Abstract
Purpose: In lung radiotherapy, variations in cycle-to-cycle breathing results in four-dimensional computed tomography imaging artifacts, leading to inaccurate beam coverage and tumor targeting. In previous studies, the effect of audiovisual (AV) biofeedback on the external respiratory signal reproducibility has been investigated but the internal anatomy motion has not been fully studied. The aim of this study is to test the hypothesis that AV biofeedback improves diaphragm motion reproducibility of internal anatomy using magnetic resonance imaging (MRI).
Methods: To test the hypothesis 15 healthy human subjects were enrolled in an ethics-approved AV biofeedback study consisting of two imaging sessions spaced ∼1 week apart. Within each session MR images were acquired under free breathing and AV biofeedback conditions. The respiratory signal to the AV biofeedback system utilized optical monitoring of an external marker placed on the abdomen. Synchronously, serial thoracic 2D MR images were obtained to measure the diaphragm motion using a fast gradient-recalled-echo MR pulse sequence in both coronal and sagittal planes. The improvement in the diaphragm motion reproducibility using the AV biofeedback system was quantified by comparing cycle-to-cycle variability in displacement, respiratory period, and baseline drift. Additionally, the variation in improvement between the two sessions was also quantified.
Results: The average root mean square error (RMSE) of diaphragm cycle-to-cycle displacement was reduced from 2.6 mm with free breathing to 1.6 mm (38% reduction) with the implementation of AV biofeedback (p-value < 0.0001). The average RMSE of the respiratory period was reduced from 1.7 s with free breathing to 0.3 s (82% reduction) with AV biofeedback (p-value < 0.0001). Additionally, the average baseline drift obtained using a linear fit was reduced from 1.6 mm/min with free breathing to 0.9 mm/min (44% reduction) with AV biofeedback (p-value = 0.012). The diaphragm motion reproducibility improvements with AV biofeedback were consistent with the abdominal motion reproducibility that was observed from the external marker motion variation.
Conclusions: This study was the first to investigate the potential of AV biofeedback to improve the motion reproducibility of internal anatomy using MRI. The study demonstrated the significant improvement in diaphragm motion reproducibility using AV biofeedback combined with MRI. This system can potentially provide clinically beneficial motion management of internal anatomy in MRI and radiotherapy.
Keywords: AV biofeedback, diaphragm
INTRODUCTION
Studies have reported that four-dimensional computed tomography (4DCT) (Refs. 1 and 2) and positron emission tomography (PET) (Ref. 3) images suffer from motion artifacts caused by respiratory irregularity. Variations in cycle-to-cycle breathing cause inadequate respiratory motion sampling in image reconstruction, resulting in image distortions of moving organs, such as blurring and overlapping structures.1, 2 These artifacts can cause incorrect target and healthy surrounding tissue delineation in treatment planning, leading to the irradiation of healthy surrounding tissues in addition to the tumor itself, which results in a substantial increase in radiation-related toxicity.4, 5, 6
To address the problem of respiratory irregularity, various approaches to patient breathing training have been applied. Recently, respiratory motion-guidance systems using an external surrogate7, 8, 9, 10, 11, 12 were utilized in treatment procedures and medical imaging, improving respiratory motion reproducibility during radiotherapy and medical imaging. In magnetic resonance imaging (MRI), an audio breath-hold prompt was used to improve coronary imaging8 and a MR-compatible active breathing control (ABC) system was applied to lung MRI.13 In previous respiratory motion-guidance studies, George et al. employed audio instruction or visual feedback in order to maintain a constant inhale/exhale position of abdominal motion during breathing.14 Lim et al. utilized a visual guidance system combined with a respiration monitoring mask to guide respiratory motion.7
Audiovisual (AV) biofeedback, developed by Venkat et al., utilized respiratory-related abdominal motion to audio-visually guide the patient to produce regular respiratory motion.9 Venkat et al. reported that AV biofeedback significantly reduced average cycle-to-cycle variations in respiratory-related abdominal motion amplitude and periods by 55% and 75%, respectively. In addition, Yang et al. reported improved PET image quality in a phantom study using the AV biofeedback motion traces derived from the Venkat study.3 They found that motion blurring artifacts were reduced using AV biofeedback. For example, the average increase in target diameter due to motion blurring artifacts was reduced from 1.3 mm with free breathing to 0.6 mm with AV biofeedback.
In previous studies, the effect of respiratory motion-guidance systems on the external respiratory signal reproducibility has been investigated but the internal anatomy has not been fully studied. Vedam et al. investigated diaphragm motion reproducibility of five lung cancer patients using fluoroscopy with visual feedback, but the effect of AV biofeedback was not tested and their sample size was small. The current study was the first to investigate the potential of AV biofeedback to improve the motion reproducibility of the internal anatomy with MRI. The aim of this study was to test the hypothesis that AV biofeedback improves the diaphragm motion reproducibility during MRI scans.
METHOD AND MATERIALS
AV biofeedback
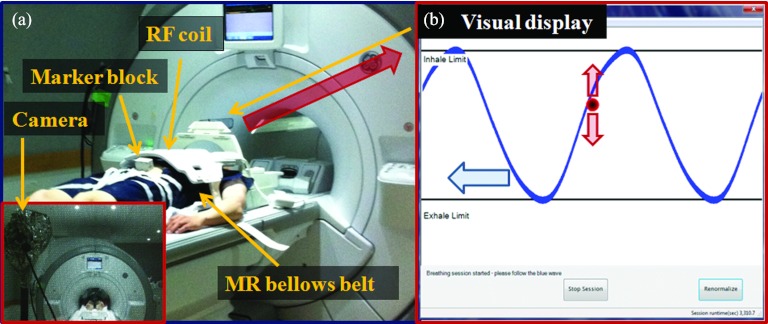
The AV biofeedback system was developed to provide respiratory guidance during medical imaging and treatment procedures.9 Real-time respiratory motion signals were obtained using the real-time position management (RPM) system (Varian Medical Systems, Palo Alto, USA) consisting of an infrared camera and a marker block on the abdomen (Fig. 1). The respiratory motion of the external-marker was utilized to compile a patient-specific visual guiding wave formulated from the patients’ own breathing within the MRI in each session. This system was combined with MR compatible AV equipment including a projector, screen, and headphones. During an AV biofeedback session, the visual component consisted of a patient-specific visual guiding wave and a red ball moving up and down; the red ball's motion corresponded to the motion of the external marker on their abdomen. Each patient can adjust their breathing so that the red ball followed the guiding wave on the screen. Simultaneously, the audio component consisted of classical music being played to the patient. If the breathing signal deviated by more than 15% from the guiding wave, the speed of the music was increased or decreased to inform the patient that an adjustment to their breathing was required.
Figure 1.
(a) AV biofeedback system in 3 Tesla GE MRI. Infrared camera and marker block on the abdomen. (b) The screen of the AV biofeedback system shows a guiding wave (curve moving to the left) and the current breathing position (ball moving up and down) in real time. The goal is to match the ball to the curve during MR scans.
MRI studies with AV biofeedback
The improvement in the diaphragm motion reproducibility using the AV biofeedback system combined with thoracic MRI was investigated across 30 studies with 15 healthy human subjects (2 female and 13 male). The range of ages of the patients was 19–53 yr with a mean of 31 yr. Our imaging protocol was approved by the institutional ethics board and informed consent was obtained from the participants. Each subject underwent two sessions for assessment of the diaphragm motion reproducibility both with AV biofeedback and without (free breathing). The second session featured the reverse order of breathing conditions (AV biofeedback followed by free breathing) in order to avoid any bias against the breathing order. The MRI acquisitions consisted of both coronal and sagittal planes according to the intersectional diaphragm position. On coronal images the thoracic cavity was clearly visible and on sagittal images the right thoracic region and the liver were observed.
In our studies, we utilized (1) the external position information of the abdomen using the RPM system (30 Hz) to guide the subjects breathing and synchronously (2) a fast 2D gradient-recalled-echo (fGRE) MR pulse sequence to measure the diaphragm position (196 ms/frame). The RPM signal and MR acquisitions were not temporally synchronized. To achieve this, we used the GE bellows belt (25 Hz) during the MR scan. The RPM signal was synchronized with the bellows belt signal by direct signal matching as these were placed in the same anatomic position (abdomen). The bellows belt signal was time synchronized with the MR acquisition by the MR scanner. Therefore, we could temporally synchronize the RPM signal with the MR acquisition. Note that the GE bellows belt could not be used directly as the respiratory signal as it has a range of motion and baseline drift correction algorithm embedded in the acquisition hardware on the scanner.
For thoracic imaging, typical MR imaging parameters were TR/TE = 2.4/1 ms, FOV = 480 × 384 mm2, slice thickness = 5 mm, image matrix = 96 × 96, and acquisition time for each image = 196 ms (512 2D images were acquired for each measurement). Each MRI was interpolated to an image matrix of 256 × 256 by the MR scanner (using the extended zero-filling method) before further image processing.
Reproducibility metrics
Visual and statistical evaluations of diaphragm and abdominal motion reproducibility were performed. In the diaphragm motion analysis, the five pixel-wide and the 60 pixel-long rectangle region of interest (ROI) was selected on the right diaphragm including the dome of the liver since the left side of the chest showed a high contribution of signal intensity from blood vessels and the heart. Each column of the signal intensity was averaged to reduce noise while enhancing diaphragm detection on each frame. The root mean square error (RMSE) of diaphragm and abdominal motion displacement and period were calculated from the diaphragm motion data and the abdominal motion data. In the analysis the average waveform from the data was calculated using a Fourier series fit.9 Each waveform was compared with the average waveform to calculate the RMSE of displacement in the phase domain. In addition, the baseline drift was quantified using the slope of the linear fit on the collected data.
The RMSE in period was also computed from each waveform. To evaluate the diaphragm motion reproducibility, the dispersion of the power spectrum scaled by fundamental frequency in Fourier space was used as a metric to quantify the complexity and irregularity of the diaphragm motion (spectral power dispersion metric: SPDM).15 This metric was the second moment around the mean frequency and a smaller value indicated a less complex breathing pattern.
Quantitative statistical comparison of RMSE in displacement and period from the different breathing conditions was conducted using the paired Student's t-test (Microsoft Excel 2007, TTEST). To investigate the variation between sessions, the paired Student t-test between the first session and the second session was performed.
RESULTS
Fifteen healthy human subjects participated across 30 MRI studies. The total MRI acquisitions across the 30 studies were 242 measurements (72 coronal and 170 sagittal). Due to a technical issue, one respiratory dataset from the RPM system with AV biofeedback was not completed.
Diaphragm motion reproducibility
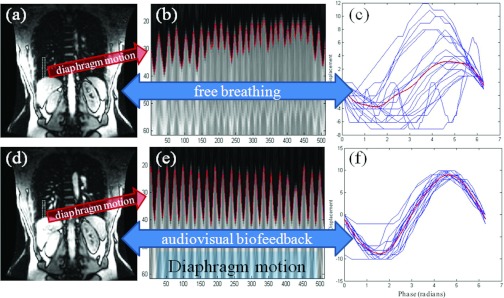
As shown in Fig. 2, the fGRE pulse sequence (196 ms acquisition) in the coronal plane was able to clearly display anatomical features such as the liver, right and left kidneys, and spleen. A 1D signal profile of the ROI, which included the diaphragm, was obtained from the 512 images, and the regularity metric was computed. Figure 2e shows that AV biofeedback was able to produce much more reproducible diaphragm motion in both period and displacement. In addition, variation in the mean diaphragm position from cycle-to-cycle was reduced by using AV biofeedback. Diaphragm motion cycles close to the average waveform in the phase domain indicate that the diaphragm motion reproducibility was improved, as shown in Fig. 2f.
Figure 2.
(a, d) ROI (region of interest) boxes on coronal images in Study 6. (b) and (e) 1D signal profile of the ROI over 512 images. Outline of diaphragm shown (solid line). (c, f) Diaphragm motion cycles (thin) and average curve (thick) shown in phase domain. By using the AV biofeedback system the diaphragm motion reproducibility has been significantly improved.
MRIs of the right thoracic region in the sagittal plane were obtained showing the diaphragm and liver. Like the coronal images, a 1D signal profile of the ROI, including the diaphragm, was obtained from the 512 images and the regularity metric was computed, demonstrating the improved diaphragm motion reproducibility due to AV biofeedback in both period and displacement. In addition, AV biofeedback reduced the baseline drift, which is consistent with the results from the coronal images.
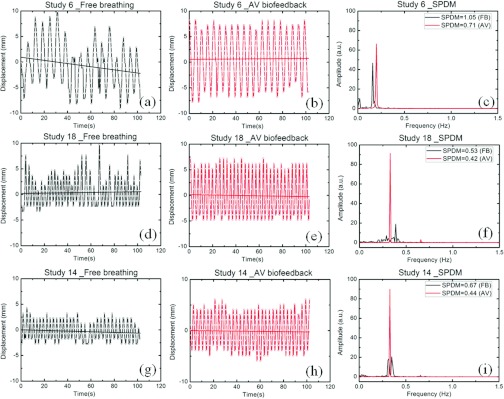
In Fig. 3, examples of three sets of diaphragm motion data with free breathing and with AV biofeedback are shown. Three of the 30 studies, representative of the 90th, 50th, and 10th percentile range of reduced RMSE in displacement due to AV biofeedback, are presented. For the 90th percentile, study 6 in Figs. 3a, 3b, there is a 65% reduction in RMSE in displacement due to AV biofeedback. For the 50th percentile, study 18 in Figs. 3d, 3e, there is a 34% reduction of RMSE. This indicates that half of the subjects had an improvement in RMSE of at least 34%. For the 10th percentile, study 14 in Figs. 3g, 3h, AV biofeedback increased the RMSE in displacement by 34%. In Figs. 3a, 3b, 3d, 3e, 3g, 3h, the solid lines are the baseline of respiratory cycles obtained using a linear fit on to the respiratory data. A substantial baseline drift is shown in Fig. 3a with free breathing for study 6. Figures 3c, 3f, 3i illustrate the SPDM of each diaphragm respiratory signal. The diaphragm motion with free breathing shows large variations in displacement, period, and baseline. However, the variations have been significantly reduced by using the AV biofeedback system.
Figure 3.
Diaphragm motion data with (a), (d), and (g) free breathing (b), (e), and (h) AV biofeedback from three of the 30 studies representative of the 90th (study 6), 50th (study 18), and 10th (study 14) percentile range of reduced RMSE in displacement due to AV biofeedback. A constant y-offset value (mean position of the diaphragm motion data for the first 15 s) has been applied to the displacement values of each dataset to increase the clarity of the figure. The solid lines are the baseline of respiratory cycles. (c), (f), and (i) Spectral power dispersion of each diaphragm motion data with SPDM are presented.
Table 1 shows average RMSE and baseline drift of diaphragm motion and results of paired Student t-test comparing the breathing models. The results are a significant reduction of RMSE in displacement and period, and baseline drift due to AV biofeedback: 31%, 71%, and 35%, respectively, for the first session and 46%, 86%, and 50%, respectively, for the second session. In the significance test, statistical significance was determined by the reduction of RMSE in displacement and period, and baseline drift.
Table 1.
Averaged RMSE and baseline drift of diaphragm motion and paired Student t-test p-values (FB denotes free breathing and AV denotes AV biofeedback).
| Diaphragm motion | RMSE in displacement (mm) | p-value | RMSE in period (s) | p-value | Baseline drift (mm/min) | p-value | |
|---|---|---|---|---|---|---|---|
| First session | FB | 2.6 | 0.026 | 1.4 | 0.004 | 1.7 | 0.201 |
| AV | 1.8 (−31%) | 0.4 (−71%) | 1.1 (−35%) | ||||
| Second session | FB | 2.6 | 0.0006 | 2.1 | <0.0001 | 1.6 | 0.019 |
| AV | 1.4 (−46%) | 0.3 (−86%) | 0.8 (−50%) | ||||
| All sessions | FB | 2.6 | <0.0001 | 1.7 | <0.0001 | 1.6 | 0.012 |
| AV | 1.6 (−38%) | 0.3 (−82%) | 0.9 (−44%) |
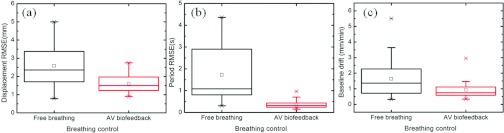
Figure 4 is a comparison between free breathing and AV biofeedback for the entire study using box plots of the RMSE in displacement and phase, and baseline drift. In Fig. 4a, the average RMSE of diaphragm motion displacement was reduced from 2.6 mm with free breathing to 1.6 mm with AV biofeedback. Figure 4b illustrates reduction in the RMSE of period from 1.7 s with free breathing to 0.35 s with AV biofeedback. In addition, average baseline drift has been reduced from 1.6 mm/min with free breathing to 0.9 mm/min with AV biofeedback [Fig. 4c].
Figure 4.
Box plots of the RMSE in (a) displacement, (b) period, and (c) baseline drift of diaphragm motion.
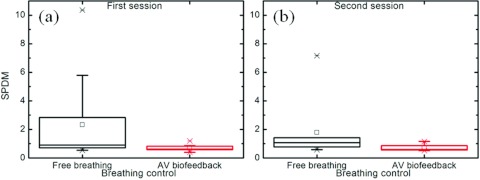
Figure 5 is an evaluation of the diaphragm motion reproducibility using the SPDM metric. There is a 70% reduction with AV biofeedback in the first session (2.3 with free breathing and 0.7 with AV biofeedback: p-value = 0.052) and a 61% reduction with AV biofeedback in the second session (1.8 with free breathing and 0.7 with AV biofeedback: p-value = 0.046). The p-value of SPDM over all studies between free breathing and AV biofeedback was 0.005 (67% reduction with AV biofeedback).
Figure 5.
Spectral power dispersion metric of the diaphragm motion in Fourier space, scaled by fundamental frequency (SPDM). (a) First session. (b) Second session. There is a 67% reduction with AV biofeedback in all sessions (2.1 with free breathing and 0.7 with AV biofeedback: p-value = 0.005).
Table 2 demonstrates the ratios between AV biofeedback and free breathing for RMSE in displacement and period in addition to baseline drift. A lower ratio indicates greater improvement in regularity with AV biofeedback. A greater improvement in regularity with AV biofeedback in the second session when compared to the first session is observed for both diaphragm and abdominal motion. However, the p-values indicate that this is not statistically conclusive (p-values > 0.05).
Table 2.
Comparison of breathing reproducibility (RMSEAV/RMSEFB and baseline driftAV/baseline driftFB) with prior AV biofeedback training (variation between the two sessions).
| Paired t-test | Diaphragm motion |
|||||
|---|---|---|---|---|---|---|
| RMSEAV/RMSEFB | RMSEAV/RMSEFB | Baseline driftAV/ | ||||
| in displacement |
in breathing period |
baseline driftFB |
||||
| Session | 1st | 2nd | 1st | 2nd | 1st | 2nd |
| Average value | 0.875 | 0.639 | 0.426 | 0.269 | 1.426 | 0.926 |
| Cases improved by AV biofeedback | 12 of 15 | 14 of 15 | 14 of 15 | 14 of 15 | 11 of 15 | 11 of 15 |
| p-value | 0.170 | 0.212 | 0.489 | |||
| Abdominal motion |
||||||
| Session |
1st |
2nd |
1st |
2nd |
1st |
2nd |
| Average value | 0.700 | 0.509 | 0.386 | 0.237 | 0.904 | 1.684 |
| Cases improved by AV biofeedback | 12 of 14 | 15 of 15 | 14 of 14 | 15 of 15 | 10 of 14 | 11 of 15 |
| p-value | 0.053 | 0.093 | 0.230 | |||
Abdominal motion reproducibility
In addition to qualitative evaluation of diaphragm motion regularity during MR scans, respiratory waveforms on the MRI computer screen and the AV biofeedback screen were visually inspected. AV biofeedback produced more regular external marker motion by reducing cycle-to-cycle variations in period and displacement. The external marker was positioned on the subject's abdomen which implies that the motion of the abdomen is more reproducible with AV biofeedback.
In Table 3, the RMSE averaged over all of the studies and Student's t-test p-values comparing the breathing methods are reported for the abdominal motion. AV biofeedback reduced RMSE in displacement by 33% in the first session and 54% in the second session. AV biofeedback also reduced RMSE in period by 67% in the first session and 84% in the second session. Additionally, AV biofeedback reduced average baseline drift by 47% and 65% in the first and second sessions, respectively. All p-values comparing the different regularity metrics were less than 0.05, indicating statistical significance in the reduction of baseline drift as well as the RMSE of both displacement and period.
Table 3.
RMSE and baseline drift of abdominal motion averaged over all of the studies and paired Student t-test p-value comparing the breathing methods (FB denotes free breathing and AV denotes AV biofeedback).
| Abdominal motion | RMSE in displacement (mm) | p-value | RMSE in period (s) | p-value | Baseline drift (mm/min) | p-value | |
|---|---|---|---|---|---|---|---|
| First session | FB | 1.2 | 0.0016 | 1.2 | 0.0008 | 0.21 | 0.007 |
| AV | 0.8 (−33%) | 0.4 (−67%) | 0.06 (−70%) | ||||
| Second session | FB | 1.3 | <0.0001 | 1.9 | 0.0008 | 0.22 | 0.011 |
| AV | 0.6 (−54%) | 0.3 (−84%) | 0.04 (−80%) | ||||
| All sessions | FB | 1.3 | <0.0001 | 1.6 | <0.0001 | 0.21 | <0.0001 |
| AV | 0.7 (−46%) | 0.3 (−81%) | 0.05 (−75%) |
DISCUSSION
In this study the improvement in internal anatomic motion using AV biofeedback in conjunction with MRI was investigated for the first time. The only other study to have investigated internal motion with visual biofeedback was that of Vedam et al.16 who used fluoroscopy in a smaller cohort (five lung cancer patients). In their study, they utilized a visual motion trace on a screen, which included two motion limits (inhale and exhale limits) for visual biofeedback. To further enhance biofeedback guidance systems, this study explicitly utilized subject-specific waveguides for each of the 15 subjects for the visual component of the AV biofeedback system.
A reduction of RMSE in average abdominal displacement (46%) and period (81%) with AV biofeedback was found in this study. This reduction is consistent with the results reported by Venkat et al., who observed a reduction in RMSE for average abdominal displacement of 55% and a reduction in RMSE for period of 75%.9 In addition, Venkat et al. observed a reduction in baseline drift for abdominal motion, which is consistent with our findings and in agreement with the findings of George et al.10
Unlike previous studies, this study directly investigated the improvement of diaphragm motion reproducibility due to the implementation of AV biofeedback through the use of thoracic MR images. To avoid any bias against the breathing order, the second session was conducted with a reversed order of breathing methods. That is, the first session used free breathing followed by AV biofeedback, while the second session started with AV biofeedback. In the results, most of the volunteers breathing reproducibility improved when using AV biofeedback but there were a small number that did not show improvement. This was due to some subjects having regular free breathing or having difficulty following the waveguide. Clinically, a short free breathing session for each patient should be performed to assess their suitability for the use of AV biofeedback.
As shown in Table 1, the motion reproducibility with AV biofeedback in the second session showed improvement over that in the first session, suggesting that there may be a benefit to prior AV biofeedback training in addition to the breathing order. However, in the paired Student's t-test, comparing the improvement of breathing reproducibility (the ratio between AV biofeedback and free breathing for RMSE in displacement and period) with prior breathing training, it was demonstrated that the prior breathing training did not have a statistically significant effect on diaphragm displacement, breathing period, and baseline drift. This could be due to the sample size (15 subjects) or low number of repetitions (1 repetition/subject).
Two separate respiratory signals were utilized: (1) the RPM signal which was fed into the AV biofeedback system for audio and visual guidance and (2) the diaphragm respiratory signal which was obtained from thoracic MR images simultaneously with the RPM signal. In the experimental setup, the GE cardiac RF coil was placed on the chest of each subject on top of the MR bellows belt. For a clear view from the RPM camera, the marker block was positioned on the subject's abdomen, far enough away from RF coil such that the coil did not disrupt the marker's motion. Therefore, a possible time discrepancy may exist between two respiratory signals measured at different anatomic locations. However, any significant impact on the findings was not observed. If any time discrepancy occurred, systematic variations would have been present in both the results of AV biofeedback and free breathing. Note that the improvement for the diaphragm motion reproducibility was similar to the improvement for the abdominal motion reproducibility which indicates that any systematic errors are likely to be small or insignificant.
fGRE is a fast MR pulse sequence available on a 3T GE clinical MRI. In this study, the high sample rate (5 Hz) with thoracic MR imaging made diaphragm motion measurements available during AV biofeedback. Anatomical features, such as the liver, kidneys, and spleen, were clearly observable in addition to the diaphragm because internal organs have a higher proton density than lung tissue and the MR signal of the organs was more prominent than that of the lung tissue. Distinct motion artifacts such as ghost artifact along the phase encoding direction were not detected in the images. Wrap-around artifacts were avoided by using a large field of view, and motion artifacts were avoided by utilizing a fast image acquisition time. The use of a small image matrix (96 × 96) significantly reduced MRI acquisition time but uncertainty in diaphragm position increased due to the large size of the image pixels. However, the extended zero-filling method of interpolation produces improved apparent spatial resolution to improve accuracy of edge detection while real image resolution remains.17, 18 Unlike directly interpolating the image, the zero-filling method increases the apparent image resolution by zero-filling to the end of the sampled data which interpolates the continuous shape of objects and reduces partial volume artifacts.17 In this paper, RMSE in displacement has been analyzed using the apparent image resolution (1.875 × 1.875 mm2) and signal averaging from the 5 pixel-wide and the 60 pixel-long rectangular ROI.
This study investigated the effect of AV biofeedback on the improvement of diaphragm motion reproducibility with healthy human subjects and not cancer patients. These results may be applicable to some cancer patients typically without compromised lung function, such as those with liver, kidney, or pancreatic tumors. To translate the findings from this study to patients typically with compromised lung function, such as lung cancer patients, several challenges need to be addressed. First, potential weak correlations between patient abdominal and internal anatomic motion has been observed in some patients.19 The weak correlations could degrade the improvement of internal anatomic motion reproducibility using the AV biofeedback system. To overcome this problem, the AV biofeedback system can be integrated with devices to monitor internal markers, such as the Calypso system, to allow AV guidance with an internal marker. This would eliminate the need to establish a correlation between internal and external marker positions. Second, some patients have difficulties following the instructions or the guiding wave and training sessions prior to medical imaging or radiation treatment may reduce the patient's anxiety and improve familiarity with and performance of the AV biofeedback system. Third, long medical imaging and treatment sessions could lead to a gradual decline in performance. Each imaging session within this study took about 1 h to complete across the two breathing sessions. Although this study could not mimic treatment sessions fully, the designated AV biofeedback session time of about 30 min is comparable to real treatment durations.
This study demonstrated the feasibility of AV biofeedback to improve internal motion reproducibility. Using the AV biofeedback system can reduce average cycle-to-cycle variations in breathing, leading to improved image quality, with fewer artifacts and more reproducible intrafractional motion applicable to a course of fractionated radiotherapy.
CONCLUSION
This study demonstrated the improvement in diaphragm motion reproducibility using AV biofeedback combined with MRI. This system can potentially provide clinically beneficial motion management of internal anatomy in MRI and radiotherapy.
ACKNOWLEDGMENTS
This work was supported by Sydney Medical School New Staff/Early Career Researcher Scheme Grant, NIH/NCI Grant No. R01CA93626 and an NHMRC Australia Fellowship. The authors thank Julie Baz and Casey Willoughby for their review of this paper, Michael Graf for technical support on MRI measurements, and Dr. Elaine Ryan for preparing the IRB application.
References
- Yamamoto T., Langner U., B. W.LooJr., Shen J., and Keall P. J., “Retrospective analysis of artifacts in four-dimensional CT images of 50 abdominal and thoracic radiotherapy patients,” Int. J. Radiat. Oncol., Biol., Phys. 72, 1250–1258 (2008). 10.1016/j.ijrobp.2008.06.1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner U. W. and Keall P. J., “Quantification of artifact reduction with real-time cine four-dimensional computed tomography acquisition methods,” Int. J. Radiat. Oncol., Biol., Phys. 76, 1242–1250 (2010). 10.1016/j.ijrobp.2009.07.013 [DOI] [PubMed] [Google Scholar]
- Yang J., Yamamoto T., Cho B., Seo Y., and Keall P. J., “The impact of audio-visual biofeedback on 4D PET images: Results of a phantom study,” Med. Phys. 39, 1046–1057 (2012). 10.1118/1.3679012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo G. D., Campbell J., Zhang T., and Di Yan D. S., “Cumulative lung dose for several motion management strategies as a function of pre-treatment patient parameters,” Int. J. Radiat. Oncol., Biol., Phys. 74, 593–601 (2009). 10.1016/j.ijrobp.2008.12.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theuws J., Kwa S. L. S., Wagenaar A. C., Seppenwoolde Y., Boersma L. J., Damen E. M. F., Muller S. H., Baas P., and Lebesque J. V., “Prediction of overall pulmonary function loss in relation to the 3-D dose distribution for patients with breast cancer and malignant lymphoma,” Radiother. Oncol. 49, 233–243 (1998). 10.1016/S0167-8140(98)00117-0 [DOI] [PubMed] [Google Scholar]
- Marks L. B., Bentzen S. M., Deasy J. O., Kong F. M. S., Bradley J. D., Vogelius I. S., El Naqa I., Hubbs J. L., Lebesque J. V., and Timmerman R. D., “Radiation dose-volume effects in the lung,” Int. J. Radiat. Oncol., Biol., Phys. 76, S70–S76 (2010). 10.1016/j.ijrobp.2009.06.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S., Park S. H., Ahn S. D., Suh Y., Shin S. S., Lee S.-w., Kim J. H., Choi E. K., Yi B. Y., Kwon S. I., Kim S., and Jeung T. S., “Guiding curve based on the normal breathing as monitored by thermocouple for regular breathing,” Med. Phys. 34, 4514–4518 (2007). 10.1118/1.2795829 [DOI] [PubMed] [Google Scholar]
- Wang Y., Christy P. S., Korosec F. R., Alley M. T., Grist T. M., Polzin J. A., and Mistretta C. A., “Coronary MRI with a respiratory feedback monitor: The 2D imaging case,” Magn. Reson. Med. 33, 116–121 (1995). 10.1002/mrm.1910330118 [DOI] [PubMed] [Google Scholar]
- Venkat R. B., Sawant A., Suh Y., George R., and Keall P. J., “Development and preliminary evaluation of a prototype audiovisual biofeedback device incorporating a patient-specific guiding waveform,” Phys. Med. Biol. 53, N197 (2008). 10.1088/0031-9155/53/11/N01 [DOI] [PubMed] [Google Scholar]
- George R., Chung T. D., Vedam S. S., Ramakrishnan V., Mohan R., Weiss E., and Keall P. J., “Audio-visual biofeedback for respiratory-gated radiotherapy: Impact of audio instruction and audio-visual biofeedback on respiratory-gated radiotherapy,” Int. J. Radiat. Oncol., Biol., Phys. 65, 924–933 (2006). 10.1016/j.ijrobp.2006.02.035 [DOI] [PubMed] [Google Scholar]
- Locklin J. K., Yanof J., Luk A., Varro Z., Patriciu A., and Wood B. J., “Respiratory biofeedback during CT-guided procedures,” J. Vasc. Interv. Radiol. 18, 749–755 (2007). 10.1016/j.jvir.2007.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kini V. R., Vedam S. S., Keall P. J., Patil S., Chen C., and Mohan R., “Patient training in respiratory-gated radiotherapy,” Med. Dosim. 28, 7–11 (2003). 10.1016/S0958-3947(02)00136-X [DOI] [PubMed] [Google Scholar]
- Arnold J. F. T., Mörchel P., Glaser E., Pracht E. D., and Jakob P. M., “Lung MRI using an MR-compatible active breathing control (MR-ABC),” Magn. Reson. Med. 58, 1092–1098 (2007). 10.1002/mrm.21424 [DOI] [PubMed] [Google Scholar]
- George R., Keall P., Kini V., Vedam S., Ramakrishnan V., and Mohan R., “Is the diaphragm motion probability density function normally distributed?,” Med. Phys. 32, 396–404 (2005). 10.1118/1.1845031 [DOI] [PubMed] [Google Scholar]
- Murphy M. J. and Dieterich S., “Comparative performance of linear and nonlinear neural networks to predict irregular breathing,” Phys. Med. Biol. 51, 5903–5914 (2006). 10.1088/0031-9155/51/22/012 [DOI] [PubMed] [Google Scholar]
- Vedam S. S., Kini V. R., Keall P. J., Ramakrishnan V., Mostafavi H., and Mohan R., “Quantifying the predictability of diaphragm motion during respiration with a noninvasive external marker,” Med. Phys. 30, 505–513 (2003). 10.1118/1.1558675 [DOI] [PubMed] [Google Scholar]
- Bernstein Matt A., King K. F., and Zhou X. J., Handbook of MRI Pulse Sequences (Elsiver, 2004). [Google Scholar]
- Comisarow M. B. and Melka J. D., “Error estimates for finite zero-filling in Fourier transform spectrometry,” Anal. Chem. 51, 2198–2203 (1979). 10.1021/ac50049a032 [DOI] [Google Scholar]
- Keall P., Mageras G., Balter J., Emery R., Forster K., Jiang S., Kapatoes J., Low D., Murphy M., Murray B., Ramsey C., Van Herk M., Vedam S., Wong J., and Yorke E., “The management of respiratory motion in radiation oncology report of AAPM Task Group 76,” Med. Phys 33, 3874–3900 (2006). 10.1118/1.2349696 [DOI] [PubMed] [Google Scholar]