Abstract
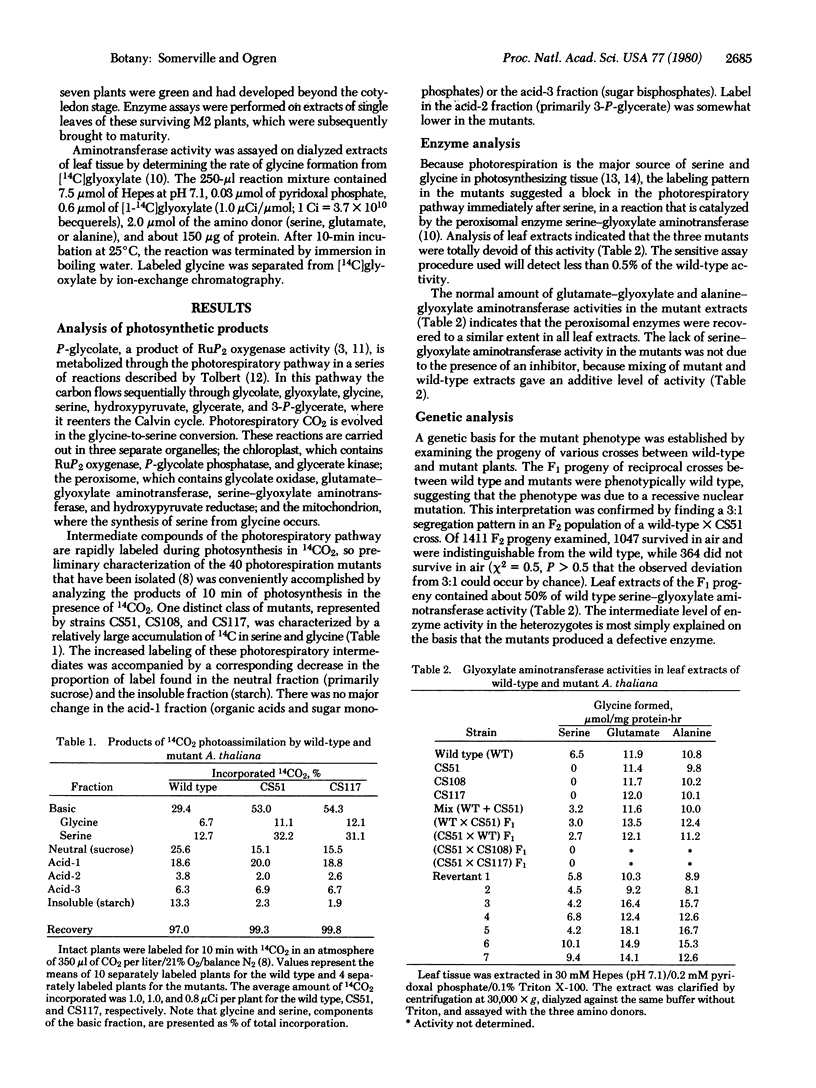
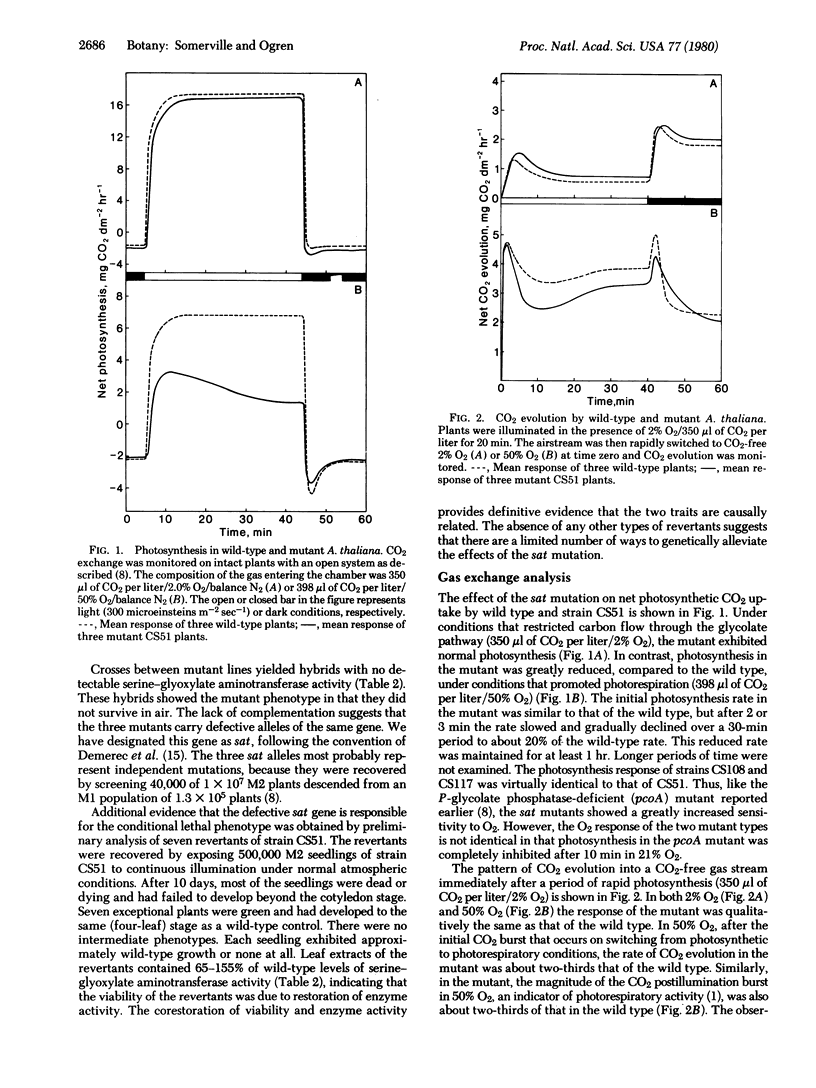
Three mutants of the crucifer Arabidopsis thaliana (Linnaeus) Heynhold were isolated that are completely lacking in activity catalyzed by serine-glyoxylate aminotransferase (EC 2.6.1.45), a peroxisomal enzyme involved in photorespiratory carbon metabolism. These mutants were viable and exhibited normal photosynthesis under conditions that suppressed photorespiration, but they were inviable and photosynthesized at greatly reduced rates under conditions that promoted photorespiration. Serine and glycine accumulated as end products of photosynthesis in the mutants, mostly at the expense of starch and sucrose. The mutants are allelic, and the segregation patterns of plant viability, photosynthetic activity, and enzyme activity in the F1 and F2 generations indicated that all the observed effects were caused by a single recessive nuclear mutation. This conclusion was confirmed by the isolation of seven revertants in which viability, photosynthetic capacity, and enzyme activity were simultaneously restored. Mutants of the type described here, in which photorespiration is changed from a merely wasteful process into one that is lethal, may permit the direct selection of secondary mutations that reduce photorespiration.
Keywords: photosynthesis; ribulose-1,5-bisphosphate carboxylase-oxygenase; revertants; peroxisome
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Lorimer G. H., Tolbert N. E. Ribulose diphosphate oxygenase. I. Synthesis of phosphoglycolate by fraction-1 protein of leaves. Biochemistry. 1973 Jan 2;12(1):11–18. doi: 10.1021/bi00725a003. [DOI] [PubMed] [Google Scholar]
- Bowes G., Ogren W. L., Hageman R. H. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1971 Nov 5;45(3):716–722. doi: 10.1016/0006-291x(71)90475-x. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIMENEZ E., BALDWIN R. L., TOLBERT N. E., WOOD W. A. Distribution of C14 in sucrose from glycolate-C14 and serine 3-C14 metabolism. Arch Biochem Biophys. 1962 Jul;98:172–175. doi: 10.1016/0003-9861(62)90163-7. [DOI] [PubMed] [Google Scholar]
- Laing W. A. Regulation of Soybean Net Photosynthetic CO(2) Fixation by the Interaction of CO(2), O(2), and Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1974 Nov;54(5):678–685. doi: 10.1104/pp.54.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin B. J., Marker A. F., Whittingham C. P. The metabolism of glycine and glycollate by pea leaves in relation to photosynthesis. Biochim Biophys Acta. 1966 Jun 8;120(2):266–273. doi: 10.1016/0926-6585(66)90346-3. [DOI] [PubMed] [Google Scholar]
- RABSON R., TOLBERTNE, KEARNEY P. C. Formation of serine and glyceric acid by the glycolate pathway. Arch Biochem Biophys. 1962 Jul;98:154–163. doi: 10.1016/0003-9861(62)90161-3. [DOI] [PubMed] [Google Scholar]
- Rehfeld D. W., Tolbert N. E. Aminotransferases in peroxisomes from spinach leaves. J Biol Chem. 1972 Aug 10;247(15):4803–4811. [PubMed] [Google Scholar]
- Servaites J. C. Chemical inhibition of the glycolate pathway in soybean leaf cells. Plant Physiol. 1977 Oct;60(4):461–466. doi: 10.1104/pp.60.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm J. M., Ogren W. L. Photorespiratory-induced senescence of plants under conditions of low carbon dioxide. Proc Natl Acad Sci U S A. 1969 Jul;63(3):668–675. doi: 10.1073/pnas.63.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Pathways of carbon fixation in green plants. Annu Rev Biochem. 1975;44:123–145. doi: 10.1146/annurev.bi.44.070175.001011. [DOI] [PubMed] [Google Scholar]



