Abstract
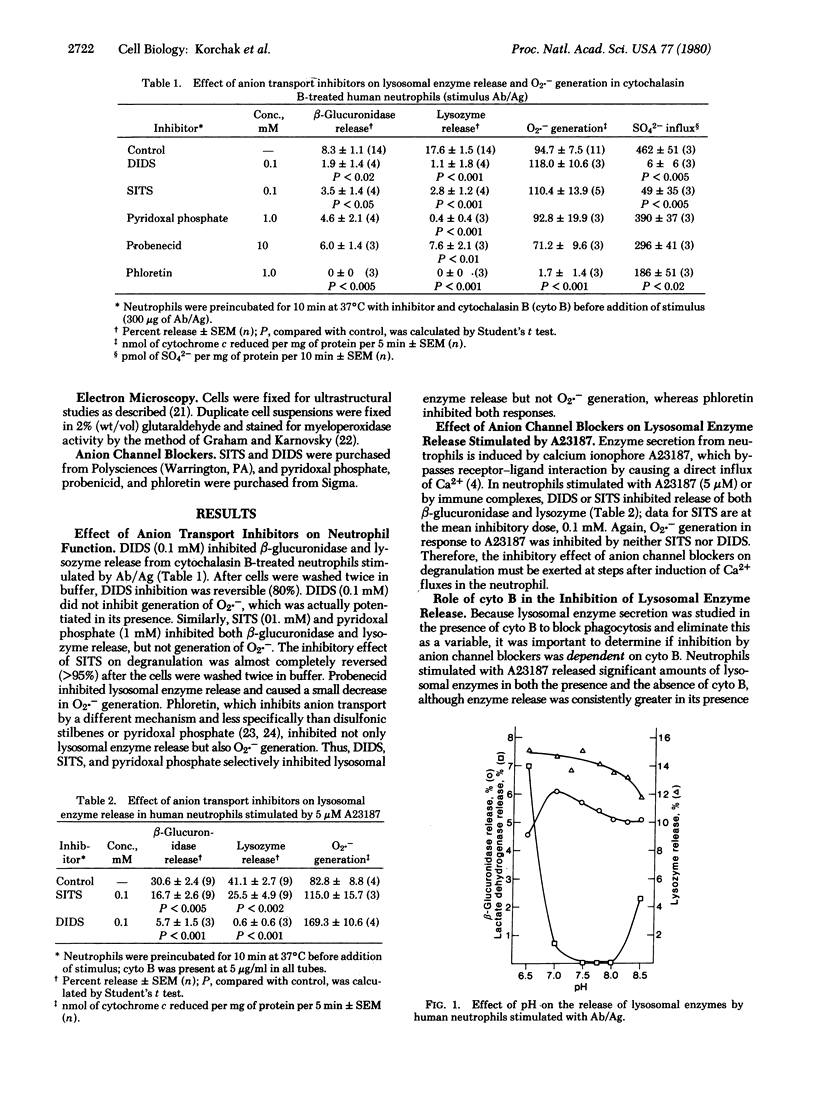
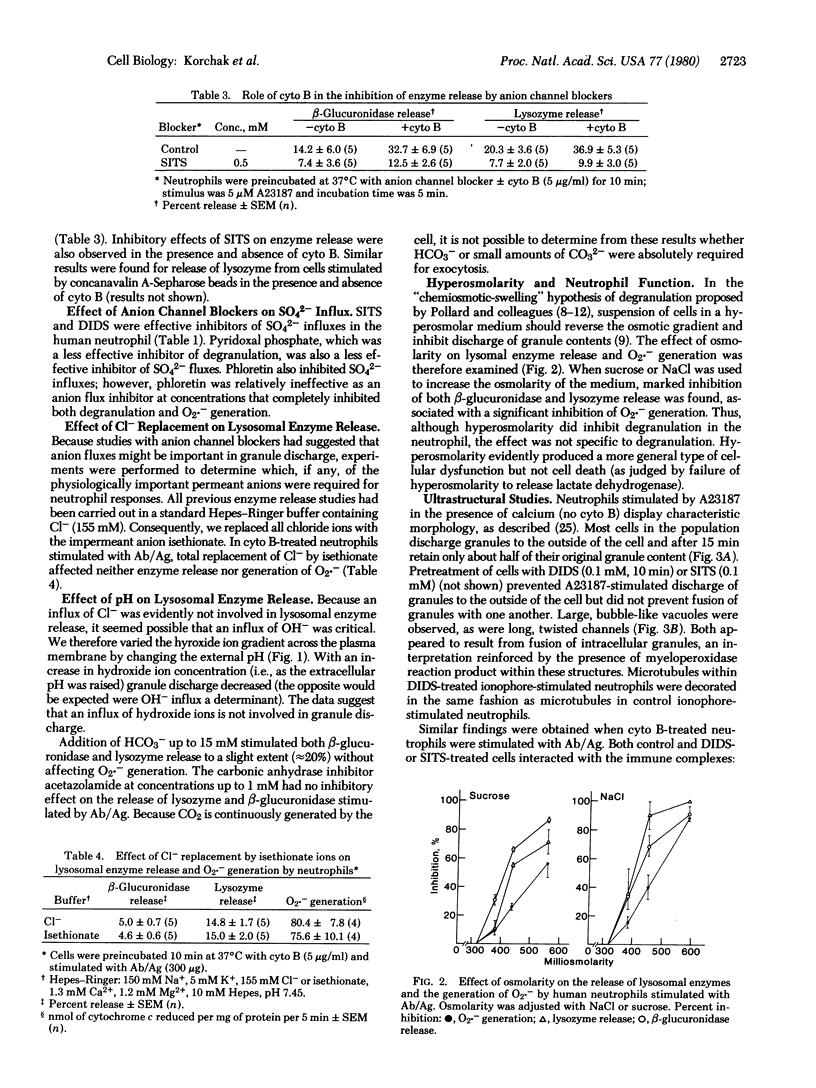
The role of permeant anions in lysosomal enzyme secretion from human neutrophils was investigated by means of anion-channel-blocking agents: 4,4'-diisothiocyanostilbene-2,2'-disulfonic acid (DIDS), 4-acetamido-4'-isothiocyanostilbene-2,2'-disulfonic acid (SITS), and pyridoxal phosphate. Lysosomal enzyme release from cytochalasin B-treated human neutrophils stimulated by immune complexes (bovine serum albumin and IgG anti-bovine serum albumin) was inhibited by DIDS, SITS, and pyridoxal phosphate at concentrations that inhibited sulfate fluxes. Enzyme secretion triggered by calcium ionophore A23187 was also inhibited by DIDS and SITS; these agents acted on secretory events subsequent to Ca2+ influx. Neither the species of permeant anion(s) nor the role of anion fluxes in degranulation was identified, although influxes of chloride, hydroxide, or phosphate ions were not critical. In contrast to degranulation, generation of superoxide anions (O2.-) stimulated by immune complex or A23187 was not inhibited by these agents. Ultrastructural cytochemical studies demonstrated that, although lysosomal contents were not discharged from stimulated cells, vacuole formation and lysosome-lysosome fusion were unaffected by SITS or DIDS. Data suggest that anion channel blockers specifically inhibit fusion of lysosomes with the plasma membrane or its invaginations.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briggs R. T., Drath D. B., Karnovsky M. L., Karnovsky M. J. Localization of NADH oxidase on the surface of human polymorphonuclear leukocytes by a new cytochemical method. J Cell Biol. 1975 Dec;67(3):566–586. doi: 10.1083/jcb.67.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. M., Pazoles C. J., Creutz C. E., Aurbach G. D., Pollard H. B. Role of anions in parathyroid hormone release from dispersed bovine parathyroid cells. Proc Natl Acad Sci U S A. 1978 Feb;75(2):876–880. doi: 10.1073/pnas.75.2.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cabantchik Z. I., Knauf P. A., Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of 'probes'. Biochim Biophys Acta. 1978 Sep 29;515(3):239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Dewald B., Baggiolini M., Curnutte J. T., Babior B. M. Subcellular localization of the superoxide-forming enzyme in human neutrophils. J Clin Invest. 1979 Jan;63(1):21–29. doi: 10.1172/JCI109273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Involvement of calcium in exocytosis and the exocytosis--vesiculation sequence. Biochem Soc Symp. 1974;(39):1–28. [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennaro R., Romeo D. The release of superoxide anion from granulocytes: effect of inhibitors of anion permeability. Biochem Biophys Res Commun. 1979 May 14;88(1):44–49. doi: 10.1016/0006-291x(79)91694-2. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Brai M., Osler A. G., Weissmann G. Lysosomal enzyme release from human leukocytes: mediation by the alternate pathway of complement activation. J Immunol. 1973 Jul;111(1):33–37. [PubMed] [Google Scholar]
- Goldstein I. M., Cerqueira M., Lind S., Kaplan H. B. Evidence that the superoxide-generating system of human leukocytes is associated with the cell surface. J Clin Invest. 1977 Feb;59(2):249–254. doi: 10.1172/JCI108635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Horn J. K., Kaplan H. B., Weissmann G. Calcium-induced lysozyme secretion from human polymorphonuclear leukocytes. Biochem Biophys Res Commun. 1974 Sep 23;60(2):807–812. doi: 10.1016/0006-291x(74)90312-x. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hoffstein S., Weissmann G. Microfilaments and microtubules in calcium ionophore-induced secretion of lysosomal enzymes from human polymorphonuclear leukocytes. J Cell Biol. 1978 Sep;78(3):769–781. doi: 10.1083/jcb.78.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak H. M., Weissmann G. Changes in membrane potential of human granulocytes antecede the metabolic responses to surface stimulation. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3818–3822. doi: 10.1073/pnas.75.8.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lynch R. E., Fridovich I. Permeation of the erythrocyte stroma by superoxide radical. J Biol Chem. 1978 Jul 10;253(13):4697–4699. [PubMed] [Google Scholar]
- Melnik E., Latorre R., Hall J. E., Tosteson D. C. Phloretin-induced changes in ion transport across lipid bilayer membranes. J Gen Physiol. 1977 Feb;69(2):243–257. doi: 10.1085/jgp.69.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard J. A., Gerard K. W., Schneider D. L. The isolation from rat peritoneal leukocytes of plasma membrane enriched in alkaline phosphatase and a B-type cytochrome. Biochem Biophys Res Commun. 1979 Sep 12;90(1):312–319. doi: 10.1016/0006-291x(79)91626-7. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Changes in ionic movements across rabbit polymorphonuclear leukocyte membranes during lysosomal enzyme release. Possible ionic basis for lysosomal enzyme release. J Cell Biol. 1977 Dec;75(3):635–649. doi: 10.1083/jcb.75.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazoles C. J., Pollard H. B. Evidence for stimulation of anion transport in ATP-evoked transmitter release from isolated secretory vesicles. J Biol Chem. 1978 Jun 10;253(11):3962–3969. [PubMed] [Google Scholar]
- Pollard H. B., Pazoles C. J., Creutz C. E., Ramu A., Strott C. A., Ray P., Brown E. M., Aurbach G. D., Tack-Goldman K. M., Shulman N. R. A role for anion transport in the regulation of release from chromaffin granules and exocytosis from cells. J Supramol Struct. 1977;7(3-4):277–285. doi: 10.1002/jss.400070302. [DOI] [PubMed] [Google Scholar]
- Pollard H. B., Tack-Goldman K., Pazoles C. J., Creutz C. E., Shulman N. R. Evidence for control of serotonin secretion from human platelets by hydroxyl ion transport and osmotic lysis. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5295–5299. doi: 10.1073/pnas.74.12.5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showell H. J., Naccache P. H., Sha'afi R. I., Becker E. L. The effects of extracellular K+, Na+ and Ca++ on lysosomal enzyme secretion from polymorphonuclear leukocytes. J Immunol. 1977 Sep;119(3):804–811. [PubMed] [Google Scholar]
- ULMER D. D., VALLEE B. L., WACKER W. E. Metalloenzymes and myocardial infarction. II. Malic and lactic dehydrogenase activities and zinc concentrations in serum. N Engl J Med. 1956 Sep 6;255(10):450–456. doi: 10.1056/NEJM195609062551001. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Zvaifler N. J. Quantitative phagocytosis by neutrophils. I. A new method with immune complexes. J Immunol. 1973 Dec;111(6):1771–1776. [PubMed] [Google Scholar]
- Zurier R. B., Hoffstein S., Weissmann G. Cytochalasin B: effect on lysosomal enzyme release from human leukocytes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):844–848. doi: 10.1073/pnas.70.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]







