated neutrophils secrete TNFα, TRAIL and FASL, TNFα appears to be the primary mediator of their anticancer action when combined with Smac mimetics.
Immunotherapy with BCG administered intravesically stimulates a potent inflammatory response with a Th1 profile, whereby neutrophils are recruited much earlier than other immune cells. Neutrophils are shown to be required for the efficacy of BCG immunotherapy in mouse models,2 and proportionately, neutrophils account for about 75% of the immune cells recruited in response to BCG immunotherapy in patients.3 Neutrophil priming/activation in this setup can be either due to the BCG lipopolysaccharides, which enable neutrophil self-priming, or to interferon γ (IFNγ) secreted from other immune cells (which helps neutrophil priming).4 Stimulated neutrophils (notably in response to mycobacterial products) are good sources of tumor necrosis factor α (TNFα), TRAIL and FAS ligand (FASL).5,6 However, BCG-stimulated neutrophils by themselves are not capable to kill bladder cancer cells indicating the cancer cell-intrinsic alterations might contribute to the therapeutic efficacy of BCG.5
Smac mimetics are known to sensitize cancer cells to apoptosis by interfering with intracellular interactors of death receptors (such as c-IAPs) and caspase inhibitors (such as XIAP).7 We examined the ability of a Smac mimetic to sensitize bladder cancer cells to BCG-stimulated neutrophils and found failing c-IAP2 stabilization and XIAP downregulation as a signature associated with cell death induced by BCG-stimulated neutrophils plus Smac mimetics (Fig. 1).5 Using recombinant forms of TNFα, TRAIL and FASL, and their corresponding neutralizing antibodies, we indetified TNFα as the primary mediator of the cell death program triggered by BCG-stimulated neutrophils in combination with Smac mimetics.5 Thus, Smac mimetics might be useful for overcoming the resistance of bladder cancer cells to BCG-stimulated neutrophils in vivo, a concept that is worth testing in clinical trials.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20928
References
- 1.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suttmann H, Riemensberger J, Bentien G, Schmaltz D, Stöckle M, Jocham D, et al. Neutrophil granulocytes are required for effective Bacillus Calmette-Guérin immunotherapy of bladder cancer and orchestrate local immune responses. Cancer Res. 2006;66:8250–7. doi: 10.1158/0008-5472.CAN-06-1416. [DOI] [PubMed] [Google Scholar]
- 3.de Boer EC, de Jong WH, van der Meijden AP, Steerenberg PA, Witjes F, Vegt PD, et al. Leukocytes in the urine after intravesical BCG treatment for superficial bladder cancer. A flow cytofluorometric analysis. Urol Res. 1991;19:45–50. doi: 10.1007/BF00294021. [DOI] [PubMed] [Google Scholar]
- 4.Ethuin F, Gérard B, Benna JE, Boutten A, Gougereot-Pocidalo MA, Jacob L, et al. Human neutrophils produce interferon gamma upon stimulation by interleukin-12. Lab Invest. 2004;84:1363–71. doi: 10.1038/labinvest.3700148. [DOI] [PubMed] [Google Scholar]
- 5.Jinesh GG, Chunduru S, Kamat AM. Smac mimetic enables the anticancer action of BCG-stimulated neutrophils through TNF-alpha but not through TRAIL and FasL. J Leukoc Biol. 2012 doi: 10.1189/jlb.1211623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemp TJ, Ludwig AT, Earel JK, Moore JM, Vanoosten RL, Moses B, et al. Neutrophil stimulation with Mycobacterium bovis bacillus Calmette-Guerin (BCG) results in the release of functional soluble TRAIL/Apo-2L. Blood. 2005;106:3474–82. doi: 10.1182/blood-2005-03-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fulda S, Vucic D. Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov. 2012;11:109–24. doi: 10.1038/nrd3627. [DOI] [PubMed] [Google Scholar]