Abstract
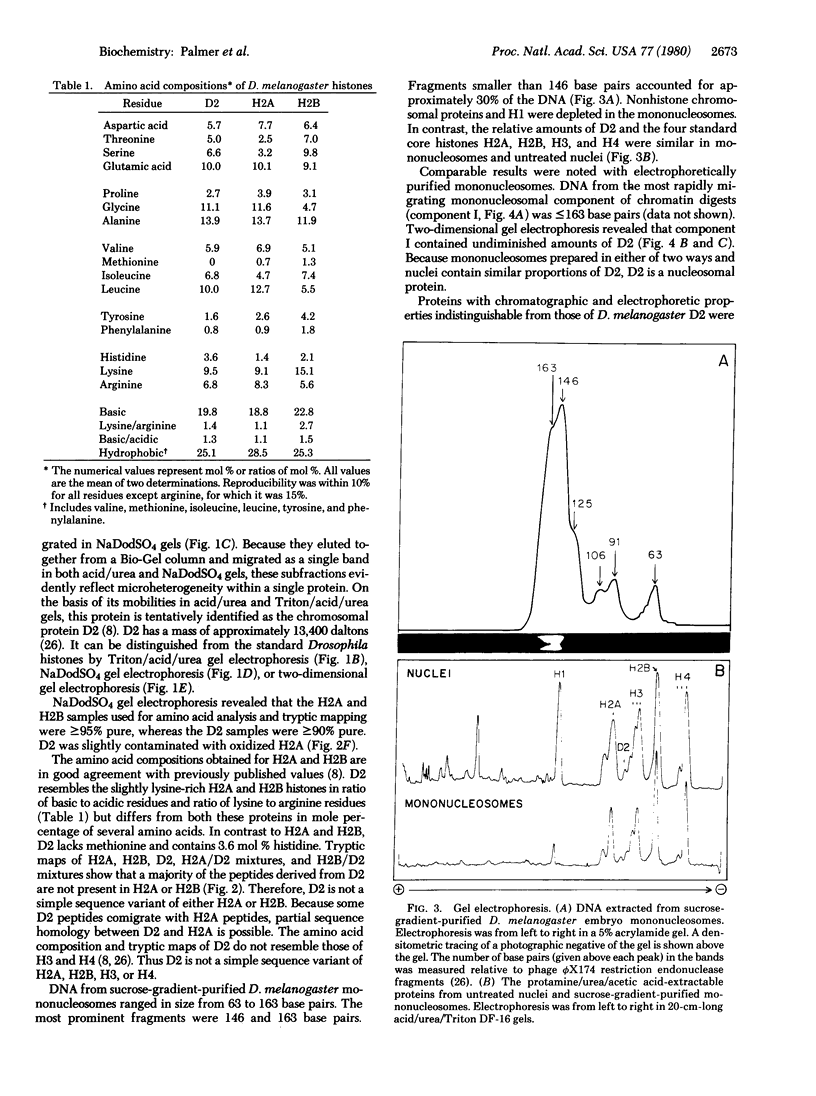
Mononucleosomes prepared from Drosophila melanogaster nuclei contain the four core histones H2A, H2B, H3, and H4 plus an additional histone-like, acid-soluble, chromosomal protein. It is probably the protein designated D2 by Alfageme et al. [Alfageme, C.R., Zweidler, A., Mahowald, A. & Cohen, L.H. (1974) J. Biol. Chem. 249, 3729-3736]. D2 elutes with histone H2A from a Bio-Gel P-100 column, but can be distinguished electrophoretically from H2A and from the other standard Drosophila core histones. The amino acid composition of D2 resembles the compositions of H2A and H2B. However, peptide mapping reveals that D2 is not a simple sequence variant of either H2A or H2B. D2 is present in nuclei from embryos and adult heads, and thus is not restricted to a narrowly defined developmental period. It is present in D. melanogaster and D. virilis, and thus appears to be conserved during the evolution of Drosophila. D2 is present in D. melanogaster chromatin with an approximate frequency of one molecule per five nucleosomes, and must therefore be associated with a subset of nucleosomes. The function of this protein is not known. Its presence in nucleosomes, evolutionary conservation, and comparatively large abundance all suggest that it is an important nucleosomal element. It will be interesting to learn whether this histone-like protein is encoded in a subset of the Drosophila histone gene cluster or is encoded separately.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright S. C., Nelson P. P., Garrard W. T. Histone molar ratios among different electrophoretic forms of mono- and dinucleosomes. J Biol Chem. 1979 Feb 25;254(4):1065–1073. [PubMed] [Google Scholar]
- Alfageme C. R., Rudkin G. T., Cohen L. H. Locations of chromosomal proteins in polytene chromosomes. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2038–2042. doi: 10.1073/pnas.73.6.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfageme C. R., Zweidler A., Mahowald A., Cohen L. H. Histones of Drosophila embryos. Electrophoretic isolation and structural studies. J Biol Chem. 1974 Jun 25;249(12):3729–3736. [PubMed] [Google Scholar]
- Billings P. C., Orf J. W., Palmer D. K., Talmage D. A., Pan C. G., Blumenfeld M. Anomalous electrophoretic mobility of Drosophila phosphorylated H1 histone: is it related to the compaction of satellite DNA into heterochromatin? Nucleic Acids Res. 1979;6(6):2151–2164. doi: 10.1093/nar/6.6.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankstein L. A., Levy S. B. Changes in histone f2a2 associated with proliferation of Friend leukaemic cells. Nature. 1976 Apr 15;260(5552):638–640. doi: 10.1038/260638a0. [DOI] [PubMed] [Google Scholar]
- Blankstein L. A., Stollar B. D., Franklin S. G., Zweidler A., Levy S. B. Biochemical and immunological characterization of two distinct variants of histone H2A in Friend leukemia. Biochemistry. 1977 Oct 18;16(21):4557–4562. doi: 10.1021/bi00640a003. [DOI] [PubMed] [Google Scholar]
- Blumenfeld M., Fox A. S., Forrest H. S. A family of three related satellite DNAs in Drosophila virilis. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2772–2775. doi: 10.1073/pnas.70.10.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld M., Orf J. W., Sina B. J., Kreber R. A., Callahan M. A., Mullins J. I., Snyder L. A. Correlation between phosphorylated H1 histones and satellite DNAs in Drosophila virilis. Proc Natl Acad Sci U S A. 1978 Feb;75(2):866–870. doi: 10.1073/pnas.75.2.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt W. F., Strickland W. N., Strickland M., Carlisle L., Woods D., von Holt C. A histone programme during the life cycle of the sea urchin. Eur J Biochem. 1979 Feb 15;94(1):1–10. doi: 10.1111/j.1432-1033.1979.tb12864.x. [DOI] [PubMed] [Google Scholar]
- Franklin S. G., Zweidler A. Non-allelic variants of histones 2a, 2b and 3 in mammals. Nature. 1977 Mar 17;266(5599):273–275. doi: 10.1038/266273a0. [DOI] [PubMed] [Google Scholar]
- Goldknopf I. L., Busch H. Isopeptide linkage between nonhistone and histone 2A polypeptides of chromosomal conjugate-protein A24. Proc Natl Acad Sci U S A. 1977 Mar;74(3):864–868. doi: 10.1073/pnas.74.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldknopf I. L., French M. F., Musso R., Busch H. Presence of protein A24 in rat liver nucleosomes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5492–5495. doi: 10.1073/pnas.74.12.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G. H., Woodhead L., Johns E. W. The presence of high mobility group non-histone chromatin proteins in isolated nucleosomes. FEBS Lett. 1977 Jan 15;73(1):85–88. [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Holmgren P., Rasmuson B., Johansson T., Sundquist G. Histone content in relation to amount of heterochromatin and developmental stage in three species of Drosophila. Chromosoma. 1976 Feb 13;54(2):99–116. doi: 10.1007/BF00292833. [DOI] [PubMed] [Google Scholar]
- Honda B. M., Baillie D. L., Candido E. P. Properties of chromatin subunits from developing trout testis. J Biol Chem. 1975 Jun 25;250(12):4643–4647. [PubMed] [Google Scholar]
- Houston L. L. Amino acid analysis of stained bands from polyacrylamide gels. Anal Biochem. 1971 Nov;44(1):81–88. doi: 10.1016/0003-2697(71)90348-4. [DOI] [PubMed] [Google Scholar]
- Hunt L. T., Dayhoff M. O. Amino-terminal sequence identity of ubiquitin and the nonhistone component of nuclear protein A24. Biochem Biophys Res Commun. 1977 Jan 24;74(2):650–655. doi: 10.1016/0006-291x(77)90352-7. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D. Structure of chromatin. Annu Rev Biochem. 1977;46:931–954. doi: 10.1146/annurev.bi.46.070177.004435. [DOI] [PubMed] [Google Scholar]
- LUCK J. M., RASMUSSEN P. S., SATAKE K., TSVETIKOV A. N. Further studies on the fractionation of calf thymus histone. J Biol Chem. 1958 Dec;233(6):1407–1414. [PubMed] [Google Scholar]
- Newrock K. M., Cohen L. H., Hendricks M. B., Donnelly R. J., Weinberg E. S. Stage-specific mRNAs coding for subtypes of H2A and H2B histones in the sea urchin embryo. Cell. 1978 Jun;14(2):327–336. doi: 10.1016/0092-8674(78)90118-6. [DOI] [PubMed] [Google Scholar]
- Noll M., Kornberg R. D. Action of micrococcal nuclease on chromatin and the location of histone H1. J Mol Biol. 1977 Jan 25;109(3):393–404. doi: 10.1016/s0022-2836(77)80019-3. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Deficiency in histone acetylation in nontransforming host range mutants of polyoma virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1092–1096. doi: 10.1073/pnas.73.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer K. R., Chalkley R. A new method for fractionating histones for physical and chemical studies. Biochemistry. 1974 Feb 26;13(5):1022–1027. doi: 10.1021/bi00702a029. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban M. K., Franklin S. G., Zweidler A. Isolation and characterization of the histone variants in chicken erythrocytes. Biochemistry. 1979 Sep 4;18(18):3952–3960. doi: 10.1021/bi00585a017. [DOI] [PubMed] [Google Scholar]
- Woodcock C. L., Sweetman H. E., Frado L. L. Structural repeating units in chromatin. II. Their isolation and partial characterization. Exp Cell Res. 1976 Jan;97:111–119. doi: 10.1016/0014-4827(76)90660-1. [DOI] [PubMed] [Google Scholar]