Abstract
B cells infiltrating into solid tumors are poorly investigated despite their described positive prognostic value. Whether this antitumor potential comes from either the antigen presentation or the antibody production capacity of B cells, or both, is unknown. Our recently published method on tumor-infiltrating B lymphocyte cloning may prove helpful in unraveling the actual relevance of these cells for tumor development and response to therapy.
Keywords: B cell subtypes, tumor host interaction, tumor immunology, tumor-infiltrating B cells, tumor-infiltrating lymphocytes
The degree and the composition of lymphocytes infiltrating human malignant tumors have strong positive prognostic relevance.1 Most studies performed so far focused on the cell type with best antitumoral potential, i.e., CD8+ T cells. However, when tumor-infiltrating B cells (TiBcs) were analyzed, their presence was frequently found to be the next-best predictor of positive disease outcomes.2 In addition to their antibody (Ab)-producing capacity, TiBcs boost T-cell responses via stimulatory cytokines and chemokines, by serving as local antigen-presenting cells, and form tertiary lymphoid structures in mutual cooperation with T cells and dendritic cells.3 In a classical dogma of tumor immunology, T-cell responses are considered to be good whereas humoral responses oppose the latter and are consequently bad. Decades of experimental work with inbreed mouse strains have support this point of view. However, novel clear cut data from the fields of autoimmunity and transplantation demonstrate the extremely strong tissue damaging potential of B cells, especially when they interact with T cells. Thus, as far as B cells are concerned, we see the above outlined dogma breaking into parts.
Breast cancer is the tumor entity in which TiBcs have been best analyzed, their presence being a clear positive prognostic factor.4 In this setting, tumor antigen specific Ab responses have repeatedly been found. Possibly the most impressive example is a humoral immune response directed against β-actin, exposed on apoptotic mammary carcinoma cells, using recombinant Ab cloning techniques.5 In our recent work, clonal TiBc cultures were established by EBV-immortalization starting from fresh colorectal cancers. TiBcs were antigen-experienced and secreted immunoglobulins (Igs), and IgGs derived from several TiBc clones strongly bound to allogeneic tumor cell lines.6 These exemplary analyses demonstrated that a proportion of TiBcs accumulating in solid tumor tissues can produce Ab specific for antigens present on tumor cells. If these Ig-producing TiBcs are exclusively CD38+ plasma cells in situ remains an open question. In any case, TiBc-derived IgGs will be helpful in identifying novel tumor specific antigens.7
The identification of antigens recognized by TiBc-produced Ig might be much more convenient when taking advantage of recombinant antibody library technologies.8 However, on top of functional Igs, our cloning strategy provides live TiBcs, which are applicable to cell-based assays. Thus, we may be able to unravel the true TiBc potential to functionally suppress or promote tumor growth either directly or via interaction with (T) lymphocyte subpopulations. We are not aware of any comparable technique. Of note, beside colorectal cancer, TiBc clones were so far successfully established from pancreatic, lung and mammary cancer cases (unpublished data).
So far, TiBcs have most often been characterized by immunohistochemistry. In colorectal cancer, TiBcs typically reside at the invasive margin in tertiary lymphoid structures together with follicular dendritic cells. These aggregates, also termed “Crohn’s like reaction,” represent cooperative interactions between tumor-infiltrating leukocyte populations and can be interpreted as an immune-mediated antitumor effect. This may have implications for prognosis, since it was found that tumors containing both antigen-presenting (B cells or dendritic cells) and effector (T cells) cells are associated with better survival than those containing single immune cell populations.4,9 Nevertheless, the precise contribution of TiBcs to disease outcome remains largely illusive. This may be, at least partly, attributable to the paucity of methods used for analyzing (in situ) B cell function. Present immunohistochemical staining techniques only give an overview on the presence or absence of certain immune cell subsets. In our recent work, we found high expression of MHC Class I and II molecules as well as of co-stimulatory adhesion molecules and activation markers (CD80, CD23) on cultured TiBc clones. We interpreted this as remnants of a specific antitumoral effector function in situ. Yet, to gain deeper insights into the functional relationship between TiBcs and other immune cells, dual or even multicolor stainings followed by functional analyses are warranted.
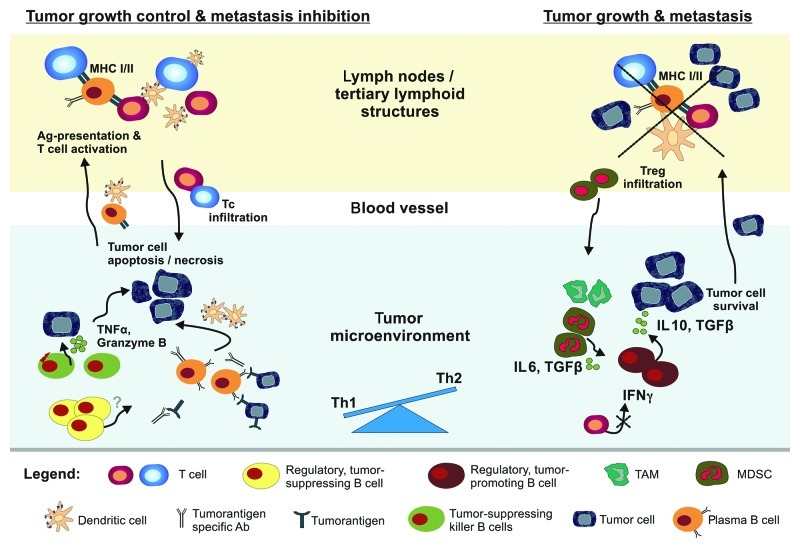
In analogy to tumor-infiltrating T lymphocytes, we would like to hypothesize that TiBcs consist of many different functional subclasses (Fig. 1). Thus, beside classical plasma cells, at least tumor-suppressing killer B cells (analog to CD8+ T cells) and regulatory B cells with tumor-suppressive (comparable to Th1-type CD4+ T cells) or tumor-promoting capacity (analog to CD4+ regulatory T cells) can be expected.4 The latter two types may be similar to, if not identical, Be-1 and Be-2-polarized B cells differentiated after mutual interaction with Th1 and Th2 CD4+ T cells.10
Figure 1. Potential interactions of tumor-infiltrating B cells (TiBcs) with cancer and immune cells. TiBc may either inhibit or promote tumor growth. Tumor-infiltrating plasma cells secrete specific antibodies that favor opsonisation, complement-mediated lysis, or antibody-dependent cellular cytotoxicity. Tumor-suppressing killer B cells have the capacity to directly eliminate tumor cells via lytic proteins (i.e., TNFα, granzyme B). A subset of regulatory B cells exerts tumor-suppressive capacity by secretion of unknown (but most likely proinflammatory and T cell stimulatory) factors. TiBcs take up antigens, migrate to sentinel lymph nodes and present processed antigens to T cells. Activated and expanded antitumoral T cells subsequently infiltrate the tumor. Contrary, a suppressive subset of regulatory B cells may support tumor growth and metastasis by IL-10 and TGFβ production. This favors Th2 and inhibits Th1 immune responses (in particular the secretion of IFNγ). As a consequence, Tregs and other immunosuppressive cell populations (TAM, MDSC) are recruited to the tumor. This generally inhibits proinflammatory anti-tumor T cells.
In summary, we would like to conclude that investigations on the natural role of TiBcs in tumor biology are an obvious task with a high pay-back potential. A more refined knowledge on the functional orientation, the mutual interactions with other immune cells but also with tumor cells, and ultimately the antigen specificities of TiBcs should nourish a deeper understanding of the tumor-host interaction. Such a deeper knowledge of TiBcs will surely favor the development of novel and more effective anticancer immunotherapies.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20641
References
- 1.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 2.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72:1070–80. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt M, Böhm D, von Törne C, Steiner E, Puhl A, Pilch H, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008;68:5405–13. doi: 10.1158/0008-5472.CAN-07-5206. [DOI] [PubMed] [Google Scholar]
- 4.Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185:4977–82. doi: 10.4049/jimmunol.1001323. [DOI] [PubMed] [Google Scholar]
- 5.Coronella-Wood JA, Hersh EM. Naturally occurring B-cell responses to breast cancer. Cancer Immunol Immunother. 2003;52:715–38. doi: 10.1007/s00262-003-0409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen MH, Nielsen HV, Ditzel HJ. Translocation of an intracellular antigen to the surface of medullary breast cancer cells early in apoptosis allows for an antigen-driven antibody response elicited by tumor-infiltrating B cells. J Immunol. 2002;169:2701–11. doi: 10.4049/jimmunol.169.5.2701. [DOI] [PubMed] [Google Scholar]
- 7.Maletzki C, Jahnke A, Ostwald C, Klar E, Prall F, Linnebacher M. Ex-vivo clonally expanded B lymphocytes infiltrating colorectal carcinoma are of mature immunophenotype and produce functional IgG. PLoS One. 2012;7:e32639. doi: 10.1371/journal.pone.0032639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavoni E, Monteriù G, Santapaola D, Petronzelli F, Anastasi AM, Pelliccia A, et al. Tumor-infiltrating B lymphocytes as an efficient source of highly specific immunoglobulins recognizing tumor cells. BMC Biotechnol. 2007;7:70. doi: 10.1186/1472-6750-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milne K, Köbel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, et al. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One. 2009;4:e6412. doi: 10.1371/journal.pone.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20:332–8. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]