Abstract
To investigate the intracellular transport of sterols in etiolated leek (Allium porrum L.) seedlings, in vivo pulse-chase experiments with [1-14C]acetate were performed. Then, endoplasmic reticulum-, Golgi-, and plasma membrane (PM)-enriched fractions were prepared and analyzed for the radioactivity incorporated into free sterols. In leek seedlings sterols are present as a mixture in which (24R)-24-ethylcholest-5-en-3β-ol is by far the major compound (around 60%). The other sterols are represented by cholest-5-en-3β-ol, 24-methyl-cholest-5-en-3β-ol, (24S)-24-ethylcholesta-5,22E-dien-3β-ol, and stigmasta-5,24(241)Z-dien-3β-ol. These compounds are shown to reside mainly in the PM. Our results clearly indicate that free sterols are actively transported from the endoplasmic reticulum to the PM during the first 60 min of chase, with kinetics very similar to that of phosphatidylserine. Such a transport was found to be decreased at low temperature (12°C) and following treatment with monensin and brefeldin A. These data are consistent with a membrane-mediated process for the intracellular transport of sterols to the PM, which likely involves the Golgi apparatus.
Whereas mammalian and fungal cells mainly contain one major sterol, cholesterol and ergosterol, respectively, higher plant cells are characterized by a mixture of sterols in which sitosterol, stigmasterol, and 24-methylcholesterol often predominate. Sterol biosynthesis in plants has been extensively studied (Benveniste, 1986). From the conversion of farnesyl diphosphate into squalene and end products, this pathway represents a sequence of more than 30 enzyme-catalyzed reactions, all associated with membranes. It is now well established that sterols are synthesized at the level of the ER, but mainly accumulate in the PM (Hartmann and Benveniste, 1987). Thus, the neosynthesized sterols must be transferred from the ER to the PM.
In contrast to recent advances in the understanding of intracellular movement of membrane proteins, relatively little attention has been paid to the intracellular transport of membrane lipids. Recent studies have been devoted to the transport of phospholipids in etiolated leek seedlings (Moreau et al., 1988; Bertho et al., 1991; Sturbois et al., 1994). It has been shown that the different phospholipid classes do not follow the same route from the ER to the PM. Moreover, low temperatures and treatment with monensin have been shown to block the transfer of only some molecular species (Bertho et al., 1991; Moreau and Cassagne, 1994; Sturbois-Balcerzak et al., 1995). As no information on the mechanisms involved in the delivery of sterol molecules to the PM is so far available, we have taken the advantage of this plant system to investigate the intracellular transport of sterols in higher plant cells. In vivo pulse-chase experiments with [1-14C]acetate clearly indicate that the sterols synthesized in the ER membranes are transferred to the PM with kinetics similar to that of PS. The effects of low temperature and treatment with monensin and brefeldin A on the transport of sterols were also investigated.
MATERIALS AND METHODS
Leek (Allium porrum L.) seeds were purchased from Vilmorin (La Ménitré, France) and stored overnight at 4°C before being hydrated with distillated water for 2 h. The seeds were allowed to germinate in the dark for 7 d at 22 to 24°C, as described previously (Moreau et al., 1988).
Chemicals
All chemicals were purchased from Sigma. [1-14C]Acetate was obtained from CEA (Saclay, France).
Pulse-Chase Experiments
For each experimental value, 10 batches of 20 seedlings (cut into 5- to 10-mm segments, including roots) were first incubated in 0.2 mL of 3.5 × 105 Bq of [1-14C]acetate (2 × 1012 Bq mol−1) for 120 min at 24°C or 12°C, in the presence or absence of monensin (5 μm) or brefeldin A (100 or 500 μm). Chase was made with 0.5 mL of 0.2 m unlabeled acetate for periods of time ranging from 30 to 120 min.
Isolation of ER, Golgi, and PM Fractions
Leek seedlings were homogenized in a buffer consisting of 10 mm KH2PO4, pH 8.2, with 0.5 m sorbitol, 5% (w/v) PVP 40, 0.5% (w/v) BSA, 2 mm salicylhydroxamic acid, and 1 mm PMSF. The homogenate was submitted to differential centrifugations at 1,000g for 10 min, 10,000g for 10 min, and 150,000g for 60 min. The resulting microsomal pellet was resuspended in 10 mm KH2PO4 and 0.5 m sorbitol. One-half of the suspension was loaded onto a discontinuous Suc-density gradient consisting of 2.5 mL of 37% (w/v) Suc, 3.5 mL of 25% (w/v) Suc, and 3.5 mL of 18% (w/v) Suc. After centrifugation at 80,000g for 150 min, membranes at the 18/25% (ER fraction) and 25/37% (Golgi fraction) Suc interface were collected, diluted with 30 mm Hepes-KCl, pH 6.8, and centrifuged at 100,000g for 60 min.
PMs were isolated by phase partitioning using PEG 4000 and dextran T500. The other half of the microsomal suspension was mixed with a polymer (PEG/dextran mixture) in 0.5 m sorbitol containing 10 mm KH2PO4 and 40 mm NaCl, pH 7.8, to obtain final PEG 4000 and dextran T500 concentrations of 6.0% (w/w). The solution (final volume, 28 mL) was centrifuged for 15 min at 1000g and the PEG-enriched upper phase (12 mL) was recovered without disturbing the interface. Membranes were then recovered after centrifugation at 150,000g for 60 min and resuspended in 30 mm Hepes-KCl, pH 6.8.
Specific membrane compartments were identified by assays for the following markers: ER, NADPH-Cyt c reductase and CDP-choline phosphotransferase; Golgi apparatus, IDPase; and PM, glucan synthetase II (Moreau et al., 1988; Bertho et al., 1991). Setting the specific activities of the marker enzymes at 1 in the homogenate, we obtained the following relative enrichments in the membrane fractions: ER fraction, 8.5 for NADPH-Cyt c reductase, 3.5 for CDP-choline phosphotransferase, 2.5 for IDPase, and 0.1 for glucan synthetase II; Golgi fraction, 2.4 for NADPH-Cyt c reductase, 0.5 for CDP-choline phosphotransferase, 9.5 for IDPase, and 0.6 for glucan synthetase II; PM fraction, 0.1 for NADPH-Cyt c reductase, 0.15 for CDP-choline phosphotransferase, 0.35 for IDPase, and 4.4 for glucan synthetase II. The specific activity of succinodeshydrogenase (a mitochondrial marker) was < 0.1 in the microsomes compared with the homogenate.
A low contamination of ER and Golgi fractions by plastid envelope membranes was determined by the presence of small amounts of galactolipids (< 10% of the total glycerolipids). Protein concentrations were determined by the method of Bradford (1976) using BSA as standard.
Lipid Analyses
Lipids were extracted by chloroform:methanol (1:1, v/v) for 30 min at room temperature, and then washed three times with distilled water. The solvent was evaporated and lipids were resuspended in an appropriate volume of chloroform:methanol (1:1, v/v) according to procedures already described (Moreau et al., 1988; Bertho et al., 1991).
PS isolation was carried out on HPTLC plates (60F254, Merck, Darmstadt, Germany) developed with methylacetate:n-propanol:chloroform:methanol:aqueous 0.25% (w/v) KCl (25:25:25:10:9, v/v) according to the method of Heape et al. (1985). Neutral lipids were isolated onto HPTLC plates developed with hexane:chloroform:methanol (100:60:10, v/v) to separate into sterols (RF 0.54), fatty alcohols (RF 0.64), and free fatty acids (RF 1); with hexane:ethylether:acetic acid (90:15:2, v/v) to give diacylglycerols (RF 0.08), 4-demethylsterols (RF 0.17), fatty alcohols (RF 0.22), and free fatty acids (RF 0.29).
After identification by comparison with standards, the different lipids were scraped off directly into vials and their radioactivity was determined by liquid scintillation counting (model 2000CA counter, Packard Instruments, Meriden, CT). Radioactivity of the lipids was also determined after autoradiography of the HPTLC plates (Hyperfilm MP-RPN 1675, Amersham) and scanning with a densitometer (model 76510, Camag, Muttenz, Switzerland). Both methods gave similar results and were alternately used.
Sterols were identified and quantified as previously reported (Hartmann and Benveniste, 1987). After extraction from membrane fractions with hexane, sterols were subjected to TLC with dichloromethane as the developing solvent for two runs. The 4-demethylsterols (end products) were eluted and acetylated before being analyzed by GC on a glass capillary column (30 m long, 0.25-mm i.d., coated with DB-1). The temperature program used includes a fast rise from 60°C to 230°C (30°C/min), then a slow rise from 230°C to 280°C (2°C/min). A cholesterol standard (not acetylated) was added to the samples prior to analysis. Sterol identification was made by GC-MS (Rahier and Benveniste, 1989).
RESULTS
Free Sterol Composition of Membrane Fractions
ER, Golgi, and PM fractions were prepared from 7-d-old etiolated leek seedlings and characterized by enzymatic markers (Moreau et al., 1988; Bertho et al., 1991) and labeling by anti-HDEL (Napier et al., 1992) and JIM 84 (Horsley et al., 1993) antibodies for ER and Golgi fractions, respectively (B. Sturbois-Balcerzak, L. Maneta-Peyret, M. Duvert, B. Satiat-Jeunemaitre, P. Vincent, C. Cassagne, and P. Moreau, unpublished data). These fractions were analyzed for their free 4-demethylsterol content. Data are shown in Table I. In leek seedlings, like in other plant tissue, the PM was found to be the richest membrane in free sterols (expressed in micrograms per milligram of protein). A few sterol molecules were present in the ER. The Golgi fraction contained an intermediate concentration of free sterols. PM was also characterized by the highest sterol-to-phospholipid molar ratio. In all of the fractions, sterols are present as a mixture in which sitosterol is largely predominent (62%–70%). The other sterols are represented by 24-methylcholesterol, stigmasterol, cholesterol, and isofucosterol. Such a sterol composition and the relatively high content of cholesterol (10%) are in agreement with published data concerning other plants belonging to Liliaceae (Itoh et al., 1977).
Table I.
Sterol composition of membrane fractions isolated from 7-d-old etiolated leek seedlings
| Membrane Fraction | Phospholipids | Sterols | Molar Ratio of Sterols to Phospholipids | Relative Sterol Compositionsa
|
||||
|---|---|---|---|---|---|---|---|---|
| Ch | 24-m | St | Si | Is | ||||
| μg · mg−1 protein | % | |||||||
| ER | 580 | 8.8 | 0.025 | 10.5 | 8.5 | 5.0 | 69.5 | 6.5 |
| Golgi | 500 | 18.9 | 0.065 | 8.5 | 8.0 | 3.0 | 66.5 | 14.0 |
| PM | 420 | 45.5 | 0.18 | 5.0 | 8.0 | 3.0 | 61.5 | 17.0 |
The values are from two independent lipid analyses.
Ch, cholesterol; 24-m, 24-methylcholesterol; St, stigmasterol; Si, sitosterol; Is, isofucosterol.
Transport Kinetics of Sterols to the PM
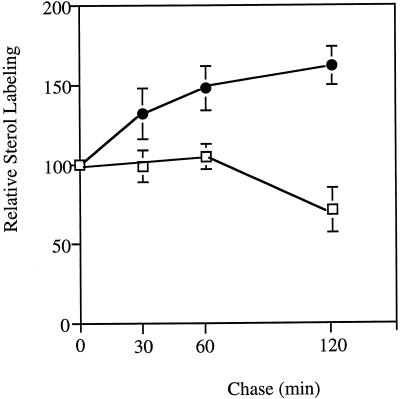
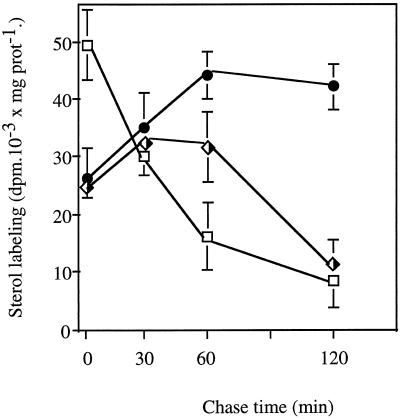
Leek seedlings were first incubated with [1-14C]acetate for 120 min, then with unlabeled acetate for 30 to 120 min (Figs. 1–3). Membrane fractions were prepared and analyzed for their radioactivity incorporated into free sterols. Figure 1 shows the comparative evolution of the radioactivity incorporated into sterols as a function of chase time in microsomes and purified PM. A significant increase in the sterol labeling of PM was observed whatever the chase time, whereas the amount of radioactivity associated with sterols of microsomes remained stable or decreased (after 120 min), indicating that the increase in the sterol labeling of PM was likely due to a delivery of newly synthesized sterols (made during the pulse period). To determine the origin of labeled sterols in the PM, we analyzed the sterol labeling of ER, Golgi, and PM fractions from leek seedlings after similar pulse-chase experiments. Results are presented in Figure 2. At time 0, i.e. at the end of the pulse, the radioactivity associated with sterols of ER was about 2-fold higher than that present in the Golgi and PM fractions, as would be expected for the involvement of ER in the synthesis of free sterols (Hartmann and Benveniste, 1987). During the chase, an increase in the sterol labeling of PM was correlated with a decrease in the radioactivity associated with sterols of ER. The Golgi fraction appeared to have an intermediate behavior, as the sterol labeling increased after 30 and 60 min of chase and decreased after 120 min.
Figure 1.
Sterol labeling in the microsomes (□) and the PM (•) as a function of chase time. Leek seedlings were first incubated with [14C]- acetate for 120 min, then with unlabeled acetate for the indicated periods of time. Microsomes and PM were prepared and the radioactivity associated with sterols determined as explained in Methods. Values are expressed as percentages of the radioactivity (dpm mg−1 protein) incorporated during the 120-min labeling period (n = 7), and correspond to an average of 4 (30- and 60-min chase) and 7 (120-min chase) determinations (±sd). Sterol labeling in the microsomes and PM fraction at the end of the pulse (i.e. 0-min chase) was 65,000 and 25,000 dpm mg−1 protein, respectively.
Figure 2.
Sterol labeling in the ER (□), Golgi (◅), and PM (•) as a function of chase time. Pulse-chase procedures and measurements of sterol labeling were as in Figure 1. PM/ER ratios increase from 0.54 ± 0.06 (120-min pulse) to 5.07 ± 0.45 (120-min chase). The values are from three independent experiments (±sd).
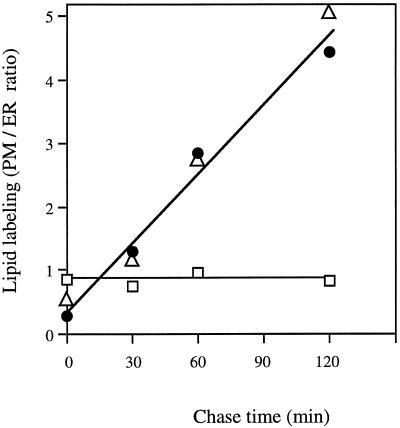
The kinetics of transfer of sterols from the ER to the PM were compared with that of PS, a phospholipid previously shown to be transported to the PM in a vesicular pathway (Sturbois-Balcerzak et al., 1995). As shown in Figure 3, the kinetics of labeling of sterols and PS, as expressed as the ratio of radioactivities associated with the PM to those associated with the ER, were quite similar, suggesting closely related mechanisms of delivery to the cell surface for these two classes of molecules. As a control, the evolution of the labeling of other lipid classes such as diacylglycerols and free fatty acids was checked. No variation in their radioactivities was observed during the chase, indicating that these lipids were not transported.
Figure 3.
PM/ER ratios of sterol and PS labeling (dpm mg−1 protein) as a function of chase time. Pulse-chase procedures and measurements of lipid labeling were as in Figure 1. The data concerning sterols were taken from Figure 2 and compared with those obtained for PS, which is considered as a marker for membrane-mediated processes (Sturbois-Balcerzak et al., 1995), with free fatty acids and diacylglycerol as negative controls. For the sake of clarity, the sds from three experiments with values between 7% and 13% were omitted. Sterol labeling in the ER and PM fractions at the end of the pulse (i.e. 0-min chase) was 50,000 and 27,000 dpm mg−1 protein, respectively. •, Sterol; ▵, PS; □, diacylglycerol plus free fatty acids.
Effect of Low Temperature on Sterol Transport
Leek seedlings were incubated with [14C]acetate for 120 min at 24°C or at 12°C, a temperature that was found to block the transport of some phospholipids (particularly PS and PE) to the PM (Sturbois-Balcerzak et al., 1995). ER, Golgi, and PM fractions were then prepared and their sterol and PS (used as a reporter for the vesicular transport) labeling was determined. To focus only on the effect of low temperature on the distribution of labeled lipids between the various cellular membranes independently of the effect of low temperature on their synthesis, we have calculated for each lipid L (PS or sterols) of each membrane fraction X (ER, Golgi, or PM) the following ratio: [L (X) 12°C/L (X) 24°C] × [L (μ) 24°C/L (μ) 12°C] (see legend of Table II). Two situations can be observed: (a) a value close to 1 for the lipid of the PM means that there is no apparent temperature block, and therefore no great change in the ratio of this lipid in the intracellular membrane fractions is expected; and (b) a value less than 1 for the lipid of the PM indicates an effect of low temperature on the transport of this lipid to the PM. The lipid not transferred would be expected to accumulate intracellulary, and thus the ratio of this lipid will be greater than 1 in one or both intracellular membrane fractions (Table II).
Table II.
Effect of low temperature (12°C) on the delivery of free sterols and PS to the PM
| Membrane Fraction | Radioactivitya(12°C/24°C
Ratios)
|
|
|---|---|---|
| Free sterols | PS | |
| ER | 1.88 ± 0.12 | 1.42 ± 0.18 |
| Golgi | 2.70 ± 0.23 | 1.85 ± 0.24 |
| PM | 0.41 ± 0.07 | 0.34 ± 0.08 |
Leek seedlings were labeled at 24°C or 12°C with [14C]acetate for 120 min. ER, Golgi, and PM were isolated and the radioactivity of free sterols and PS was determined.
We have calculated for each lipid L of each membrane fraction X the following expression: [L (X) 12°C/L (X) 24°C] X [L (μ) 24°C/L (μ) 12°C], where L(X) 12°C and L(X) 24°C are the radioactivities (dpm mg−1 protein) of the lipid L of the membrane fraction X at 12°C and 24°C, respectively, and L (μ) 12°C and L (μ) 24°C are the radioactivities (dpm mg−1 protein) of the lipid L of microsomes at 12°C and 24°C, respectively. These calculations have been made from five experiments (±sd).
The value obtained for free sterols was ≪ 1 for the PM, suggesting that sterols were less efficiently delivered to the PM at 12°C. Moreover, a sterol accumulation was observed in the ER and Golgi fractions (ratios > 1). In agreement with previous results (Sturbois-Balcerzak et al., 1995), the value for PS in the PM was also ≪ 1 and confirmed the arrest of the transfer of this phospholipid to the PM at 12°C. PS was shown to accumulate in the ER and Golgi fractions, since the values of ratios were found to be > 1 in both fractions.
These results strongly suggest that the transport of free sterols to the PM was either slowed down or partly blocked at 12°C in a way similar to that observed for PS transfer. Their accumulation in the intracellular membranes could be explained by the decrease in the number of secretory vesicles and the increase in the surface area of the trans-Golgi/trans-Golgi network that was morphologically observed at 12°C (Sturbois-Balcerzak et al., 1995).
Effect of Monensin and Brefeldin A on Sterol Transport
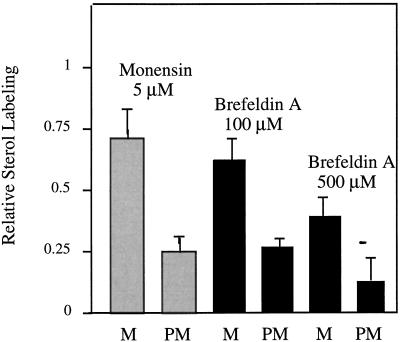
Monensin has been shown to disturb the secretory function of the Golgi apparatus (Mollenhauer et al., 1990) and was previously used to show the intermediate position of this organelle in the delivery of some phospholipids (including PS) to the PM (Bertho et al., 1991; Sturbois-Balcerzak et al., 1995). The monensin concentration (5 μm) used led to a 25% inhibition of sterol synthesis in the microsomes (Fig. 4). Under these conditions, the amount of the radioactivity associated with sterols of the PM was decreased by 75% (Fig. 4), suggesting that the delivery of these molecules to the PM was affected.
Figure 4.
Effect of monensin and brefeldin A on sterol delivery to the PM. Leek seedlings were incubated with [14C]acetate for 120 min in the absence or presence of 5 μm monensin or 100 μm or 500 μm brefeldin A. Microsomes and PM were then prepared and the radioactivity (dpm mg−1 protein) incorporated into sterols was determined. The ratios of treated to untreated were then calculated for each membrane fraction. The data are from three independent experiments and the results are expressed as arbitrary units, with the control values equal to 1. M, Microsomes. Sterol labeling in the crude microsomes and PM in the absence of drug were 56,000 and 31,000 dpm mg−1 protein, respectively.
Brefeldin A, a fungus-derived cyclic lactone, has been largely used to dissect membrane-trafficking events in mammalian cells (Klausner et al., 1992) and has only recently been implemented in plant cells (Satiat-Jeunemaître et al., 1996). Following treatment of leek seedlings with 100 and 500 μm of brefeldin A, the sterol synthesis in the microsomes was found to be inhibited by 37% and 62%, respectively. Under these conditions, the decrease in the sterol labeling of the PM reached 73% and 88%, respectively (Fig. 4), indicating that in addition to an effect on sterol synthesis, brefeldin A also inhibited the delivery of sterols to the PM.
DISCUSSION
In etiolated leek seedlings, free sterols are mainly concentrated in the PM (Table I), as they are in other plant tissues (Hartmann and Benveniste, 1987). A few sterol molecules are present in the ER membranes and an intermediate amount of sterol was found in the Golgi fraction. In all of these membrane fractions, sterols are present as a mixture, with sitosterol as the major compound, suggesting that free sterols are transported together to the PM. In vivo pulse-chase experiments with [1-14C]acetate clearly indicated that sterols are actively transferred from the ER to the PM during the first 60 min of chase. These results are totally in agreement with previous data obtained with maize coleoptiles after in vivo labeling with [5-14C]mevalonic acid (Hartmann, 1980). Such a study indicated that a few molecules of biosynthetic precursors were also transported to the PM. In contrast, steryl esters were found not to be transferred. In leek seedlings kinetics of intracellular transport of free sterols from the ER to the PM were shown to be similar to that of PS. The transport of these two classes of lipids was found to be decreased by low temperature and treatment with monensin and brefeldin A (Table II; Fig. 4), suggesting similar mechanisms of delivery to the PM.
Which mechanism(s) might be responsible for the movement of free sterols to the PM: a simple diffusion through the cytoplasm, a protein-mediated transport, or a vesicular transfer? It is generally accepted that a spontaneous diffusion through the aqueous medium is a slow phenomenon and therefore not a significant route for sterol transport, so an activation-collision mechanism was suggested (Steck et al., 1988). However, a significant sterol exchange through the aqueous phase was recently observed in fibroblasts (Frolov et al., 1996). This spontaneous exchange was faster from PM to intracellular membranes than in the reverse direction (Frolov et al., 1996). These results are therefore not in favor of a quantitative transport of sterols to the PM by simple diffusion.
Specific carrier proteins could enhance the diffusion through the aqueous phase. Frolov et al. (1996) have observed that sterol exchange between biological membranes is highly enhanced by the sterol carrier protein SCP2. In good agreement with these in vitro studies, Puglielli et al. (1995) have shown a requirement for SCP2 in sterol transport from the ER to the PM of cultured fibroblasts. Their results pointed to a predominant SCP2-mediated transport of cholesterol in normal fibroblasts but also to the occurrence of a membrane-mediated transport revealed in the SCP2-deficient fibroblasts. Thus, a protein-stimulated as well as a membrane-mediated transport of sterols has to be considered. There is no indication in the literature of the existence of specialized proteins in plant cells that are able to transfer sterols (Kader, 1996). Although the occurrence of specific sterol-carrier proteins in plants cannot be ruled out, a much more likely way to transfer sterols is via a membrane-mediated process.
Our data are compatible with such a process for several reasons. First, we estimated the t1/2 of sterol transfer to the PM to be about 30 min (Fig. 2), which is of the same order of magnitude as those obtained for phospholipids, which are known to follow a vesicular pathway (Bertho et al., 1991; Sturbois et al., 1994), and for proteins (Mitsui et al., 1985; Kappler et al., 1986). Moreover, the transport of sterols was similar to that of PS (Fig. 3), which has been shown to be membrane mediated (Sturbois et al., 1994; Sturbois-Balcerzak et al., 1995).
In addition, we have observed that low temperature (12°C) partly blocked the delivery of sterols to the PM and resulted in their intracellular accumulation (as was also the case with PS; Table II). Similar results have been obtained in animal cells treated at 15°C (Kaplan and Simoni, 1985), and the existence of cholesterol-rich intracellular membranes potentially involved in cholesterol transport has been demonstrated (Kaplan and Simoni, 1985; Lange and Steck, 1985). Sterol-rich lipid particles have also been suggested as possible structures for the transport of sterols from internal membranes to the PM of yeast (Zinser et al., 1993).
Other arguments favoring a membrane-mediated process come from experiments carried out with monensin and brefeldin A (Fig. 4). Although the effects of monensin on the plant secretory system are controversial (Sticher and Jones, 1988; Zhang et al., 1993; Satiat-Jeunemaître et al., 1994), it has been shown that in our system monensin led to a transport block of some phospholipid species and to their accumulation in internal membranes, including a Golgi-enriched fraction (Bertho et al., 1991; Sturbois-Balcerzak et al., 1995). We found that at a monensin concentration that only slightly affected the de novo synthesis of sterols, their delivery to the PM was dramatically inhibited (Fig. 4). Therefore, it is possible that monensin induces an accumulation of sterol molecules in an intracellular compartment resembling the Golgi-derived swollen vesicles described by Zhang et al. (1996).
The other drug used in these experiments is brefeldin A, and its effect on the Golgi apparatus in plant cells has been extensively described (Satiat-Jeunemaître et al., 1996). Contrary to monensin, the effect of brefeldin A on plant cells has been widely reproduced (Satiat-Jeunemaître et al., 1996) with only few exceptions (Robinson, 1993). The results with brefeldin A are similar to those obtained with monensin (compare 5 μm monensin and 100 μm brefeldin A, Fig. 4). As a consequence, it is likely that a disturbance of the Golgi apparatus was at least to some extent at the origin of the inhibition of sterol transport to the PM.
These data clearly differ from those obtained with Chinese hamster ovary cells, in which none of these drugs affected the intracellular transport of cholesterol (Kaplan and Simoni, 1985; Urbani and Simoni, 1990). In this case, the delivery of newly synthesized cholesterol to the PM would be mediated by lipid-rich vesicles not related to the Golgi apparatus (Urbani and Simoni, 1990; Liscum and Underwood, 1995). The potential occurrence of a direct ER-to-PM pathway for intracellular transport of sterols in leek seedlings also has to be considered (Kristen et al., 1987; Sturbois-Balcerzak et al., 1995).
In conclusion, the present data clearly favor a membrane-mediated process for the transport of free sterols from the ER to the PM in leek seedlings. Free sterols are known to play a key role in regulating the physical properties of the PM as well as the activity of some membrane-bound enzymes such as H+-ATPase. Consequently, levels of these molecules within the PM have to be tightly regulated. Mechanisms contributing to homeostasis of sterols in higher plant cells remain to be elucidated, but certainly differ fundamentally from those operating in mammalian cells. One argument is the absence of the classic low-density lipoprotein pathway in plants. It now appears crucial to isolate membrane vesicles participating in lipid transport from plant tissues. Such experiments are currently being performed in our laboratory and are expected to determine whether free sterols and PS molecules are transported together in the same vesicles, and to shed more light on sterol trafficking in plant cells.
ACKNOWLEDGMENT
We thank Mr. John F. Ackerson for critically reading the English text.
Abbreviations:
- cholesterol
cholest-5-en-3β-ol
- HPTLC
high-performance TLC
- isofucosterol
stigmasta-5,24(241)Z-dien-3β-ol
- PM
plasma membrane
- PS
phosphatidylserine
- sitosterol
(24R)-24-ethylcholest-5-en-3β-ol
- stigmasterol
(24S)-24-ethylcholesta-5,22E-dien-3β-ol
Footnotes
This work was supported by the Centre National de la Recherche Scientifique and the University Victor Segalen Bordeaux 2.
LITERATURE CITED
- Benveniste P. Sterol biosynthesis. Annu Rev Plant Physiol. 1986;37:275–308. [Google Scholar]
- Bertho P, Moreau P, Morré DJ, Cassagne C. Monensin blocks the transfer of very long chain fatty acid containing lipids to the plasma membrane of leek seedlings: evidence for lipid sorting based on fatty acyl chain length. Biochim Biophys Acta. 1991;1070:127–134. doi: 10.1016/0005-2736(91)90154-z. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Frolov A, Woodford JK, Murphy EJ, Billheimer JT, Schroeder F. Spontaneous and protein-mediated sterol transfer between intracellular membranes. J Biol Chem. 1996;271:16075–16083. doi: 10.1074/jbc.271.27.16075. [DOI] [PubMed] [Google Scholar]
- Hartmann MA (1980) These de Doctorat d'Etat, Université Louis Pasteur, Strasbourg, France
- Hartmann MA, Benveniste P. Plant membrane sterols: isolation, identification and biosynthesis. Methods Enzymol. 1987;148:632–650. [Google Scholar]
- Heape AM, Juguelin H, Boiron F, Cassagne C. Improved one-dimensional thin-layer chromatographic technique for polar lipids. J Chromatogr. 1985;322:391–395. doi: 10.1016/s0021-9673(01)97702-7. [DOI] [PubMed] [Google Scholar]
- Horsley D, Coleman J, Evans D, Crooks K, Peart J, Satiat-Jeunemaitre B, Hawes C. A monoclonal antibody, JIM 84, recognizes the Golgi apparatus and plasma membrane in plant cells. J Exp Bot. 1993;44:223–229. [Google Scholar]
- Itoh T, Tamura T, Mitsuhashi T, Matsumoto T. Sterols of Liliaceae. Phytochem. 1977;16:140–141. [Google Scholar]
- Kader JC. Lipid-transfer proteins in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:627–654. doi: 10.1146/annurev.arplant.47.1.627. [DOI] [PubMed] [Google Scholar]
- Kaplan MR, Simoni RD. Transport of cholesterol from the endoplasmic reticulum to the plasma membrane. J Cell Biol. 1985;101:446–453. doi: 10.1083/jcb.101.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler R, Kristen U, Morré DJ. Membrane flow in plants: fractionation of growing pollen tubes of tobacco by preparative free-flow electrophoresis and kinetics of labeling of endoplasmic reticulum and Golgi apparatus with [3H] leucine. Protoplasma. 1986;132:38–50. [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristen U, Lockhausen J, Kandasamy MK. Structural aspects of protein secretion in higher plant cells. Phyton. 1987;28:183–191. [Google Scholar]
- Lange Y, Steck TL. Cholesterol-rich intracellular membranes: a precursor to the plasma membrane. J Biol Chem. 1985;260:15592–15597. [PubMed] [Google Scholar]
- Liscum L, Underwood KW. Intracellular cholesterol transport and compartmentation. J Biol Chem. 1995;270:15443–15446. doi: 10.1074/jbc.270.26.15443. [DOI] [PubMed] [Google Scholar]
- Mitsui T, Akazawa T, Christeller JT, Tartakoff AM. Biosynthesis of rice seed α-amylase: two pathways of amylase secretion by the scutellum. Arch Biochem Biophys. 1985;241:315–328. doi: 10.1016/0003-9861(85)90388-1. [DOI] [PubMed] [Google Scholar]
- Mollenhauer HH, Morré DJ, Rowe LD. Alteration of intracellular traffic by monensin: mechanism, specificity and relationship to toxicity. Biochim Biophys Acta. 1990;1031:225–246. doi: 10.1016/0304-4157(90)90008-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P, Cassagne C. Phospholipid trafficking and membrane biogenesis. Biochim Biophys Acta. 1994;1197:257–290. doi: 10.1016/0304-4157(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Moreau P, Juguelin H, Lessire R, Cassagne C. Plasma membrane biogenesis in higher plants: in vivo transfer of lipids to the plasma membrane. Phytochemistry. 1988;27:1631–1638. [PubMed] [Google Scholar]
- Napier RM, Fowke L, Hawes C, Lewis M, Pelham HRB. Immunological evidence that plants use both HDEL and KDEL for targeting proteins to the endoplasmic reticulum. J Cell Sci. 1992;102:261–271. doi: 10.1242/jcs.102.2.261. [DOI] [PubMed] [Google Scholar]
- Puglielli L, Rigotti A, Greco AV, Santos MJ, Nervi F. Sterol carrier protein-2 is involved in cholesterol transfer from the endoplasmic reticulum to the plasma membrane in human fibroblasts. J Biol Chem. 1995;270:18723–18726. doi: 10.1074/jbc.270.32.18723. [DOI] [PubMed] [Google Scholar]
- Rahier A, Benveniste P (1989) Mass spectral identification of phytosterols. In WD Nes, E Parish, eds, Analysis of Sterols and Other Significant Steroids. Academic Press, New York, pp 223–250
- Robinson D. Brefeldin A: a tool for plant cell biologists? Bot Acta. 1993;106:107–109. [Google Scholar]
- Satiat-Jeunemaître B, Cole L, Bourett T, Howard R, Hawes C . Brefeldin A effects in plant and fungal cells: something new about vesicle trafficking? J Microscopy. 1996;181:162–177. doi: 10.1046/j.1365-2818.1996.112393.x. [DOI] [PubMed] [Google Scholar]
- Satiat-Jeunemaître B, Fitchette-Lainé AC, Alabouvette J, Marty-Mazars D, Hawes C, Faye L, Marty F. Differential effects of monensin on the plant secretory pathway. J Exp Bot. 1994;45:685–698. [Google Scholar]
- Steck TL, Ferenc JK, Lange Y. An activation-collision mechanism for cholesterol transfer between membranes. J Biol Chem. 1988;263:13023–13031. [PubMed] [Google Scholar]
- Sticher L, Jones RL. Monensin inhibits the secretion of α-amylase but not polysaccharide slime from seedling tissues of Zea mays. Protoplasma. 1988;142:36–45. [Google Scholar]
- Sturbois B, Moreau P, Maneta-Peyret L, Morré DJ, Cassagne C. Cell-free transfer of phospholipids between the endoplasmic reticulum and the Golgi apparatus of leek seedling. Biochim Biophys Acta. 1994;1189:31–37. doi: 10.1016/0005-2736(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Sturbois-Balcerzak B, Morré DJ, Loreau O, Noël JP, Moreau P, Cassagne C. Effects of low temperatures on the transfer of phospholipids to the plasma membrane and on the morphology of the ER-Golgi apparatus-plasma membrane pathway of leek cells. Plant Physiol Biochem. 1995;33:625–637. [Google Scholar]
- Urbani L, Simoni RD. Cholesterol and vesicular stomatitis virus G protein take separate routes from the endoplasmic reticulum to the plasma membrane. J Biol Chem. 1990;265:1919–1923. [PubMed] [Google Scholar]
- Zhang GF, Driouich A, Staehelin LA. Effect of monensin on plant Golgi: re-examination of the monensin-induced changes in cisternal architecture and functional activities of the Golgi apparatus of sycamore suspension-cultured cells. J Cell Sci. 1993;104:819–831. doi: 10.1242/jcs.104.3.819. [DOI] [PubMed] [Google Scholar]
- Zhang GF, Driouich A, Staehelin LA. Monensin-induced redistribution of enzymes and products from Golgi stacks to swollen vesicles in plant cells. Eur J Cell Biol. 1996;71:332–340. [PubMed] [Google Scholar]
- Zinser E, Paltauf F, Daum G. Sterol composition of yeast organelle membranes and subcellular distribution of enzymes involved in sterol metabolism. J Bacteriol. 1993;175:2853–2858. doi: 10.1128/jb.175.10.2853-2858.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]