Abstract
High caloric intake has been associated with an increased risk of cognitive impairment. Total caloric intake is determined by the calories derived from macronutrients. The objective of the study was to investigate the association between percent of daily energy (calories) from macronutrients and incident mild cognitive impairment (MCI) or dementia. Participants were a population-based prospective cohort of elderly persons who were followed over a median 3.7 years (interquartile range, 2.5–3.9) of follow-up. At baseline and every 15 months, participants (median age, 79.5 years) were evaluated using the Clinical Dementia Rating scale, a neurological evaluation, and neuropsychological testing for a diagnosis of MCI, normal cognition, or dementia. Participants also completed a 128-item food-frequency questionnaire at baseline; total daily caloric and macronutrient intakes were calculated using an established database. The percent of total daily energy from protein (% protein), carbohydrate (% carbohydrate), and total fat (% fat) was computed. Among 937 subjects who were cognitively normal at baseline, 200 developed incident MCI or dementia. The risk of MCI or dementia (hazard ratio [HR], [95% confidence interval]) was elevated in subjects with high % carbohydrate (upper quartile: 1.89 [1.17–3.06]; P for trend=0.004), but was reduced in subjects with high % fat (upper quartile: 0.56 [0.34–0.91]; P for trend=0.03), and high % protein (upper quartile 0.79 [0.52 – 1.20]; P for trend=0.03) in the fully adjusted models. A dietary pattern with relatively high caloric intake from carbohydrates and low caloric intake from fat and proteins may increase the risk of MCI or dementia in elderly persons.
Keywords: Mild cognitive impairment, dementia, dietary proteins, dietary fats, dietary carbohydrates, caloric intake, energy intake, prospective studies, community-based
Introduction
Dietary patterns have been associated with late life cognitive function. High intakes of fruit, vegetables, a Mediterranean style diet, and several micronutrients (vitamins B, C, E) have been reported to have beneficial effects [1–4]. A high caloric intake has also been associated with an increased risk of cognitive impairment [5], and caloric restriction with reduced amyloid-β deposition [6–8]. The primary determinants of total caloric intake and the largest component of any diet consist of macronutrients: carbohydrates, fat, and protein. Yet, the role of macronutrient intake relative to total caloric intake on cognitive function in older persons has received little attention. Given the associations of macronutrients with glucose metabolism, neuronal integrity, and neuronal function [9–11], relative intake of macronutrients may have an etiologic role or may be a marker for late life cognitive impairment. We investigated the associations of percent of daily energy (calories) derived from carbohydrate, fat, and protein with risk of mild cognitive impairment (MCI) in a population-based cohort of elderly persons.
METHODS
Study Participants
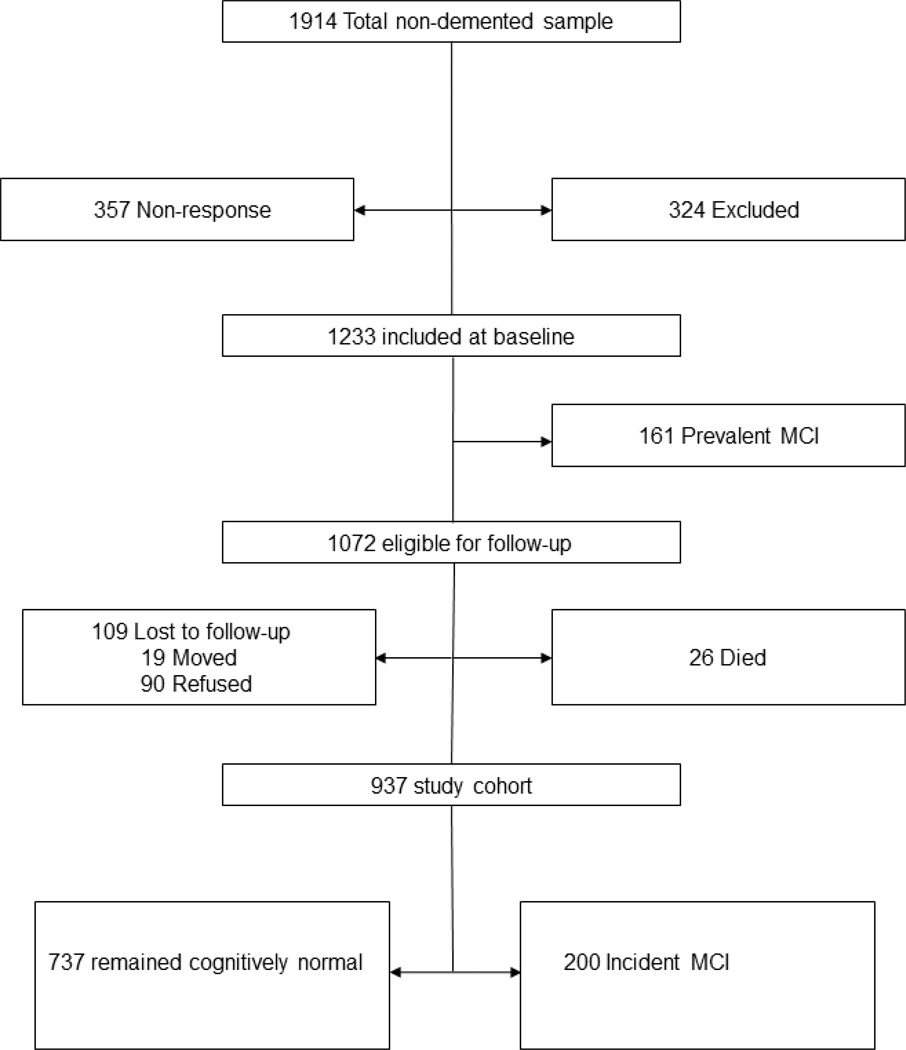
The details of the study design have been published previously [12]. Briefly, we identified all Olmsted County, MN, residents aged 70–89 years on October 1, 2004, using the medical records-linkage system of the Rochester Epidemiology Project [13, 14]. From among the 9,953 subjects that were enumerated, 4,398 were eligible: 2,719 agreed to participate (61.8% response) by telephone (n=669) or via a face-to-face evaluation (in-person evaluation; n=2,050). We mailed the food frequency questionnaire to eligible in-person participants between the first and second evaluations; 681 who were not included at baseline were similar to 1,233 non-demented participants who were included regarding sex, BMI, and APOE ε4 allele status, but were older, had a higher frequency of hypertension, coronary heart disease, stroke, type 2 diabetes, depressive symptoms, were less likely to be married, and had lower education [2, 15]. Of the 1,233, 161subjects had prevalent MCI at baseline, 26 had died, and 109 could not be contacted; 937 are included in this study (FIGURE 1).
Figure 1.
Study Flow Chart. Excluded data: 268 had ≥10 missing responses on frequency of food consumption; 56 had extreme caloric intake (kcal/day: <800 in men, <600 in women or >6000 in men, >5000 in women).
Standard Protocol Approvals, Registrations, and Patient Consent
The study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center. Written informed consent was obtained prior to participation.
Measurements
Assessment of Cognitive Status
Each study participant underwent an interview by a nurse or study coordinator, a neurological evaluation by a physician, and cognitive testing. The interview included questions about memory, date of birth, and years of education; the Clinical Dementia Rating (CDR) Scale [16] and the Functional Activities Questionnaire (FAQ) were administered to an informant [17]. The physician evaluation included the Short Test of Mental Status [18] and a complete neurological examination. The cognitive testing battery used nine tests to assess performance in four cognitive domains: memory, executive function, language, and visuospatial skills [12]. Each test score was converted to an age-adjusted Mayo’s Older American Normative Studies scaled score (mean of 10, standard deviation of 3) [19]. Domain scores were computed by summing the age-adjusted and scaled test scores within a domain and rescaling the scores [12, 20].
Diagnostic Criteria
The domain scores were compared to the means (standard deviations) of scores generated from normal subjects from the Olmsted County population [19]. Cognitive impairment was considered possible if the domain score was ≥1.0 SD below the mean. The final decision about impairment in a cognitive domain was based on a consensus agreement among the examining physician, nurse, and neuropsychologist, taking into account education, prior occupation, and visual or hearing deficits [12, 20].
A diagnosis of MCI was made by consensus according to previously published criteria: cognitive concern by participant (from interview), informant (from CDR), nurse, or physician; impairment in one or more of the four cognitive domains from the cognitive testing battery; essentially normal functional activities (from CDR and FAQ); and absence of dementia [21]. A diagnosis of dementia was made according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition criteria [22]. Subjects were considered cognitively normal if they performed within the normal cognitive range and did not meet criteria for MCI or dementia [12, 20, 21].
Assessment of Dietary Macronutrient Intake
Usual dietary intakes in the previous twelve months were assessed from a self-administered modified Block 1995 Revision of the Health Habits and History Questionnaire [23] that was mailed to in-person participants [2, 15]. The questionnaire included 128 items (103 food items and 25 beverages). For each food item, participants 1) indicated their usual portion size consumed (small, medium, or large), with the medium size specified (e.g., medium serving=1 banana, 1 cup); and 2) how often they had consumed each food (never or <1/month, 1–3/month, 1/week, 2–4/week, 5–6/week, 1/day, 2–3/day, 4–5/day, 6+/day). We analyzed the data using the Food Processor SQL nutrition analysis software (version 10.0.0., ESHA Research, Salem, OR), under the direction of a registered dietician (H.M.O) [15, 24]. We calculated the total nutrient intake in grams per day (g/d) and total daily caloric intake (kcal/d).
Assessment of Covariates
We ascertained information on history of type 2 diabetes, hypertension, and coronary heart disease, from the participant’s medical records [14]; a history of stroke was ascertained by the physician and verified in the medical record where possible [12]. We assessed depressive symptoms from an informant by interview using the Neuropsychiatric Inventory Questionnaire [24]. The frequency of moderate physical exercise in the year prior to the evaluation was assessed from self-report as: ≤1/month, 2–3/month, 1–2/week, 3–4/week, 5–6/week, and daily [25]. Body mass index (BMI) and apolipoprotein (APOE) ε4 genotyping were measured at baseline.
Longitudinal Follow-up
We evaluated participants at 15-month intervals using the same protocol that was used at baseline to determine cognitive function. Clinical and cognitive findings obtained from previous evaluations were not considered in making a diagnosis during follow-up. Subjects who declined an in-person evaluation at follow-up were invited to participate by a telephone interview (partial participation) that included the Telephone Interview of Cognitive Status-modified (TICS-m) [26, 27], the Clinical Dementia Rating Scale [16] and the Neuropsychiatric Inventory Questionnaire [24].
Statistical Analyses
Subjects who were cognitively normal at baseline were considered at risk for incident MCI or dementia. The onset of event was defined by the midpoint between the last assessment as cognitively normal and the first-ever assessments as MCI or dementia. Subjects who refused to participate, could not be contacted, or died, were censored at their last evaluation. We computed years of follow-up as the time from the baseline evaluation to onset of MCI, onset of dementia, censoring, or date of last follow-up. Our analyses included only first ever MCI diagnoses, and did not consider subjects who reverted to normal after an initial diagnosis of MCI.
We calculated the energy-adjusted values of macronutrient intake (protein, carbohydrates, and fats) using the residual method as previously described [28]. We multiplied the daily intake of carbohydrate and protein (g/d) by 4, and fat intake by 9 to obtain the daily energy derived from each macronutrient. We computed the proportion of total daily energy derived from total carbohydrates (% carbohydrate), fat (% fat), and protein (% protein); from carbohydrate components (sugar, non-sugar carbohydrate, fiber); and fat components (polyunsaturated fatty acids [PUFA], monounsaturated fatty acids [MUFA], saturated fats [saturated fats], and trans-fatty acids), and ranked participants by quartiles of intake.
We examined the association of quartiles of % macronutrient intakes with incident MCI or dementia using proportional hazards models, with age as the time variable. In model 1, we adjusted for sex, number of years of education, propensity to participate at baseline using reciprocal probability weighting to adjust for potential non-participation bias at baseline [20, 29–31], and total caloric intake [32]. In a second model, we also adjusted for additional potential confounders including Apoe ε4 carrier status, type 2 diabetes, BMI, smoking status, depressive symptoms, moderate exercise (0 vs ≥ 1 time a month), stroke, marital status, alcohol intake, and longest held primary occupation (as a surrogate for socioeconomic status). In a separate model, we excluded subjects with a history of stroke because of the strong association of stroke with cognitive impairment. We could not adjust for ethnicity since the cohort was 99% white ethnicity. Since only 8 subjects developed dementia without an intervening diagnosis or MCI, our results are in regard to a composite endpoint of MCI or dementia.
RESULTS
TABLE 1 describes the characteristics of the 937 subjects who were cognitively normal at baseline. Median age was 79.5 years, 51% were male, 40% had ≤ 12 years of education, and 65% were married. A total of 200 subjects developed incident MCI or dementia over a median follow-up of 3.7 years (interquartile range, 2.5–3.9; 2871 person-years).
Table 1.
Demographic and Clinical Characteristics of Study Participants at Baseline
| Variable | All N = 937 |
|---|---|
| Age y, Med (Q1, Q3) | 79.5 (75.3, 83.9) |
| Male Gender, n (%) | 478 (51.0) |
| Education ≤12 y, n (%) | 379 (40.4) |
| Married, n (%) | 611 (65.2) |
| BMI ≥30, Kg/m2 n (%)a | 253 (27.7) |
| Type 2 diabetes, n (%) | 149 (15.9) |
| Hypertension, n (%) | 707 (75.5) |
| Coronary artery disease, n (%) | 352 (37.6) |
| Stroke, n (%) | 73 (7.8) |
| Depressive symptoms, n (%)b | 91 (9.8) |
| APOE ε4, n (%)c | 196 (21.5) |
| Moderate exercise, n (%)d | 577 (64.3) |
| Caloric intake cal, med (Q1, Q3) | 1791 (1352, 2351) |
| Total carbohydrate (g/day) | 232 (172, 299) |
| Total protein (g/day) | 78 (58, 103) |
| Total fat (g/day) | 61 (43, 86) |
| Alcohol g/day, med (Q1, Q3) | 0.4 (0.0, 6.0) |
| % Carbohydratee | 52 (47, 58) |
| % Fate | 31 (27, 35) |
| % Proteine | 18 (16, 20) |
Med (Q1, Q1), median (25th 75th) percentiles; BMI, body mass index; MCI, mild cognitive impairment, APOE, Apolipoprotein.
23 subjects with missing data, 4 with MCI and 19 without MCI.
12 subjects with missing data, 4 with MCI and 8 without MCI.
2 subjects without MCI with missing data, 23 subjects with ε2ε4 were excluded (7 with MCI and 16 without).
39 subjects with missing data, 16 with MCI group and 23 without.
Percent of total daily energy from macronutrient.
TABLE 2 describes the demographic, clinical characteristics and dietary intakes of subjects across quartiles of % carbohydrate at baseline. Subjects in the highest % carbohydrate quartile had a higher frequency of women and incident MCI or dementia compared to the lowest quartile; they were also less likely to be married and had a lower BMI. Of note, there were no significant trends with key known risk factors for MCI or dementia: frequency of APOE ε4 allele, type 2 diabetes, stroke, depressive symptoms, moderate exercise, and years of education. Intake (as g/day or % of energy) of sugar, other carbohydrates (non-sugar, non-fiber) and fiber increased across increasing % carbohydrate quartiles, but protein, fat, and alcohol decreased. Total fruit intake increased across % carbohydrate quartiles however, vegetable intake was not different across quartiles.
Table 2.
Characteristics of Subjects by % Carbohydrate Intake
| Quartiles of % carbohydrate of total energy | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Variable | <47% N = 234 |
47 – 52% N = 234 |
53 – 58% N = 235 |
>58% N = 234 |
P value |
| N (%) | |||||
| Female | 86 (36.8) | 118 (50.4) | 113 (48.1) | 142 (60.7) | <0.001 |
| Married* | 176 (75.2) | 145 (62.0) | 153 (65.1) | 137 (58.5) | 0.001 |
| APOE ε4 carrier | 50 (22.2) | 39 (17.2) | 51 (22.0) | 56 (24.6) | 0.27 |
| Type 2 diabetes | 47 (20.1) | 29 (12.4) | 41 (17.4) | 32 (13.7) | 0.09 |
| Stroke | 17 (7.3) | 18 (7.7) | 16 (6.8) | 22(9.4) | 0.74 |
| Depressive symptoms | 30 (13.0) | 22 (9.6) | 21 (9.0) | 18 (7.8) | 0.27 |
| Moderate exercise | 140 (62.5) | 138 (60.8) | 156 (69.6) | 143 (64.1) | 0.23 |
| Incident MCI | 36 (15.4) | 42 (17.9) | 52 (22.1) | 70 (29.9) | 0.001 |
| Mean (SD) | |||||
| Age, yr, | 78.8 (4.8) | 80.1 (5.3) | 79.9 (5.0) | 80.9 (5.2) | <0.001 |
| Education, yr | 14.1 (3.1) | 14.2 (3.0) | 14.2 (3.0) | 14.0 (2.7) | 0.92 |
| BMI, kg/m2 | 28.8 (5.4) | 27.8 (5.1) | 27.4 (4.5) | 26.8 (4.9) | <0.001 |
| Total energy | 2025 (841) | 2004 (807) | 1950 (850) | 1770 (823) | 0.004 |
| Intake†, g/day | |||||
| Total carbohydrate | 189 (25) | 221 (20) | 245 (21) | 273 (31) | <0.001 |
| Sugar | 78 (18) | 99 (18) | 112 (21) | 129 (28) | <0.001 |
| Other carbohydrates‡ | 74 (17) | 80 (17) | 88 (18) | 91 (21) | <0.001 |
| Fiber | 18 (6) | 22 (7) | 24 (8) | 29 (11) | <0.001 |
| Protein | 89 (17) | 81 (14) | 78 (14) | 70 (15) | <0.001 |
| Fat | 75 (13) | 65 (10) | 59 (8) | 49 (9) | <0.001 |
| Alcohol | 9 (13) | 5 (10) | 3 (6) | 2 (5) | <0.001 |
| Vegetables | 217 (115) | 235 (118) | 224 (126) | 233 (169) | 0.43 |
| Fruit | 252 (126) | 322 (151) | 359 (190) | 469 (265) | <0.001 |
| Intake, % of energy | |||||
| % Total carbohydrate | 41 (4) | 50 (2) | 55 (2) | 63 (4) | <0.001 |
| % Sugar | 17 (4) | 22 (4) | 25 (4) | 30 (6) | <0.001 |
| % Other carbohydrate | 16 (4) | 18 (3) | 20 (4) | 21 (4) | <0.001 |
| % Saturated fat | 13 (3) | 11 (2) | 10 (2) | 8 (2) | <0.001 |
| % Protein | 20 (4) | 18 (3) | 17 (3) | 16 (4) | <0.001 |
| % Total fat | 37 (6) | 33 (4) | 30 (3) | 25 (4) | <0.001 |
| % MUFA | 13 (3) | 11 (2) | 10 (1) | 8 (2) | <0.001 |
| % PUFA | 6 (2) | 6 (2) | 5 (1) | 5 (1) | <0.001 |
| % Alcohol | 4 (6) | 2 (3) | 1 (2) | 1 (2) | <0.001 |
Married includes married or living with significant other
Estimates are age- and energy adjusted.
Other carbohydrate consists of total carbohydrate – (total sugar + total fiber).
TABLE 3 describes the association of % macronutrients with risk of MCI or dementia. The risk was elevated nearly 2-fold for the highest % carbohydrate quartile. In contrast, the risk was reduced at higher % fat and % protein quartiles. There was a trend toward increased risk with increasing % sugar. The significant trends persisted in the fully adjusted models that included adjusting for a history of stroke. The results did not change substantially even after exclusion of subjects with a history of stroke: HR (95% CI) for upper compared to lowest quartile were: 1.53 ([0.99–2.36]; p for trend = 0.08) for % carbohydrate; 0.66 ([0.42–1.03]; p for trend = 0.02) for % fat; 1.08 ([0.72–1.62]; p for trend = 0.15) for % protein; and 1.30 ([0.84–2.00]; p for trend = 0.14) for % sugar. In a multivariable model including carbohydrate, fat, and protein in the same model, carbohydrate remained significantly associated with MCI or dementia (upper vs. lowest quintile: 3.68 [1.61–8.38]; p for trend = 0.01); fat and protein no longer showed a significant trend (Table 4). There were no significant interactions of % carbohydrate, % fat, or % protein with age, sex, APOE ε4, or BMI.
Table 3.
Association of % Macronutrient (Carbohydrate, Fat, Protein, Sugar) With Incident MCI
| Variable | Quartiles |
P Value Trend a |
|||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| % Total carbohydrate | |||||
| Cutpoint (%) | < 47 | 47 – 52 | 53 – 58 | > 58 | |
| Incident MCI, N (%) | 36 (15) | 42 (18) | 52 (22) | 70 (30) | |
| HR (95% CI)b | 1.0 (referent) | 0.99 (0.64–1.55) | 1.21 (0.79–1.85) | 1.80 (1.19–2.74) | 0.007 |
| HR (95% CI)c | 1.0 (referent) | 0.91 (0.55–1.50) | 1.23 (0.76–1.99) | 1.89 (1.17–3.06) | 0.004 |
| % Total Fat | |||||
| Cutpoint (%) | < 27 | 27 – 31 | 32 – 35 | > 35 | |
| Incident MCI, N (%) | 63 (27) | 58 (25) | 38 (16) | 41 (18) | |
| HR (95% CI)b | 1.0 (referent) | 0.81 (0.56–1.18) | 0.50 (0.33–0.76) | 0.58 (0.39–0.88) | 0.005 |
| HR (95% CI)c | 1.0 (referent) | 0.78 (0.52–1.19) | 0.52 (0.32–0.84) | 0.56 (0.34–0.91) | 0.03 |
| % Total Protein | |||||
| Cutpoint (%) | < 16 | 16 – 18 | 19 – 20 | > 20 | |
| Incident MCI, N (%) | 63 (27) | 42 (18) | 41 (17) | 54 (23) | |
| HR (95% CI)b | 1.0 (referent) | 0.70 (0.47–1.03) | 0.70 (0.47–1.05) | 1.02 (0.69–1.49) | 0.09 |
| HR (95% CI)c | 1.0 (referent) | 0.60 (0.39–0.91) | 0.57 (0.36–0.89) | 0.79 (0.52–1.20) | 0.03 |
| % Total Sugar | |||||
| Cutpoint (%) | < 19 | 19 – 23 | 24 – 27 | > 27 | |
| Incident MCI, N (%) | 38 (16) | 52 (22) | 44 (19) | 66 (28) | |
| HR (95% CI)b | 1.0 (referent) | 1.22 (0.81–1.85) | 0.91 (0.59–1.42) | 1.48 (0.98–2.24) | 0.07 |
| HR (95% CI)c | 1.0 (referent) | 1.18 (0.74–1.87) | 0.84 (0.51–1.36) | 1.51 (0.94–2.41) | 0.05 |
Abbreviations: CI, confidence interval; HR, hazard ratio; MCI, mild cognitive impairment
Test for trend across quartiles.
Model 1, adjusted for sex, education (continuous), total daily energy (continuous), non-participation at baseline, and a single macronutrient.
Model 2, adjusted for model 1 variables with additional adjustment for APOE ε4, type 2 diabetes mellitus, depressive symptoms, body mass index (continuous), stroke, marital status, smoking status, alcohol (continuous), occupation (continuous), and frequency of moderate exercise.
Table 4.
Simultaneous Assessment of Association of % Carbohydrate, %Fat, and % Protein With MCI or dementia
| Variable | Quintiles |
P Value Trenda |
||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| % Total carbohydrate | ||||||
| Cutpoint (%) | < 45 | 45–50 | 51–54 | 55–59 | >59 | |
| HR (95% CI)b | 1.0 (referent) | 1.63 (0.93–2.83) | 1.47 (0.77–2.79) | 1.96 (0.96–3.97) | 3.68 (1.61–8.38) | 0.01 |
| HR (95% CI)c | 1.0 (referent) | 1.73 (0.93–3.23) | 1.21 (0.57–2.57) | 1.94 (0.81–4.65) | 3.55 (1.24–10.18) | 0.03 |
| % Total Fat | ||||||
| Cutpoint (%) | < 26 | 26–30 | 31–33 | 34–36 | >36 | |
| HR (95% CI)b | 1.0 (referent) | 0.95 (0.61–1.48) | 0.94 (0.54–1.63) | 0.96 (0.50–1.84) | 1.33 (0.64–2.77) | 0.74 |
| HR (95% CI)c | 1.0 (referent) | 1.04 (0.63–1.71) | 0.92 (0.48–1.75) | 0.94 (0.43–2.07) | 1.24 (0.50–3.07) | 0.87 |
| % Total Protein | ||||||
| Cutpoint (%) | <15 | 15–17 | 18–19 | 20–21 | >21 | |
| HR (95% CI)b | 1.0 (referent) | 0.94 (0.61–1.46) | 0.88 (0.55–1.43) | 1.13 (0.71–1.82) | 1.50 (0.89–2.56) | 0.29 |
| HR (95% CI)c | 1.0 (referent) | 0.81 (0.50–1.31) | 0.76 (0.44–1.33) | 0.96 (0.55–1.65) | 1.17 (0.61–2.24) | 0.56 |
Abbreviations: CI, confidence interval; HR, hazard ratio; MCI, mild cognitive impairment
Test for trend across quartiles.
Model 1, adjusted for sex, education (continuous), total daily energy (continuous), and non-participation at baseline and all 3 variables in the same model.
Model 2, adjusted for model 1 variables with additional adjustment for APOE ε4, type 2 diabetes mellitus, depressive symptoms, body mass index (continuous), stroke, marital status, smoking status, alcohol (continuous), occupation (continuous), and frequency of moderate exercise.
Table 5 shows associations of intake of other carbohydrate, fiber, and fat components with MCI or dementia. The risk increased with increasing % other carbohydrate and fiber intake, and decreased with increasing % PUFA and % saturated fat, but the tests for trend were not significant.
Table 5.
Association of % Other Carbohydrate, Fiber, and Fat Components with Incident MCI
| Variable | Quartiles |
P Value Trend a |
|||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| % Other carbohydratesb | |||||
| Cutpoint (%) | < 16 | 16–19 | 20 – 21 | > 21 | |
| Incident MCI, N (%) | 44 (19) | 43 (18) | 51 (22) | 62 (27) | |
| HR (95% CI)c | 1.0 (referent) | 0.99 (0.65– 1.51) | 1.24 (0.82– 1.86) | 1.37 (0.93– 2.02) | 0.26 |
| HR (95% CI)d | 1.0 (referent) | 1.19 (0.75–1.90) | 1.37 (0.87–2.17) | 1.49 (0.97–2.30) | 0.30 |
| Dietary fiber g/d | |||||
| Cutpoint (%) | < 16 | 16 – 22 | 23 – 32 | > 32 | |
| Incident MCI, N (%) | 37 (16) | 51 (22) | 58 (25) | 54 (23) | |
| HR (95% CI)b | 1.0 (referent) | 1.51 (0.98–2.32) | 1.66 (1.07–2.55) | 1.69 (1.03–2.77) | 0.11 |
| HR (95% CI)c | 1.0 (referent) | 1.60 (1.00–2.57) | 1.54 (0.94–2.53) | 1.90 (1.09–3.31) | 0.12 |
| % Polyunsaturated fatty acids | |||||
| Cutpoint (%) | < 4.3 | 4 .3 – 5.2 | 5.3 – 6.1 | > 6.1 | |
| Incident MCI, N (%) | 57 (24) | 55 (24) | 47 (20) | 41 (18) | |
| HR (95% CI)b | 1.0 referent) | 1.09 (0.75–1.59) | 0.85 (0.57–1.27) | 0.73 (0.49–1.09) | 0.22 |
| HR (95% CI)c | 1.0 (referent) | 1.24 (0.81–1.89) | 0.89 (0.58–1.38) | 0.66 (0.42–1.05) | 0.05 |
| % Monounsaturated fatty acids | |||||
| Cutpoint (%) | < 8.9 | 9 – 10.5 | 10.6 – 12.0 | > 12 | |
| Incident MCI, N (%) | 59 (25) | 55 (24) | 44 (19) | 42 (18) | |
| HR (95% CI)b | 1.0 (referent) | 0.85 (0.58–1.25) | 0.67 (0.45–1.01) | 0.67 (0.44–1.03) | 0.17 |
| HR (95% CI)c | 1.0 (referent) | 0.88 (0.57–1.36) | 0.69 (0.44–1.09) | 0.78 (0.47–1.28) | 0.45 |
| % Saturated fats | |||||
| Cutpoint (%) | < 8.4 | 8.4 – 10.2 | 10.2 – 11.9 | > 12 | |
| Incident MCI, N (%) | 64 (27) | 47 (20) | 52 (22) | 37 (16) | |
| HR (95% CI)b | 1.0 (referent) | 0.72 (0.49–1.07) | 0.67 (0.46–0.99) | 0.56 (0.37–0.85) | 0.04 |
| HR (95% CI)c | 1.0 (referent) | 0.70 (0.45–1.09) | 0.85 (0.55–1.32) | 0.64 (0.39–1.05) | 0.25 |
| % Transfatty acids | |||||
| Cutpoint (%) | < 0.23 | 0.23 – 0.36 | 0.37 – 0.57 | > 0.57 | |
| Incident MCI, N (%) | 53 (23) | 49 (21) | 50 (21) | 48 (21) | |
| HR (95% CI)b | 1.0 (referent) | 0.75 (0.50–1.12) | 0.76 (0.51–1.13) | 0.67 (0.45–1.01) | 0.25 |
| HR (95% CI)c | 1.0 (referent) | 0.85 (0.55–1.33) | 0.85 (0.54–1.33) | 0.82 (0.52–1.30) | 0.84 |
Abbreviations: CI, confidence interval; FA, fatty acids HR, hazard ratio; MCI, mild cognitive impairment; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids.
Test for trend across quartiles.
Non-sugar, non-fiber carbohydrates
Model 1, adjusted for sex, education (continuous), total daily energy (continuous), non-participation at baseline, and a single macronutrient.
Model 2, adjusted for model 1 variables and with additional adjustment for APOE ε4, type 2 diabetes mellitus, depressive symptoms, body mass index (continuous), stroke, marital status, smoking status, alcohol (continuous), occupation (continuous), and frequency of moderate exercise.
DISCUSSION
In our population-based cohort of elderly persons, high % carbohydrate intake was associated with an increased risk of MCI. In contrast, high % fat and high % protein intake were associated with a reduced risk of MCI or dementia. These findings suggest that dietary patterns consisting of a high intake of energy derived from carbohydrates and a relatively low intake from fat and protein may have adverse implications for development of MCI. In contrast, an optimal balance in the proportions of daily calories derived from carbohydrate, fat, and protein, may maintain neuronal integrity and optimal cognitive function in the elderly.
A possible explanation for the association of carbohydrate intake with MCI is that elderly subjects with a high % carbohydrate intake may consume more foods with a high glycemic index. Indeed, subjects in our study with the highest % carbohydrate intake also had the highest intake of sugars and fruit (which are high in sugar content) but not vegetables, and the lowest intake of fat and protein. Glucose is a major source of energy for brain metabolism, and glucose administration typically enhances cognitive performance [33]. However, in elderly persons, a dietary pattern high in carbohydrate intake and in simple sugars may disrupt glucose and insulin metabolism [8, 34–38]. High insulin levels may be detrimental to cognitive function [38]. Persistence of the association of high % carbohydrate with MCI risk after simultaneous adjustment for fat and protein suggests that high intake of carbohydrate may be a key promoter of the increased risk, and relative intakes of protein and fat may also play a role.
High carbohydrate and sugar intake may adversely affect cognition through several mechanisms. Hyperglycemia and diabetes may contribute to increased formation of advanced glycation endproducts (AGE), upregulation of the soluble receptors for AGEs, and may generate oxidative stress which in turn, enhances AGE formation [39–41]. AGEs and oxidative stress have also been associated with greater cognitive decline and with AD through effects on amyloid and tau metabolism [39, 41].
The increased risk of MCI with lower intake of fats and proteins may involve non-energy related pathways [33]. Fat and protein intake may be required for the integrity of neuronal membranes and fats for the integrity of the myelin sheaths in the brain. Although we did not observe significant trends with increasing quartiles of % MUFA and % PUFA intake, the hazard ratios were reduced for higher intake. These unsaturated fatty acids, and in particular essential PUFAs, may maintain cognitive function through effects on structural, functional, and synaptic integrity of neurons [42–44], reduced amyloid-β levels [42], improved insulin sensitivity and glucose metabolism [45–47], decreased cardiovascular disease [48] and stroke [49]. High intake of fish, an important source of omega 3 PUFA, has been associated with a reduced risk of cognitive impairment in elderly persons [50] since fish is also an important source of vitamin D, the reduced risk of cognitive impairment in individuals with high fish intake may be due to the combined effects of omega 3 PUFA and vitamin D [51]. Low intake of protein may be associated with low intake of essential proteins that are required for synthesis of neurotransmitters in the brain. For example, tryptophan crosses the blood brain barrier and is a precursor for brain serotonin, an important neurotransmitter. Murine studies suggest that tryptophan transport across the blood brain barrier decreases with ageing [52]. If this is true in humans, reduced intake of proteins in the elderly may adversely impact neuronal function.
Other factors besides macronutrient intake may contribute to our findings. Subjects with the highest % carbohydrate intake had the lowest total caloric intake which is consistent with the low % fat intake, but is also consistent with low BMI in these subjects, and with previously reported decreased weight loss in the years preceding onset of dementia in elderly persons [53–55]. In addition, moderate alcohol intake has been reported to reduce risk of cognitive impairment [3] and may play a role on MCI risk in our cohort. The dietary patterns observed may be causal or alternately, may be a marker for preclinical disease and risk of cognitive impairment or dementia in elderly persons. These associations need to be examined in other longitudinal studies.
Our findings are consistent with findings from several studies. In one study, subjects with AD and vascular dementia had a high predilection for sugar and sweet foods [56]. Other investigators suggest that reducing caloric intake through carbohydrate restriction may reduce risk of cognitive impairment, AD [5, 57–62], and amyloid-β deposition and pathology [63]. In a study among non-diabetics, the highest cognitive performance was observed in subjects with the best glucose regulation [37, 64]. In the National Health and Nutrition Examination Survey, a dietary pattern with a high % fat was associated with better processing speed, learning, and memory; in contrast high % carbohydrate was associated with poor processing speed [65]. Other studies suggest that phosphatidylcholine, an essential PUFA, improved memory, learning, concentration, and the ability to memorize words in elderly subjects with memory decline [33], and that protein may enhance cognitive performance [65, 66] by improving glucose homeostasis [67]. Decreasing total calories and BMI with increasing % carbohydrate quartile may be markers for imminent cognitive impairment, and are consistent with decreasing weight prior to dementia onset in elderly persons [55].
Potential limitations of our findings include recall bias in reporting of dietary nutrients. This effect may be small in part because subjects were cognitively normal at the time the food frequency questionnaire was completed, and because our previously reported cross-sectional findings on diet and cognition [2, 15] are consistent with several other studies [1, 68–72]. Although the validity of food frequency questionnaires has been questioned, this concern may have greater bearing on studies regarding cancer risk [73]. Other experts suggest that use of the food frequency questionnaire is valid for ranking subjects according to food and nutrient intake as in the present study [74–76]. We could not estimate glycemic index (or glycemic load) since this index is impacted by foods eaten together at a meal; the food frequency questionnaire only assessed usual eating habits in the previous 12 months. There is a potential for non-participation bias, but the higher frequency of vascular risk factors in non-participants suggests that the hazard ratios may be are biased toward a null association. The potential impact of reverse causality is unclear, but it is not possible to determine whether preclinical changes of AD, cerebrovascular disease, or other neurodegenerative pathology, contributed to dietary patterns at baseline. Finally, study participants were primarily of northern European ancestry and any generalizability to other ethnicities should be performed with caution.
Several strengths of our study should be noted. The study was specifically designed to investigate risk factors for MCI. The population-based design reduced selection bias and enhanced the external generalizability of the findings to the population [14]. The comprehensive evaluation of participants for MCI or dementia by 3 independent evaluators increased the internal validity of the findings. We categorized subjects on their usual macronutrient intake using data from a previously validated food frequency questionnaire [23], and assessed nutrient intakes using an established nutrition database. The prospective study design allowed us to estimate causal associations while taking into account potential confounding factors.
ACKNOWLEDGEMENTS
This research was supported by National Institutes of Health grants P50 AG016574, U01 AG006786, K01 MH068351, and K01 AG028573, and by the Robert H. and Clarice Smith and Abigail van Buren Alzheimer’s Disease Research Program, and was made possible by the Rochester Epidemiology Project (R01 AG034676 from the National Institute on Aging).
Dr. Knopman serves as a Deputy Editor for Neurology®; serves on a data safety monitoring board for Lilly Pharmaceuticals; is an investigator in a clinical trial sponsored by Elan Pharmaceuticals, and receives research support from the NIH (R01 AG011378, P50 AG016574, U01 AG006786, AG029550, AG032306, and U01 096917). Dr. Petersen serves on scientific advisory boards for Pfizer, Inc., Janssen Alzheimer Immunotherapy, Elan Pharmaceuticals, Wyeth Pharmaceuticals, and GE Healthcare; has given a CME lecture for Novartis, Inc., receives royalties from the publication of a book entitled Mild Cognitive Impairment (Oxford University Press, 2003); and receives research support from the National Institute on Aging (P50 AG016574 [Principal Investigator] and U01 AG006786 [Principal Investigator]) and the National Institutes of Health (R01 AG011378 [Co-Investigator] and U01 AG024904 [Co-Investigator]). Dr. Roberts currently receives research support from the National Institute on Aging (U01 AG006786 [Co-Investigator] and Abbott Laboratories, and previously received research support through K01 AG028573 [Principal Investigator]).
Footnotes
AUTHOR CONTRIBUTIONS
Dr. Roberts had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Roberts L, Roberts R, Geda.
Acquisition of data: Roberts R, Geda, Knopman, Petersen.
Analysis and interpretation of data: Roberts R, Roberts L, Cha, Pankratz, O’Connor.
Drafting of the manuscript: Roberts R.
Critical revision of the manuscript for important intellectual content: O’Connor, Geda, Knopman, Cha, Petersen.
Statistical analysis: Roberts R, Cha, Pankratz.
Obtaining funding: Roberts R, Knopman, Petersen.
Administrative, technical, or material support: Roberts R, Petersen.
Study supervision: Roberts R, Petersen.
CONFLICT OF INTEREST DISCLOSURES
No other financial disclosures were reported.
REFERENCES
- 1.Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, Breteler MM. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 2.Roberts RO, Geda YE, Cerhan JR, Knopman DS, Cha RH, Christianson TJ, Pankratz VS, Ivnik RJ, Boeve BF, O'Connor HM, Petersen RC. Vegetables, unsaturated fats, moderate alcohol intake, and mild cognitive impairment. Dement Geriatr Cogn Disord. 2010;29:413–423. doi: 10.1159/000305099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66:216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Psaltopoulou T, Kyrozis A, Stathopoulos P, Trichopoulos D, Vassilopoulos D, Trichopoulou A. Diet, physical activity and cognitive impairment among elders: the EPIC-Greece cohort (European Prospective Investigation into Cancer and Nutrition) Public Health Nutr. 2008;11:1054–1062. doi: 10.1017/S1368980007001607. [DOI] [PubMed] [Google Scholar]
- 5.Luchsinger JA, Tang MX, Shea S, Mayeux R. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59:1258–1263. doi: 10.1001/archneur.59.8.1258. [DOI] [PubMed] [Google Scholar]
- 6.Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, Morgan DG, Morgan TE, Finch CE. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol Aging. 2005;26:995–1000. doi: 10.1016/j.neurobiolaging.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Qin W, Chachich M, Lane M, Roth G, Bryant M, de Cabo R, Ottinger MA, Mattison J, Ingram D, Gandy S, Pasinetti GM. Calorie restriction attenuates Alzheimer's disease type brain amyloidosis in Squirrel monkeys (Saimiri sciureus) J Alzheimers Dis. 2006;10:417–422. doi: 10.3233/jad-2006-10411. [DOI] [PubMed] [Google Scholar]
- 8.Witte AV, Fobker M, Gellner R, Knecht S, Floel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci U S A. 2009;106:1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunt A, Schonknecht P, Henze M, Seidl U, Haberkorn U, Schroder J. Reduced cerebral glucose metabolism in patients at risk for Alzheimer's disease. Psychiatry Res. 2007;155:147–154. doi: 10.1016/j.pscychresns.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Rinne JO, Mosconi L, Pirraglia E, Rusinek H, DeSanti S, Kemppainen N, Nagren K, Kim BC, Tsui W, de Leon MJ. Regional analysis of FDG and PIB-PET images in normal aging, mild cognitive impairment, and Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2008;35:2169–2181. doi: 10.1007/s00259-008-0833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan RJ, Greenwood CE, Winocur G, Wolever TM. Cognitive performance is associated with glucose regulation in healthy elderly persons and can be enhanced with glucose and dietary carbohydrates. Am J Clin Nutr. 2000;72:825–836. doi: 10.1093/ajcn/72.3.825. [DOI] [PubMed] [Google Scholar]
- 12.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Petersen RC, Rocca WA. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 14.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. American journal of epidemiology. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts RO, Cerhan JR, Geda YE, Knopman DS, Cha RH, Christianson TJ, Pankratz VS, Ivnik RJ, O'Connor HM, Petersen RC. Polyunsaturated fatty acids and reduced odds of MCI: the Mayo Clinic Study of Aging. J Alzheimers Dis. 2010;21:853–865. doi: 10.3233/JAD-2010-091597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 17.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 18.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The Short Test of Mental Status. Correlations with standardized psychometric testing. Arch Neurol. 1991;48:725–728. doi: 10.1001/archneur.1991.00530190071018. [DOI] [PubMed] [Google Scholar]
- 19.Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. Mayo's Older Americans Normative Studies: WAIS-R, WMS-R and AVLT norms for ages 56 through 97. The Clinical Neuropsychologist. 1992;6:1–104. [Google Scholar]
- 20.Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, Boeve BF, Tangalos EG, Ivnik RJ, Rocca WA. Prevalence of mild cognitive impairment is higher in men: The Mayo Clinic Study of Aging. Neurology. 2010;75:889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 23.Block G, Coyle LM, Hartman AM, Scoppa SM. Revision of dietary analysis software for the Health Habits and History Questionnaire. Am J Epidemiol. 1994;139:1190–1196. doi: 10.1093/oxfordjournals.aje.a116965. [DOI] [PubMed] [Google Scholar]
- 24.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, Lopez OL, DeKosky ST. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 25.Geda YE, Roberts RO, Knopman DS, Christianson TJ, Pankratz VS, Ivnik RJ, Boeve BF, Tangalos EG, Petersen RC, Rocca WA. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol. 2010;67:80–86. doi: 10.1001/archneurol.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welsh KA, Breitner JCS, Magruder-Habib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1993;6:103–110. [Google Scholar]
- 27.Knopman DS, Roberts RO, Geda YE, Pankratz VS, Christianson TJ, Petersen RC, Rocca WA. Validation of the Telephone Interview for Cognitive Status-modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology. 2010;34:34–42. doi: 10.1159/000255464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 29.Kessler RC, Little RJ, Groves RM. Advances in strategies for minimizing and adjusting for survey nonresponse. Epidemiologic Reviews. 1995;17:192–204. doi: 10.1093/oxfordjournals.epirev.a036176. [DOI] [PubMed] [Google Scholar]
- 30.D'Agostino RB, Jr, Rubin DB. Estimating and using propensity scores with partially missing data. J. Amer. Statist. Assoc. 2000;95:749–759. [Google Scholar]
- 31.Geda YE, Roberts RO, Knopman DS, Petersen RC, Christianson TJ, Pankratz VS, Smith GE, Boeve BF, Ivnik RJ, Tangalos EG, Rocca WA. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry. 2008;65:1193–1198. doi: 10.1001/archpsyc.65.10.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michels KB, Bingham SA, Luben R, Welch AA, Day NE. The effect of correlated measurement error in multivariate models of diet. Am J Epidemiol. 2004;160:59–67. doi: 10.1093/aje/kwh169. [DOI] [PubMed] [Google Scholar]
- 33.Bourre JM. Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 2 : macronutrients. J Nutr Health Aging. 2006;10:386–399. [PubMed] [Google Scholar]
- 34.Hoyer S. The brain insulin signal transduction system and sporadic (type II) Alzheimer disease: an update. J Neural Transm. 2002;109:341–360. doi: 10.1007/s007020200028. [DOI] [PubMed] [Google Scholar]
- 35.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33:2477–2483. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.J SR-F, Sa-Roriz TM, Rosset I, Camozzato AL, Santos AC, Chaves ML, Moriguti JC, Roriz-Cruz M. (Pre)diabetes, brain aging, and cognition. Biochim Biophys Acta. 2009;1792:432–443. doi: 10.1016/j.bbadis.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Vanhanen M, Koivisto K, Kuusisto J, Mykkanen L, Helkala EL, Hanninen T, Riekkinen P, Sr, Soininen H, Laakso M. Cognitive function in an elderly population with persistent impaired glucose tolerance. Diabetes care. 1998;21:398–402. doi: 10.2337/diacare.21.3.398. [DOI] [PubMed] [Google Scholar]
- 38.Stolk RP, Breteler MM, Ott A, Pols HA, Lamberts SW, Grobbee DE, Hofman A. Insulin and cognitive function in an elderly population. The Rotterdam Study. Diabetes care. 1997;20:792–795. doi: 10.2337/diacare.20.5.792. [DOI] [PubMed] [Google Scholar]
- 39.Yaffe K, Lindquist K, Schwartz AV, Vitartas C, Vittinghoff E, Satterfield S, Simonsick EM, Launer L, Rosano C, Cauley JA, Harris T. Advanced glycation end product level, diabetes, and accelerated cognitive aging. Neurology. 2011;77:1351–1356. doi: 10.1212/WNL.0b013e3182315a56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernanz A, De la Fuente M, Navarro M, Frank A. Plasma aminothiol compounds, but not serum tumor necrosis factor receptor II and soluble receptor for advanced glycation end products, are related to the cognitive impairment in Alzheimer's disease and mild cognitive impairment patients. Neuroimmunomodulation. 2007;14:163–167. doi: 10.1159/000110641. [DOI] [PubMed] [Google Scholar]
- 41.Reddy VP, Zhu X, Perry G, Smith MA. Oxidative stress in diabetes and Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2009;16:763–774. doi: 10.3233/JAD-2009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calon F, Lim GP, Yang F, Morihara T, Teter B, Ubeda O, Rostaing P, Triller A, Salem N, Jr, Ashe KH, Frautschy SA, Cole GM. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron. 2004;43:633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez GH, Ilincheta de Boschero MG, Castagnet PI, Giusto NM. Age-associated changes in the content and fatty acid composition of brain glycerophospholipids. Comp Biochem Physiol B Biochem Mol Biol. 1995;112:331–343. doi: 10.1016/0305-0491(95)00079-8. [DOI] [PubMed] [Google Scholar]
- 44.Youdim KA, Martin A, Joseph JA. Essential fatty acids and the brain: possible health implications. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2000;18:383–399. doi: 10.1016/s0736-5748(00)00013-7. [DOI] [PubMed] [Google Scholar]
- 45.Clarke SD. Polyunsaturated fatty acid regulation of gene transcription: a molecular mechanism to improve the metabolic syndrome. The Journal of nutrition. 2001;131:1129–1132. doi: 10.1093/jn/131.4.1129. [DOI] [PubMed] [Google Scholar]
- 46.Huang T, Wahlqvist ML, Xu T, Xu A, Zhang A, Li D. Increased plasma n-3 polyunsaturated fatty acid is associated with improved insulin sensitivity in type 2 diabetes in China. Molecular nutrition & food research. 2010;54(Suppl 1):S112–S119. doi: 10.1002/mnfr.200900189. [DOI] [PubMed] [Google Scholar]
- 47.Lee JS, Pinnamaneni SK, Eo SJ, Cho IH, Pyo JH, Kim CK, Sinclair AJ, Febbraio MA, Watt MJ. Saturated, but not n-6 polyunsaturated, fatty acids induce insulin resistance: role of intramuscular accumulation of lipid metabolites. Journal of applied physiology. 2006;100:1467–1474. doi: 10.1152/japplphysiol.01438.2005. [DOI] [PubMed] [Google Scholar]
- 48.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa FG ISSI-Prevenzione Investigators. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 49.Keli SO, Feskens EJ, Kromhout D. Fish consumption and risk of stroke. The Zutphen Study. Stroke. 1994;25:328–332. doi: 10.1161/01.str.25.2.328. [DOI] [PubMed] [Google Scholar]
- 50.Barberger-Gateau P, Letenneur L, Deschamps V, Peres K, Dartigues JF, Renaud S. Fish, meat, and risk of dementia: cohort study. BMJ. 2002;325:932–933. doi: 10.1136/bmj.325.7370.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Annweiler C, Rolland Y, Schott AM, Blain H, Vellas B, Herrmann FR, Beauchet O. Higher Vitamin D Dietary Intake Is Associated With Lower Risk of Alzheimer's Disease: A 7-Year Follow-up. The journals of gerontology. Series A, Biological sciences and medical sciences. 2012 doi: 10.1093/gerona/gls107. [DOI] [PubMed] [Google Scholar]
- 52.Tang JP, Melethil S. Effect of aging on the kinetics of blood-brain barrier uptake of tryptophan in rats. Pharmaceutical research. 1995;12:1085–1091. doi: 10.1023/a:1016283003747. [DOI] [PubMed] [Google Scholar]
- 53.Cronin-Stubbs D, Beckett LA, Scherr PA, Field TS, Chown MJ, Pilgrim DM, Bennett DA, Evans DA. Weight loss in people with Alzheimer's disease: a prospective population based analysis. BMJ. 1997;314:178–179. doi: 10.1136/bmj.314.7075.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nourhashemi F, Deschamps V, Larrieu S, Letenneur L, Dartigues JF, Barberger-Gateau P. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003;60:117–119. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- 55.Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007;69:739–746. doi: 10.1212/01.wnl.0000267661.65586.33. [DOI] [PubMed] [Google Scholar]
- 56.Mungas D, Cooper JK, Weiler PG, Gietzen D, Franzi C, Bernick C. Dietary preference for sweet foods in patients with dementia. J Am Geriatr Soc. 1990;38:999–1007. doi: 10.1111/j.1532-5415.1990.tb04423.x. [DOI] [PubMed] [Google Scholar]
- 57.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 58.Feinman RD, Volek JS. Low carbohydrate diets improve atherogenic dyslipidemia even in the absence of weight loss. Nutr Metab (Lond) 2006;3:24. doi: 10.1186/1743-7075-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forsythe CE, Phinney SD, Fernandez ML, Quann EE, Wood RJ, Bibus DM, Kraemer WJ, Feinman RD, Volek JS. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids. 2008;43:65–77. doi: 10.1007/s11745-007-3132-7. [DOI] [PubMed] [Google Scholar]
- 60.Seshadri P, Iqbal N, Stern L, Williams M, Chicano KL, Daily DA, McGrory J, Gracely EJ, Rader DJ, Samaha FF. A randomized study comparing the effects of a low-carbohydrate diet and a conventional diet on lipoprotein subfractions and C-reactive protein levels in patients with severe obesity. Am J Med. 2004;117:398–405. doi: 10.1016/j.amjmed.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 61.Krauss RM, Blanche PJ, Rawlings RS, Fernstrom HS, Williams PT. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. Am J Clin Nutr. 2006;83:1025–31. doi: 10.1093/ajcn/83.5.1025. quiz 205. [DOI] [PubMed] [Google Scholar]
- 62.Westman EC, Yancy WS, Jr, Olsen MK, Dudley T, Guyton JR. Effect of a low-carbohydrate, ketogenic diet program compared to a low-fat diet on fasting lipoprotein subclasses. Int J Cardiol. 2006;110:212–216. doi: 10.1016/j.ijcard.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 63.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease--is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 64.Messier C, Desrochers A, Gagnon M. Effect of glucose, glucose regulation, and word imagery value on human memory. Behavioral neuroscience. 1999;113:431–438. doi: 10.1037//0735-7044.113.3.431. [DOI] [PubMed] [Google Scholar]
- 65.Zhang J, McKeown RE, Muldoon MF, Tang S. Cognitive performance is associated with macronutrient intake in healthy young and middle-aged adults. Nutritional neuroscience. 2006;9:179–187. doi: 10.1080/10284150600955172. [DOI] [PubMed] [Google Scholar]
- 66.La Rue A, Koehler KM, Wayne SJ, Chiulli SJ, Haaland KY, Garry PJ. Nutritional status and cognitive functioning in a normally aging sample: a 6-y reassessment. Am J Clin Nutr. 1997;65:20–29. doi: 10.1093/ajcn/65.1.20. [DOI] [PubMed] [Google Scholar]
- 67.Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C, Christou DD. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. The Journal of nutrition. 2003;133:411–417. doi: 10.1093/jn/133.2.411. [DOI] [PubMed] [Google Scholar]
- 68.Solfrizzi V, Colacicco AM, D'Introno A, Capurso C, Torres F, Rizzo C, Capurso A, Panza F. Dietary intake of unsaturated fatty acids and age-related cognitive decline: a 8.5-year follow-up of the Italian Longitudinal Study on Aging. Neurobiol Aging. 2006;27:1694–1704. doi: 10.1016/j.neurobiolaging.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 69.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, Aggarwal N, Schneider J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60:940–946. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 70.Solfrizzi V, Colacicco AM, D'Introno A, Capurso C, Del Parigi A, Capurso SA, Argentieri G, Capurso A, Panza F. Dietary fatty acids intakes and rate of mild cognitive impairment. The Italian Longitudinal Study on Aging. Exp Gerontol. 2006;41:619–627. doi: 10.1016/j.exger.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 71.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006;67:1370–1376. doi: 10.1212/01.wnl.0000240224.38978.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Requejo AM, Ortega RM, Robles F, Navia B, Faci M, Aparicio A. Influence of nutrition on cognitive function in a group of elderly, independently living people. Eur J Clin Nutr. 2003;57(Suppl 1):S54–S57. doi: 10.1038/sj.ejcn.1601816. [DOI] [PubMed] [Google Scholar]
- 73.Kristal AR, Potter JD. Not the time to abandon the food frequency questionnaire: counterpoint. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15:1759–1760. doi: 10.1158/1055-9965.EPI-06-0727. [DOI] [PubMed] [Google Scholar]
- 74.Willett W. Nutritional Epidemiology. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- 75.Willett WC. Invited commentary: comparison of food frequency questionnaires. Am J Epidemiol. 1998;148:1157–1159. doi: 10.1093/oxfordjournals.aje.a009600. discussion 62-5. [DOI] [PubMed] [Google Scholar]
- 76.Willett W. Commentary: Dietary diaries versus food frequency questionnaires-a case of undigestible data. Int J Epidemiol. 2001;30:317–319. doi: 10.1093/ije/30.2.317. [DOI] [PubMed] [Google Scholar]