Abstract
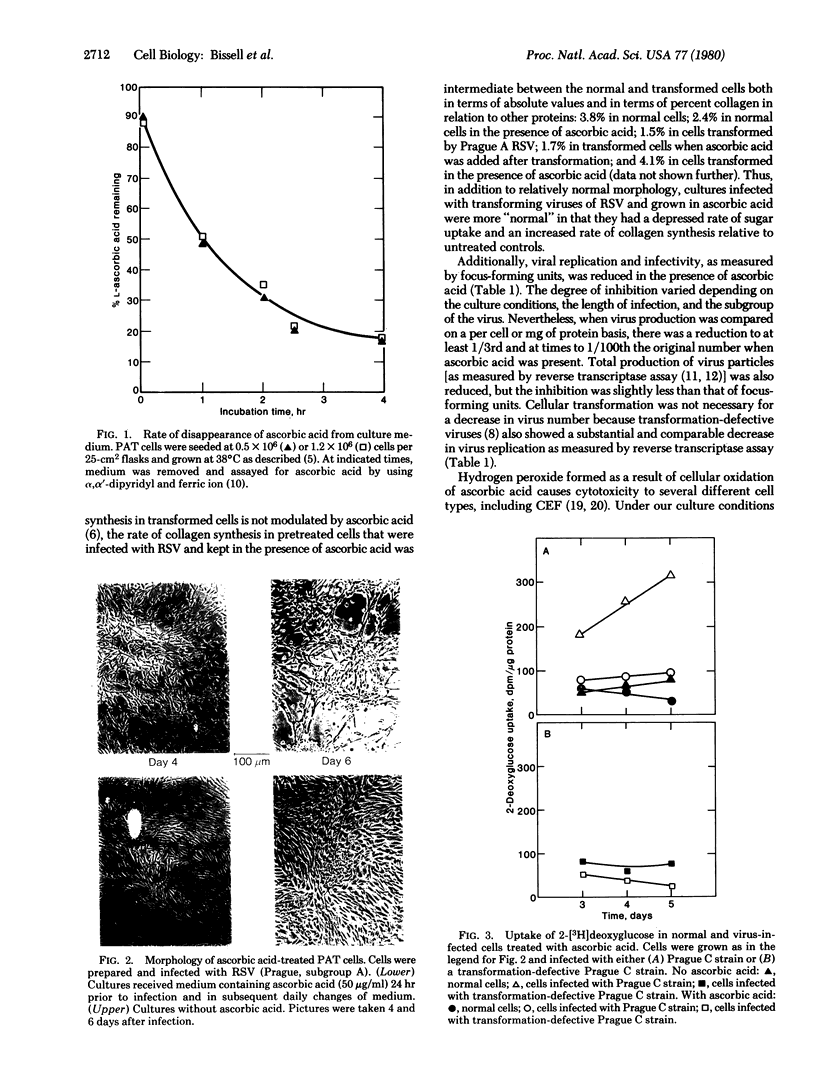
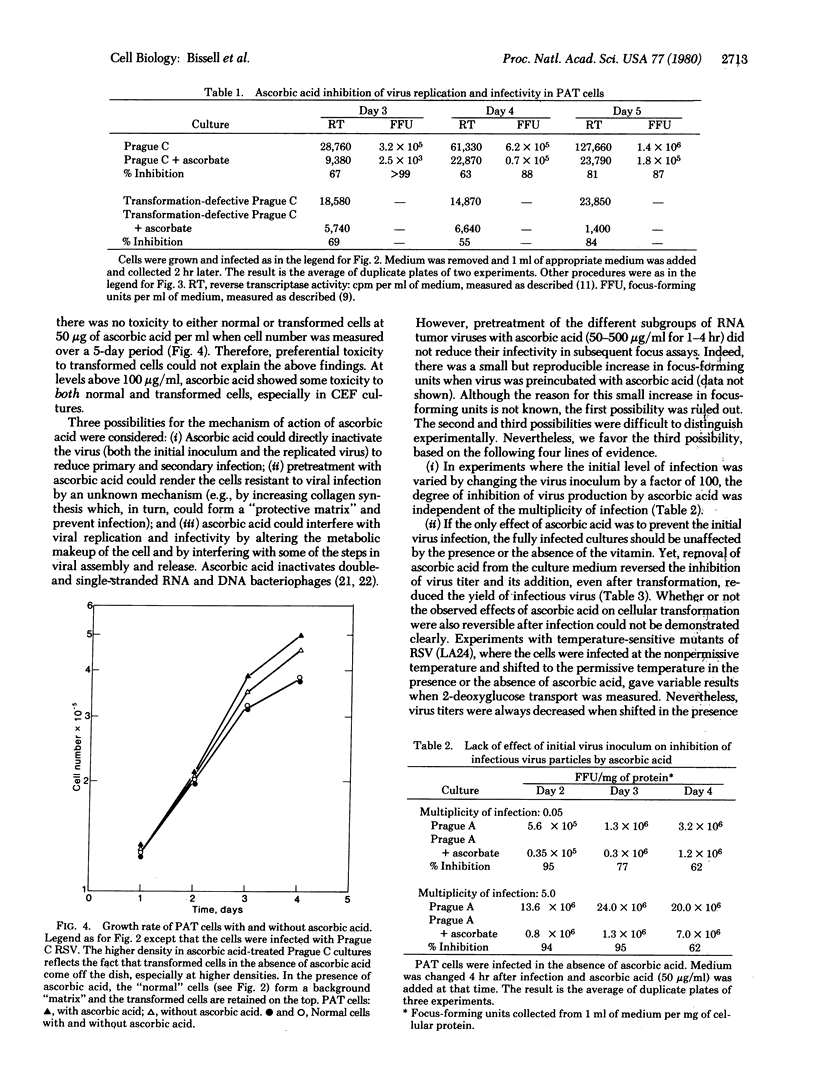
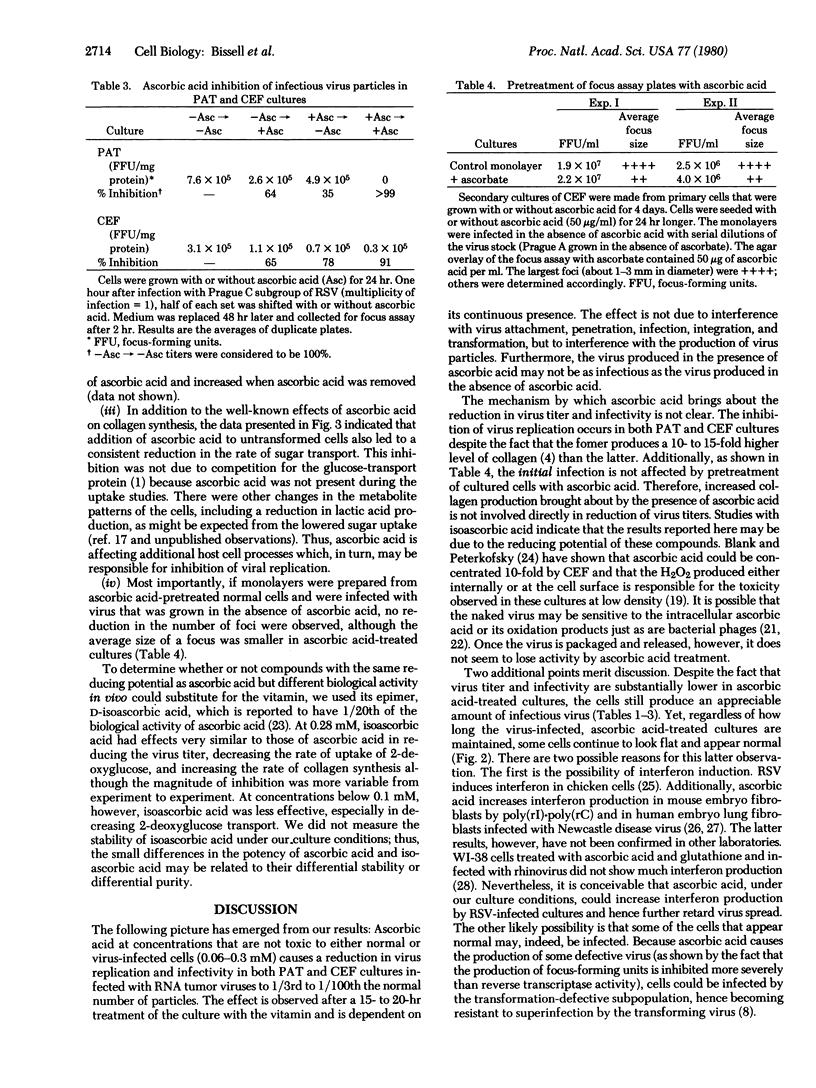
Ascorbic acid, at nontoxic concentrations, causes a substantial reduction in the ability of avian tumor viruses to replicate in both primary avian tendon cells and chicken embryo fibroblasts. The virus-infected cultures appear to be less transformed in the presence of ascorbic acid by the criteria of morphology, reduced glucose uptake, and increased collagen synthesis. The vitamin does not act by altering the susceptibility of the cells to initial infection and transformation, but instead appears to interfere with the spread of infection through a reduction in virus replication and virus infectivity. The effect is reversible and requires the continuous presence of the vitamin in the culture medium.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BADER J. P. Production of interferon by chick embryo cells exposed to Rous sarcoma virus. Virology. 1962 Apr;16:436–443. doi: 10.1016/0042-6822(62)90224-6. [DOI] [PubMed] [Google Scholar]
- Bissell M. J., Farson D., Tung A. S. Cell shape and hexose transport in normal and virus-transformed cells in culture. J Supramol Struct. 1977;6(1):1–12. doi: 10.1002/jss.400060102. [DOI] [PubMed] [Google Scholar]
- Bissell M. J., Hatie C., Tischler A. N., Calvin M. Preferential inhibition of the growth of virus-transformed cells in culture by rifazone-82, a new rifamycin derivative. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2520–2524. doi: 10.1073/pnas.71.6.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M. J. Transport as a rate limiting step in glucose metabolism in virus-transformed cells: studies with cytochalasin B. J Cell Physiol. 1976 Dec;89(4):701–709. doi: 10.1002/jcp.1040890430. [DOI] [PubMed] [Google Scholar]
- Blanck T. J., Peterkofsky B. The stimulation of collagen secretion by ascorbate as a result of increased proline hydroxylation in chick embryo fibroblasts. Arch Biochem Biophys. 1975 Nov;171(1):259–267. doi: 10.1016/0003-9861(75)90031-4. [DOI] [PubMed] [Google Scholar]
- Cameron E., Pauling L., Leibovitz B. Ascorbic acid and cancer: a review. Cancer Res. 1979 Mar;39(3):663–681. [PubMed] [Google Scholar]
- Chang S. K., Harrington G. W., Rothstein M., Shergalis W. A., Swern D., Vohra S. K. Accelerating effect of ascorbic acid on N-nitrosamine formation and nitrosation by oxyhyponitrite. Cancer Res. 1979 Oct;39(10):3871–3874. [PubMed] [Google Scholar]
- Dahl H., Degré M. The effect of ascorbic acid on production of human interferon and the antiviral activity in vitro. Acta Pathol Microbiol Scand B. 1976 Oct;84B(5):280–284. doi: 10.1111/j.1699-0463.1976.tb01938.x. [DOI] [PubMed] [Google Scholar]
- Dolberg D. S., Bassham J. A., Bissell M. J. Selective inhibition of the facilitated mode of sugar uptake by cytochalasin B in cultured chick fibroblasts. Exp Cell Res. 1975 Nov;96(1):129–137. doi: 10.1016/s0014-4827(75)80045-0. [DOI] [PubMed] [Google Scholar]
- Duesberg P., Helm K. V., Canaani E. Properties of a soluble DNA polymerase isolated from Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):747–751. doi: 10.1073/pnas.68.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Melcher A. H., Brunette D. M., Moe H. K. Determination of L-ascorbic acid levels in culture medium: concentrations in commercial media and maintenance of levels under conditions of organ culture. In Vitro. 1977 Feb;13(2):91–99. doi: 10.1007/BF02615072. [DOI] [PubMed] [Google Scholar]
- Koch C. J., Biaglow J. E. Toxicity, radiation sensitivity modification, and metabolic effects of dehydroascorbate and ascorbate in mammalian cells. J Cell Physiol. 1978 Mar;94(3):299–306. doi: 10.1002/jcp.1040940307. [DOI] [PubMed] [Google Scholar]
- Levinson W., Bhatnagar R. S., Liu T. Z. Loss of ability to synthesize collagen in fibroblasts transformed by rous sarcoma virus. J Natl Cancer Inst. 1975 Oct;55(4):807–810. doi: 10.1093/jnci/55.4.807. [DOI] [PubMed] [Google Scholar]
- Murata A., Oyadomari R., Ohashi T., Kitagawa K. Mechanism of inactivation of bacteriophage deltaA containing single-stranded DNA by ascorbic acid. J Nutr Sci Vitaminol (Tokyo) 1975;21(4):261–269. doi: 10.3177/jnsv.21.261. [DOI] [PubMed] [Google Scholar]
- Murata A., Uike M. Mechanism of inactivation of bacteriophage MS2 containing single-stranded RNA by ascorbic acid. J Nutr Sci Vitaminol (Tokyo) 1976;22(5):347–354. doi: 10.3177/jnsv.22.347. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Prather W. Cytotoxicity of ascorbate and other reducing agents towards cultured fibroblasts as a result of hydrogen peroxide formation. J Cell Physiol. 1977 Jan;90(1):61–70. doi: 10.1002/jcp.1040900109. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B. The effect of ascorbic acid on collagen polypeptide synthesis and proline hydroxylation during the growth of cultured fibroblasts. Arch Biochem Biophys. 1972 Sep;152(1):318–328. doi: 10.1016/0003-9861(72)90221-4. [DOI] [PubMed] [Google Scholar]
- RUBIN H. An analysis of the assay of Rous sarcoma cells in vitro by the infective center technique. Virology. 1960 Jan;10:29–49. doi: 10.1016/0042-6822(60)90004-0. [DOI] [PubMed] [Google Scholar]
- Schwarz R. I., Bissell M. J. Dependence of the differentiated state on the cellular environment: modulation of collagen synthesis in tendon cells. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4453–4457. doi: 10.1073/pnas.74.10.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. I., Farson D. A., Soo W. J., Bissell M. J. Primary avian tendon cells in culture. An improved system for understanding malignant transformation. J Cell Biol. 1978 Dec;79(3):672–679. doi: 10.1083/jcb.79.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerdt P. R., Schwerdt C. E. Effect of ascorbic acid on rhinovirus replication in WI-38 cells. Proc Soc Exp Biol Med. 1975 Apr;148(4):1237–1243. doi: 10.3181/00379727-148-38724. [DOI] [PubMed] [Google Scholar]
- Siegel B. V. Enhancement of interferon production by poly(rI)-poly(rC) in mouse cell cultures by ascorbic acid. Nature. 1975 Apr 10;254(5500):531–532. doi: 10.1038/254531a0. [DOI] [PubMed] [Google Scholar]
- Stich H. F., Wei L., Whiting R. F. Enhancement of the chromosome-damaging action of ascorbate by transition metals. Cancer Res. 1979 Oct;39(10):4145–4151. [PubMed] [Google Scholar]
- Szabo C., Bissell M. J. Antiviral action of a rifamycin derivative: formation Rous sarcoma virus particles deficient in 60 to 70S RNA. J Virol. 1978 Mar;25(3):944–947. doi: 10.1128/jvi.25.3.944-947.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M. J., Friis R. R. Dissociation of transformation parameters using temperature-conditional mutants of Rous sarcoma virus. Cell. 1979 Jan;16(1):25–32. doi: 10.1016/0092-8674(79)90184-3. [DOI] [PubMed] [Google Scholar]
- Zannoni V., Lynch M., Goldstein S., Sato P. A rapid micromethod for the determination of ascorbic acid in plasma and tissues. Biochem Med. 1974 Sep;11(1):41–48. doi: 10.1016/0006-2944(74)90093-3. [DOI] [PubMed] [Google Scholar]




