Abstract
Evidence suggests that cocaine addiction may involve progressive neuroadaptive changes in the dorsolateral caudate putamen (dlCPu). While cocaine seeking following abstinence from chronic self-administration requires intact dlCPu function, in vivo neurotransmitter release in the dlCPu has not been investigated. The current study measured dlCPu dopamine (DA) and glutamate (GLU) release during drug seeking following limited or extended abstinence, as well as in response to a cocaine priming injection alone. Male, Sprague-Dawley rats self-administered cocaine (0.2 mg/50 µl infusion, i.v.) for 10 days (2-h/day). In vivo microdialysis occurred in the self-administration chamber after 1 and 14 days of abstinence (experiment 1). A separate set of animals that completed self-administration as well as drug naïve controls received a cocaine priming injection (20 mg/kg) during concurrent microdialysis (experiment 2). DA release increased during drug seeking in the self-administration context at both 1 and 14 days post abstinence. In contrast, GLU release only increased after 1 day of abstinence. Furthermore, animals with a cocaine self-administration history showed enhanced DA and GLU release following cocaine challenge as compared to drug naïve controls. These results indicate that chronic cocaine self-administration enhances dlCPu DA and GLU under both drug-paired context and drug-primed conditions.
Keywords: cocaine, dopamine, dorsal, glutamate, self-administration, striatum
1. Introduction
Drug addiction can be characterized as a chronic relapsing disorder that involves the progression from initial reward driven drug use to habitual and compulsive drug taking. Understanding the neural mechanisms underlying relapse to drug use is imperative in order to develop effective treatments. The dorsolateral striatum (i.e., caudate putamen) has been extensively implicated in habitual stimulus-response (S-R) learning (Graybiel, 2008, Packard and Knowlton, 2002), and has been of growing interest as a key substrate in cocaine addiction.
Neuroimaging studies in cocaine dependent humans have demonstrated increased metabolic activity (Garavan et al., 2000) and dopamine (DA) release (Volkow et al., 2006) in the dorsal striatum in response to cocaine associated cues. Non-human primate studies have demonstrated changes in glucose utilization that progressively shift from the ventral striatum to regions of the dorsal striatum following extensive cocaine self-administration (Porrino et al., 2004). Rodent studies have shown increases in DA release in the dorsolateral striatum in response to cocaine contingent cues (Ito et al., 2002) and blockade of DA receptors in the dorsolateral striatum impaired cocaine self-administration under a second order schedule of reinforcement (Vanderschuren et al., 2005). Additionally, electrolytic lesions (Suto et al., 2011) and dopamine receptor antagonism (Veeneman et al., 2012) in the dorsal striatum reduced responding for cocaine under a progressive ratio schedule of reinforcement. In previous studies, we found that the dorsolateral caudate putamen (dlCPu) is necessary for cocaine seeking when rats are returned to the drug taking environment following abstinence from daily cocaine self-administration (Fuchs et al., 2006, See et al., 2007), as well as cue-induced and cocaine-primed reinstatement following daily extinction (Gabriele and See, 2011, See et al., 2007).
A number of studies have indicated that dysregulation of corticostriatal glutamatergic systems may be critical in the transition to addiction. Specifically, altered glutamate (GLU) homeostasis in the prefrontal cortex to NAc pathway may lead to reduced cortical control of striatal processes, causing increased involvement of motor output circuits (Kalivas, 2009). Similar to the ventral striatum, the dorsal striatum also consists primarily of medium spiny GABA neurons (Kemp and Powell, 1971, Wilson and Groves, 1980) that receive glutamatergic input from the prefrontal cortex and dopaminergic input from the midbrain (Bolam and Smith, 1990, Sesack et al., 2003). Furthermore, DA receptors on corticostriatal terminals regulate GLU release (Bamford et al., 2004a, Bamford et al., 2004b) and long term synaptic changes in the dorsal striatum arise largely from DA and GLU co-activation of these medium spiny neurons (Centonze et al., 2001).
While pharmacological inactivation studies have demonstrated a clear role for the dlCPu in cocaine seeking following abstinence (Fuchs et al., 2006, Pacchioni et al., 2011, See et al., 2007), no studies to date have examined neurochemical adaptations in DA and GLU release in the dorsolateral striatum after cocaine self-administration and abstinence. Therefore, the present study investigated changes in extracellular DA and GLU release in the dlCPu during drug seeking when subjects were returned to the cocaine-taking environment following differing periods of abstinence. Additionally, in order to investigate changes in dlCPu responses to cocaine upon initial exposure as compared to exposure following a cocaine self-administration history, we also assessed changes in DA and GLU release after acute cocaine challenge in drug naïve and cocaine experienced animals.
2. Materials and methods
2.1. Subjects
Male, Sprague-Dawley rats (Charles-River; 250–275 g) were individually housed on a 12:12 h reverse light-dark cycle, with lights off from 6:00 a.m. to 6:00 p.m. All animals received standard rat chow (Harlan, Indianapolis, IN) and water ad libitum for the duration of the experiment. Housing and care of the rats were carried out in accordance with the ‘‘Guide for the Care and Use of Laboratory Rats’’ (Institute of Laboratory Animal Resources on Life Sciences, National Research Council) and the MUSC IACUC approved all experimental procedures.
2.2. Apparatus
Testing was conducted in standard self-administration chambers (30×20×20 cm, Med-Associates, St Albans, VT) linked to a computerized data collection program (MED PC). Each chamber was equipped with two retractable levers, a white stimulus light, a tone generator (ENV-223HAM, Med Associates), and a house light on the wall opposite the levers. Each chamber was contained within a sound-attenuating cubicle equipped with a ventilation fan.
2.3. Surgery
Animals were anesthetized using a mixture of ketamine hydrochloride and xylazine (66 and 1.33 mg/kg, respectively, i.p.), followed by equithesin (0.5 ml/kg with a solution of 9.72 mg/ml pentobarbital sodium, 42.5 mg/ml chloral hydrate, and 21.3 mg/ml magnesium sulfate heptahydrate dissolved in a 44% propylene glycol, 10% ethanol solution, i.p.). Ketorolac (2.0 mg/kg, i.p.) was administered immediately prior to surgery for analgesia. For jugular catheter implantation, an indwelling catheter (Silastic tubing; 0.51 mm i.d. and 0.94 mm o.d.; Dow Corning, Midland, MI) was inserted into the right jugular vein and securely sutured. The other end was led subcutaneously to a back incision, where it was connected to an external silicone harness (Plastics One, Roanoke, VA). Stylets were inserted into the catheters when the rats were not connected to infusion pumps. Immediately following catheter surgery, animals were placed into a stereotaxic frame (Stoelting, Wood Dale, IL) for microdialysis guide cannulae implantation. For cannulae implantation, bilateral stainless steel guide cannulae (20 gauge; Plastics One, Roanoke, VA) were directed towards the dlCPu using the appropriate coordinates in mm (dlCPu: AP +1.2, ML ±3.4, DV −3.6). Guide cannulae were secured to the skull using steel jeweler’s screws and dental acrylic. Following surgery, stylets were placed into the guide cannulae to prevent blockage.
For five days following surgery, catheters were flushed daily with 0.1 ml each of 70 U/ml heparinized saline (Elkins-Sinn, Cherry Hill, NJ) and an antibiotic solution of cefazolin (10 mg/0.1 ml, Schein Pharmaceuticals, Florham Park, NJ) to maintain catheter patency. During the entire self-administration period, rats received an infusion of 10 U heparinized saline immediately prior to each session, and the cefazolin and 70 U/ml heparinized saline regimen following the session. Catheter patency was occasionally assessed by administration of 2% methohexital sodium (10.0 mg/ml i.v.; Eli Lilly and Co., Indianapolis, IN), a short-acting barbiturate that produces rapid and reversible muscle flaccidity.
2.4. Cocaine self-administration
Five days following surgery, rats began cocaine self-administration. Infusion tubing for cocaine administration was enclosed in a wire coil and screwed to the external catheter mount on the rat’s back. A weighted swivel apparatus (Instech, Plymouth Meeting, PA) allowed for free movement within the chamber. Cocaine hydrochloride (National Institute on Drug Abuse, Research Triangle Park, NC) was mixed in 0.9% sterile saline and filtered (0.45 µm) prior to self-administration, with infusions (0.2 mg/50 µl bolus, i.v.) delivered by syringe pumps located outside the cubicle. The house light signaled the initiation of the session and remained illuminated throughout the session. Cocaine reinforcement was available along a fixed-ratio 1 (FR-1) schedule for daily 2 h sessions. During each session, a response on the active lever resulted in a 2 sec cocaine infusion. Responding during the time-out or on the inactive lever was recorded, but resulted in no programmed consequences. Animals in both experiments 1 and 2 received identical self-administration experience, during which daily self-administration sessions progressed until animals reached a criterion of 10 sessions with at least 10 cocaine infusions per session. Cocaine naïve animals in experiment 2 underwent jugular catheterization, but remained in their home cage with the exception of daily handling and weighing throughout the study, without any exposure to cocaine or the self-administration chamber until testing.
2.5. Experiment 1: Abstinence tests
24 h following completion of cocaine self-administration, animals returned to the self-administration chamber for a single 2 h session (T1) for microdialysis sampling (see below), with levers present, however responding had no programmed consequences (i.e., no cocaine infusions or cocaine-paired cues). Following this first test, animals underwent a 13-day abstinence period during which animals remained in their home cages after self-administration for days 1–7. Daily handling and placement in an alternate environment were conducted on days 8–13, whereby rats were placed for 2 h/day in a room distinctly different from the self-administration testing room (alternate environment) in plastic holding chambers (27×16×20 cm high, Allentown Caging, Allentown, PA). This alternate environment procedure is identical to that utilized in previous studies (Fuchs et al., 2006, Pacchioni et al., 2011, See et al., 2007) and was developed to minimize effects of dishabituation on testing without interfering with associations formed in the cocaine-paired self-administration chamber. On day 14, animals underwent a second 2 h session (T14) identical to T1.
2.6. Experiment 2: Acute cocaine challenge
In order to test the effects of an acute cocaine challenge, a separate experiment was conducted at 24 h following completion of cocaine self-administration. Cocaine experienced and cocaine naïve animals were placed in the self-administration chamber for microdialysis sampling with no levers present. Levers were not available during the test in order to isolate the effects of the cocaine challenge on DA and GLU efflux without the confounding effects of operant behavior. After 1 h, all animals received a cocaine-priming injection (20 mg/kg, i.p.) and remained in the chamber for an additional hour.
2.7. Microdialysis
Concurrent microdialysis was conducted during both abstinence tests (experiment 1) and the cocaine challenge test (experiment 2). Dialysis probes were constructed with dialysis membrane (250 µm outer diameter; 3 mm length), based on previously described methods (Robinson et al., 1988). The probe was inserted 16–18 h prior to beginning sample collection. Dialysis probes were perfused with modified Ringer’s solution (NaCl, 147 mM; CaCl2, 1.8 mM; KCl, 2.7 mM; MgCl2, 1.2 mM; Na2HPO4, 0.5 mM; adjusted to pH 7.4) at a rate of 2 µl/min. Perfusate samples were collected in 10 µl of 0.05 N HCl. Three consecutive samples were collected at 20 min intervals and served as the baseline, which was collected in a chamber adjacent to the self-administration chamber. Samples were collected every 20 min during the 2 h test session.
2.8. High K+ stimulation
A nonspecific, but common test for the neuronal origin of microdialysis analytes is to measure increases after exposure to depolarizing K+ concentrations. High K+-stimulated neurotransmitter increases occur via K+-induced channel opening by depolarization, vesicular emptying, Ca++ influx, and neurotransmitter reuptake. In order to assess K+-stimulated release, rats in experiment 1 were removed at the end of the 2 h session in the self-administration chamber and placed back into the adjacent chamber for K+-stimulation. Following collection of three consecutive baseline samples at 20 min intervals, a high K+ solution (100 mM KCl, 49.7 mM NaCl) was perfused through the microdialysis probe for 20 min. Samples were collected during the K+ infusion and for 60 min after termination of the K+ infusion.
2.9. HPLC
Microdialysis samples were stored at −80°C before being analyzed by HPLC. For analysis of DA, samples were injected by an autosampler (ESA model 542, Chelmsford, MA) onto a reverse-phase column (ESA 3µ, 3.2×150 mm, MD-150) maintained at 30°C. Mobile phase consisted of 50 mM citric acid, 90 mM NaH2PO4, 1.7 mM octanesulfonic acid, 0.05 mM ethylenediamine-tetraacetic acid, 9% MeOH, and 13% acetonitrile (pH = 5.6). An HPLC pump was utilized at a flow rate of 0.6 ml/min. DA was detected by using coulometric detection (Coulochem Detector III, Microdialysis cell 5014B, ESA). Three electrodes were used: a guard cell (350 mV), a reduction analytical electrode (−150 mV), and an oxidation analytical electrode (200 mV). Dialysate concentrations were determined by comparing peak areas with that of a standard curve and data analyzed by a computerized chromatography analysis system (ESA). For analysis of GLU, precolumn derivatization of GLU with O-phthaldelhyde was performed using a Shimadzu Autosampler (Model SIL-10AXL). Mobile phase consisted of 25 % methanol (v/v), 100 mM Na2HPO4, pH 6.75. GLU was separated using a reversed phase column (3.0 µm, 100 × 4.6 mm, Prevail C18, Alltech), and detected by a fluorescence detector (Shimadzu) with an excitation wavelength of 320 nm and emission wavelength of 400 nm. Dialysate concentrations were determined by comparing peak areas with that of a standard curve. Data was analyzed by a computerized chromatography analysis system (Shimadzu).
2.10. Histology and data analysis
Following testing, animals were anesthetized and transcardially perfused with PBS and 10% formaldehyde solution. Brains were sectioned at a thickness of 75 µm, and stained with cresyl violet. The sections were examined under light microscopy to determine cannula placement and the most ventral point of each cannula track was mapped onto schematics from a rat brain atlas (Paxinos and Watson, 1997). Data were analyzed using one- or two-way analysis of variance (ANOVA) or t tests, where appropriate. The alpha level for all analyses was set at p < 0.05. Data points that were two standard deviations above the mean were excluded from the analyses. All data were analyzed using GraphPad Prism 5 (GraphPad Software Inc. La Jolla, CA).
3. Results
3.1. Histology
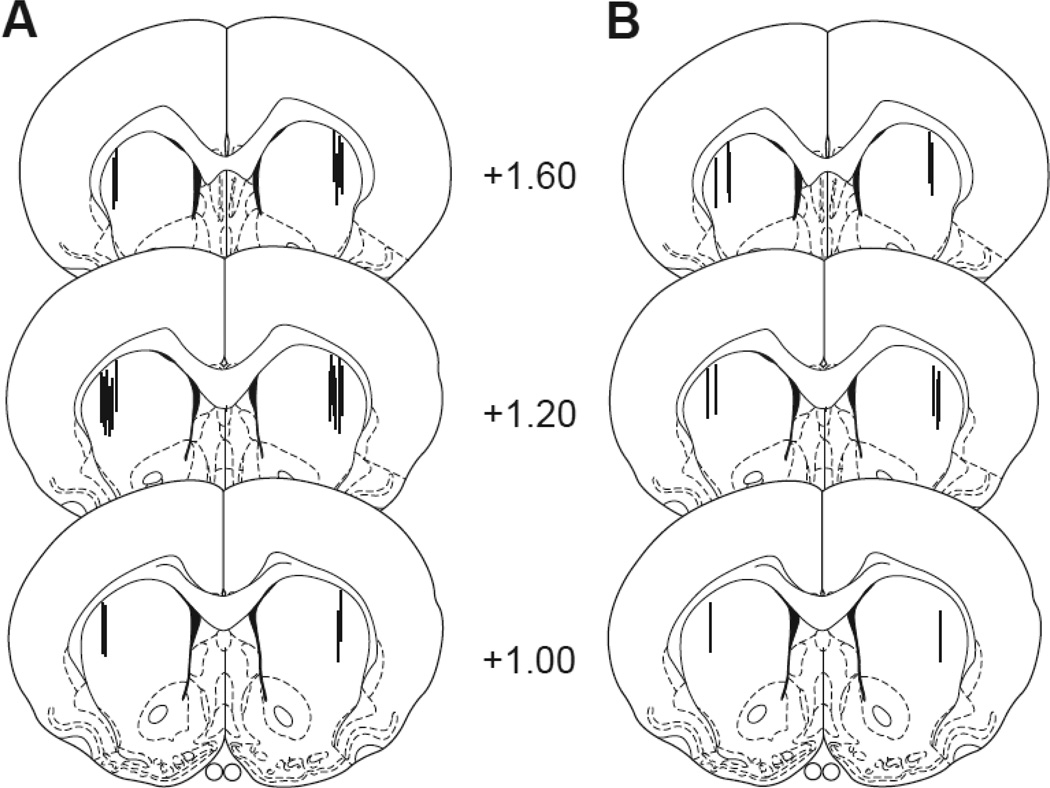
Schematic representations of the dlCPu microdialysis probe placements are indicated in Fig. 1. Probe locations within the dlCPu ranged from +1.00 to +1.60 mm anterior from bregma. Animals with one or both microdialysis cannulae tracks located outside of the target region were not included in the data analyses (n = 2). The final n/group for each analyte in Experiment 1 was: DA T1, n = 11; GLU T1, n = 12; DA T14, n = 9; and GLU T14, n = 10. For Experiment 2, the final n = 6/group (cocaine self-administration and cocaine naïve).
Fig. 1. Microdialysis probe placements.
Schematic diagrams (Paxinos and Watson, 1997) of coronal sections (anterior in mm from bregma) of microdialysis cannulae placements for the dorsolateral caudate putamen (dlCPu) in: A) Experiment 1 and B) Experiment 2.
3.2. Experiment 1
3.2.1. Self-administration and cocaine seeking
All animals readily acquired cocaine self-administration and showed stable responding (mean ± SEM for active lever responses/day across the last 5 days of self-administration = 44.15 ± 7.36) and cocaine intake (mean ± SEM in mg/kg across the last 5 days of self-administration = 14.10 ± 0.88) during the self-administration period. Inactive lever responding across all sessions was routinely very low and did not show any apparent trends (mean ± SEM for inactive lever responses/day across the last 5 days of self-administration = 1.63 ± 0.72). Following both 1 and 14 days of abstinence, animals showed similar levels of responding when returned to the self-administration chamber, even in the absence of cocaine reinforcement (mean ± SEM for active lever responses during the test: T1 = 55.29 ± 12.41, T14 = 33.82 ± 5.35). Although responding decreased between trials, there were no significant differences between the two tests.
3.2.2. DA and GLU release
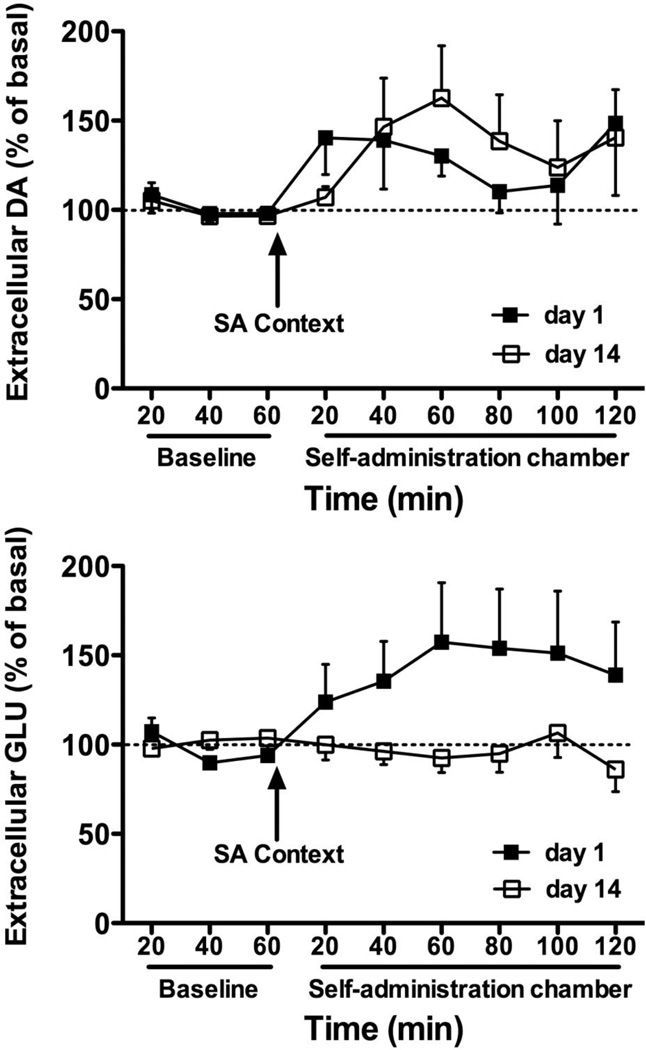
There were no differences between basal DA levels prior to either test (mean ± SEM of basal DA in nM: T1 = 1.32 ± 0.39, T14 = 0.87 ± 0.23). A two-way time × test ANOVA conducted on DA levels expressed as a percent of the baseline values indicated no significant interaction or main effect for test. A main effect for time (F8,144 = 2.19, p < 0.05) indicated DA release increased during drug seeking on both withdrawal days (Fig. 2). As with DA, were no differences between basal GLU levels prior to either abstinence test (mean ± SEM of basal GLU in µM: T1 = 1.53 ± 1.00, T14 = 1.66 ± 0.79). A two-way time × test ANOVA conducted on GLU levels expressed as percent of the baseline values indicated a significant interaction (F8,160= 2.03, p < 0.05). Additional analyses indicated that while GLU release increased at T1 (F8,88 = 2.19, p < 0.05), no significant changes in GLU release from baseline were apparent at T14 (Fig. 2).
Fig. 2. DA and GLU efflux during contextual drug seeking.
Extracellular release of dlCPu DA (top) and GLU (bottom) expressed as percent of basal levels at days 1 and 14 of abstinence. Samples were first collected for 1 h outside of the self-administration (SA) context for baseline measures immediately before placement into the SA chamber for 2 h.
3.2.3. K+ stimulation
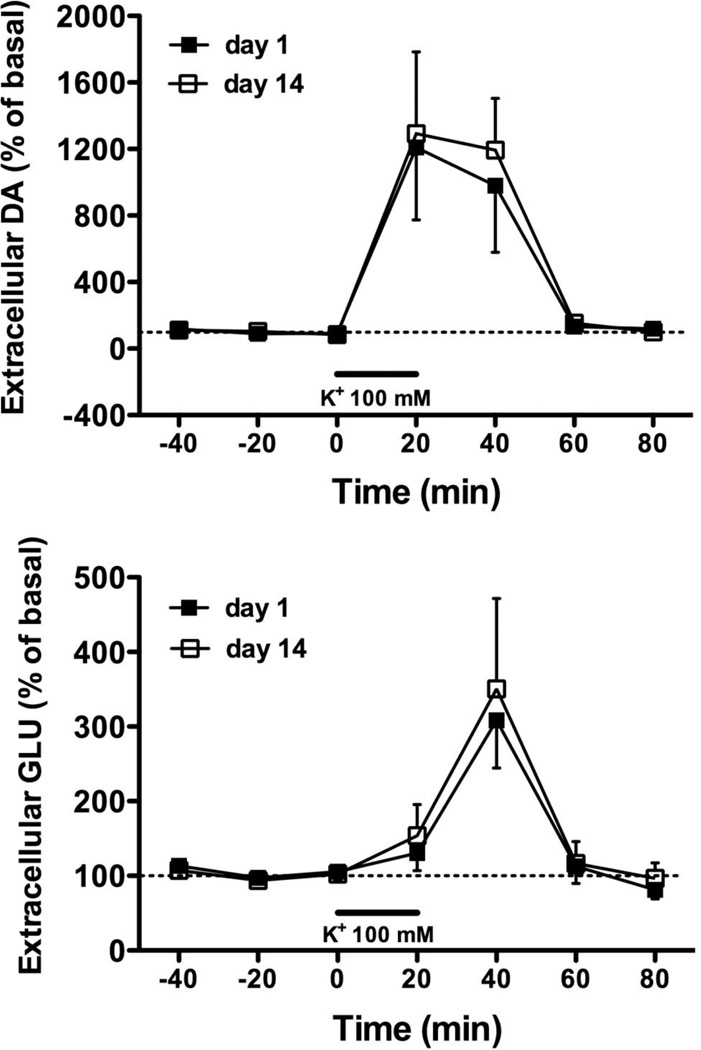
A two-way time × test ANOVA conducted on DA levels expressed as percent of the baseline indicated no significant interaction or main effect for test. A main effect for time (F6,78 = 11.45, p < 0.001) showed predictably robust increases in DA release in response to high K+ stimulation at both test sessions (Fig. 3). Likewise for GLU release, there was a main effect for time (F6,78 = 8.16, p < 0.001), but no significant interaction or main effect for test (Fig. 3).
Fig. 3. K+-stimulated DA and GLU efflux.
High K+-stimulated release of dlCPu DA (top) and GLU (bottom) expressed as percent of basal levels at days 1 and 14 of abstinence. All samples were collected outside of the self-administration (SA) context. High K+ (100 mM) was infused for 20 min as indicated by the bar.
3.3. Experiment 2
3.3.1. Self-administration and cocaine seeking
All animals readily acquired cocaine self-administration and showed stable responding (mean ± SEM for active lever responses/day across the last 5 days of self-administration = 28.72 ± 1.86) and cocaine intake (mean ± SEM in mg/kg across the last 5 days of self-administration = 13.67 ± 0.49) during the self-administration period. Inactive lever responding across all sessions was routinely very low and did not show any apparent trends (mean ± SEM for inactive lever responses/day across the last 5 days of self-administration = 0.31 ± 0.11).
3.3.2. DA and GLU release
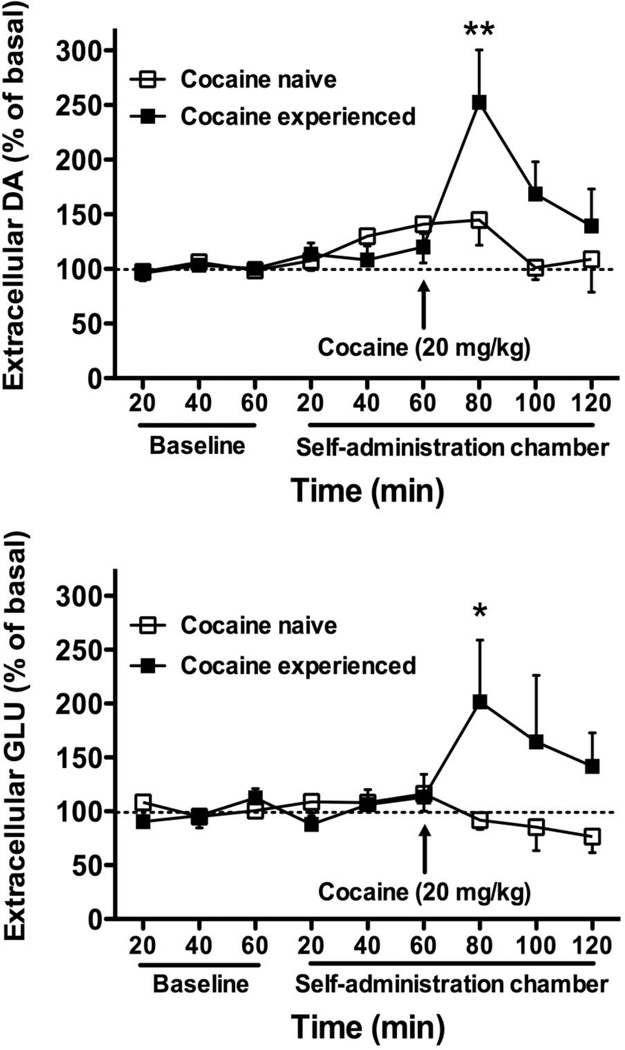
There were no differences between basal DA levels prior to the cocaine challenge (mean ± SEM of basal DA in nM: cocaine experienced = 0.48 ± 0.08, cocaine naive = 0.85 ± 0.18). A two-way group × time ANOVA conducted on DA levels in rats that had undergone cocaine self-administration as compared to cocaine naïve rats after administration of a cocaine challenge injection (Fig. 4) indicated a significant main effect for time (F8,80 = 4.42, p < 0.001), a significant interaction effect between time and group (F8,80 = 2.16, p < 0.05), and a non-significant effect for group. No differences in DA efflux were seen between cocaine experienced and cocaine naïve animals in response to exposure to the cocaine self-administration chamber alone without levers present. However, post-hoc analyses (Bonferroni post test) revealed significant differences in DA efflux between cocaine experienced and cocaine naïve animals during the time point immediately following the cocaine injection (t = 3.55, p < 0.01).
Fig. 4. DA and GLU efflux in response to cocaine challenge.
Extracellular release of dlCPu DA (top) and GLU (bottom) expressed as percent of basal levels before and after a challenge injection of cocaine (20 mg/kg, i.p.) in cocaine naive and cocaine-experienced rats. Samples were collected for 1 h outside of and 1 h within the self-administration context prior to cocaine injection. Significant differences between groups are indicated (Bonferroni post test, *p < 0.05; **p < 0.01).
Analyses conduced on basal GLU levels prior to the cocaine challenge (mean ± SEM of basal GLU in µM: cocaine experienced = 1.61 ± 0.33, cocaine naive = 0.83 ± 0.09) indicated that basal GLU levels were significantly higher in animals that had experienced cocaine self-administration (t = 2.24, p < 0.05). A two-way group × time ANOVA indicated a significant interaction effect between time and group (F8,80 = 2.09, p < 0.05), but no significant main effects for group and time (Fig. 4). No differences in GLU efflux were seen between cocaine experienced and cocaine naïve animals in response to exposure to the cocaine self-administration chamber alone without levers present. However, post-hoc analyses (Bonferroni post test) revealed significant differences in GLU efflux between cocaine experienced and cocaine naïve animals immediately following the cocaine injection (t = 3.17, p < 0.05).
4. Discussion
The current study demonstrated that following both 1 and 14 days of abstinence, drug seeking after returning to the cocaine self-administration context caused similar increases in dlCPu extracellular DA. In contrast, while GLU release increased following 1 day of abstinence, no changes in GLU release occurred following 14 days of abstinence. These results demonstrate that release of DA and GLU in the dlCPu do not proceed in tandem and that time dependent differences may play a role in withdrawal emergent changes after cocaine self-administration. Furthermore, the increased efflux of both DA and GLU after an acute injection of cocaine in drug experienced rats suggests that a history of cocaine self-administration can produce enhanced DA and GLU release in the dlCPu in a manner akin to that previously seen in the NAc (Baker et al., 2003, McFarland et al., 2003).
While very few studies have used in vivo methodology to examine DA release in the dorsal striatum after cocaine self-administration, extensive work has focused on the NAc. Both microdialysis and voltammetry studies have demonstrated increases in NAc DA efflux in response to ongoing cocaine self-administration (Ahmed et al., 2003, Gratton and Wise, 1994, Kiyatkin and Stein, 1995, Pettit and Justice, 1989) and cocaine associated cues (Ito et al., 2000, Weiss et al., 2000). NAc neurons also demonstrated patterned firing in response to reinforced drug seeking behavior (Carelli and Ijames, 2000), which was associated with phasic DA release at these same sites (Owesson-White et al., 2009). NAc neurons that fired directly following a drug seeking response showed attenuated firing at the time of non-reinforced drug seeking, with increases after a cocaine priming injection (Carelli and Ijames, 2000). Additionally, DA release in the NAc showed a small, yet significant increase during initial extinction training (Suto et al., 2010), as well as during cocaine associated cue-induced reinstatement (Weiss et al., 2000). Given that interactions between the NAc and dlCPu are critical for drug seeking (Belin and Everitt, 2008), it is not surprising that drug seeking in the cocaine-associated context led to increased DA release in the dlCPu. Furthermore, inactivation of the substantia nigra impaired contextual drug seeking following abstinence, indicating the necessity of DA inputs to the dlCPu for cocaine seeking (See et al., 2007). Drug associated cues similarly caused increases in dlCPu DA release (Ito et al., 2002). Recent evidence has shown that repeated cocaine exposure led to increased spine density and dendritic branching in dorsal striatal medium spiny neurons, both of which were attenuated by DA D1 antagonism, while increases in spine density were partially reduced by DA D2 antagonism (Ren et al., 2010). These findings support possible cocaine-induced DA mediated morphological changes in dorsal striatal neurons that may impact release patterns of DA.
Various neuroadaptations in the dorsal striatum have been examined after repeated cocaine self-administration at both early and late abstinence time points. In cases where comparisons have been made, some evidence suggests similar changes occur at both early and late time points. For example, a significant increase in the proportion of high affinity DA D2 receptors in the dorsal striatum was seen at both 3 and 30 days of abstinence after cocaine self-administration (Briand et al., 2008). Further, increased DA transporter (DAT) binding in the dorsal striatum has been demonstrated following cocaine self-administration after 4 h (Wilson et al., 1994) or 14 days of abstinence (Ben-Shahar et al., 2006) as well as increased DAT surface expression in the dorsal striatum following three weeks of abstinence (Samuvel et al., 2008). Such changes in D2 receptor affinity states and DAT binding following cocaine self-administration and abstinence may impact dorsal striatum DA release in the presence of cocaine or cocaine associated cues. Notably, our results showed that DA increases were similar at both time points in response to high K+ stimulation, suggesting that extended abstinence does not significantly alter DA vesicular stores.
In contrast to DA, dlCPu GLU only showed a significant increase at 1 day of abstinence, with no changes from baseline seen at 14 days. This was somewhat surprising, given the reported role of GLU release in the NAc following chronic cocaine self-administration (Knackstedt and Kalivas, 2009), whereby GLU release increases upon acute cocaine challenge in rats with a history of cocaine self-administration (McFarland et al., 2003, Miguens et al., 2008), an effect also seen in the dlCPu in the current study. However, while NAc GLU has been reported to increase in response to cues associated with prior noncontingent cocaine administration (Hotsenpiller et al., 2001), GLU release after cocaine self-administration has not been assessed in any brain region in the presence of drug-paired cues or context. Our results show that context activated GLU release, at least in the dlCPu, is potentiated during early, but not late withdrawal. High K+ stimulation did not reveal differences between the two abstinence test days, suggesting that the differences in release during context exposure are not likely due to presynaptic GLU storage or capacity for release. While few studies have examined striatal GLU function at the same time points as the current study, previous evidence has demonstrated differences in striatal GLU receptor subtype function, with patterns emerging at differing periods of abstinence. Following cocaine self-administration, surface expression of AMPA type glutamate receptor (AMPAR) GluR1 subunit was decreased and surface expression of AMPAR GluR2 subunit was increased in the dorsal striatum at two weeks of abstinence (Hearing et al., 2011). Further, at early (16–18 h) withdrawal following cocaine self-administration, an increase in GluR1 subunits was seen in the dorsal striatum (Hemby et al., 2005). Similarly, following self-administration and binge access, decreased dorsal striatal GluR2/3 expression was seen immediately following the binge, but increases in GluR1, GluR2/3 and GluR4 expression were seen at extended withdrawal relative to controls (Tang et al., 2004). As calcium permeability in AMPARs can regulate synaptic plasticity (Cull-Candy et al., 2006, Liu and Zukin, 2007), fluctuations in striatal AMPAR subunits at differing periods of abstinence may indicate changes in striatal responsiveness to glutamatergic inputs that direct drug seeking. Interestingly, BDNF infusions into the medial prefrontal cortex at this early time point of one day after cocaine self-administration (but not later) have been found to attenuate subsequent cocaine seeking and cocaine-stimulated GLU release (Berglind et al., 2007, Berglind et al., 2009).
Recent evidence has indicated that the shift from goal directed to habitual drug seeking reflects in part a transition from ventral to more dorsal striatum mediated behavior (Belin et al., 2009). The results from experiment 2 demonstrate that cocaine induces robust increases in both DA and GLU efflux in animals with a cocaine self-administration history as compared to drug naïve controls, supporting the progressive changes in the dorsal striatum as a result of cocaine self-administration experience. Further, we found that cocaine-experienced animals had higher basal levels of GLU (but not DA) when compared to cocaine-naive rats. These results must be viewed with some caution, as we did not conduct no net flux experiments for assessment of basal levels. However, the increased GLU in the dlCPu is of interest, as previous studies (McFarland et al., 2003, Berglind et al., 2009) have reported decreased NAc GLU levels after chronic cocaine self-administration. These differences in basal GLU may reflect a regionally selective difference in cocaine induced dorsal vs. ventral tonic regulation of GLU. Additionally, 24 h following a single cocaine exposure, dorsal striatal GLU levels have been demonstrated to initially increase, and then decrease over time (McKee and Meshul, 2005). It is possible that this transient increase in basal GLU may be further impacted by an extensive cocaine self-administration history.
Importantly, during the acute cocaine priming test in experiment 2, animals with cocaine self-administration experience only showed increased DA and GLU efflux following the cocaine injection and not in response to the self-administration chamber prior to the injection when levers were retracted, despite being familiar with the cocaine context. These results from experiment 2 suggest that the increases in neurotransmitter efflux seen during drug seeking examined in experiment 1 can be attributed, at least in part, to active drug seeking (i.e., lever pressing) in the absence of drug reinforcement in the self-administration chamber as opposed to simply the associations with the self-administration context. While animals in experiment 1 displayed robust lever responding during the cocaine seeking tests, the magnitude of responding was less than seen in previous studies from our laboratory utilizing this paradigm of testing after abstinence (Fuchs et al., 2006, See et al., 2007). This attenuation may have been due in part to the attached microdialysis tether apparatus inhibiting lever responding.
Previous studies (Barrot et al., 2001, Maisonneuve and Kreek, 1994) have reported modest increases in dorsal striatal DA in response to cocaine in drug naïve animals not tested in an operant testing chamber. We did not see this effect, perhaps due in part to transfer of the drug naïve rats to the novel environment of the chamber just prior to cocaine injection. The modest DA increases in response to the self-administration chamber prior to the cocaine injection may have masked any effects of cocaine itself in that novel environments have been shown to mask and/or dampen the response to cocaine (Rebec et al., 1997, Saigusa et al., 1999). While this drug naïve group is comparable to experimental groups used in studies that do not involve daily exposure to an operant setting, future studies are warranted to further examine the role of novelty as well as the possible effects of varying the visual and spatial characteristics of the self-administration context.
5. Conclusions
In summary, these data demonstrate that drug seeking in the cocaine associated environment results in increased dlCPu DA following both limited and extended abstinence, but increased dlCPu GLU release only following limited abstinence, suggesting that GLU release changes as a function of abstinence length. While these data implicate changes in DA and GLU release in the dlCPu during drug seeking following abstinence from chronic cocaine self-administration, further research is necessary to investigate how these neurotransmitter systems interact to affect dorsal striatal mediated cocaine seeking behavior. Finally, the enhanced response of dorsal striatal DA and GLU release to acute cocaine challenge following cocaine self-administration provides further evidence of the engagement of dorsal striatum neuroplasticity following a history of chronic cocaine.
Highlights.
In vivo microdialysis of dorsal striatum after cocaine self-administration and withdrawal.
Increased DA and GLU after 1 day and increased DA after 14 days of abstinence.
Cocaine prime increased DA and GLU in cocaine experienced, as compared to naïve rats.
Acknowledgements
The authors thank Shannon Ghee, Clifford Chan, and Andrew Novak for excellent technical assistance. This research was supported by National Institute on Drug Abuse grant DA10462 (RES) and NIH grants T32007288 and C06 RR015455.
Abbreviations used
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMPAR
AMPA-type glutamate receptor
- DA
dopamine
- dlCPu
dorsolateral caudate putamen
- FR1
fixed ratio 1
- GLU
glutamate
- NAc
nucleus accumbens
- NMDA
N-methyl-d-aspartate
- S-R
stimulus-response
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest to declare.
References
- Ahmed SH, Lin D, Koob GF, Parsons LH. Escalation of cocaine self-administration does not depend on altered cocaine-induced nucleus accumbens dopamine levels. J Neurochem. 2003;86:102–113. doi: 10.1046/j.1471-4159.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK. Dopamine modulates release from corticostriatal terminals. J Neurosci. 2004a;24:9541–9552. doi: 10.1523/JNEUROSCI.2891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, et al. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004b;42:653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- Barrot M, Abrous DN, Marinelli M, Rouge-Pont F, Le Moal M, Piazza PV. Influence of glucocorticoids on dopaminergic transmission in the rat dorsolateral striatum. Eur J Neurosci. 2001;13:812–818. doi: 10.1046/j.1460-9568.2001.01434.x. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: Relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Moscarello JM, Ettenberg A. One hour, but not six hours, of daily access to self-administered cocaine results in elevated levels of the dopamine transporter. Brain Res. 2006;1095:148–153. doi: 10.1016/j.brainres.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr, Miller SW, et al. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW, Jr, LaLmiere RT, Kalivas PW, McGinty JF. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29:3715–3719. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Smith Y. The GABA and substance P input to dopaminergic neurones in the substantia nigra of the rat. Brain Res. 1990;529:57–78. doi: 10.1016/0006-8993(90)90811-o. [DOI] [PubMed] [Google Scholar]
- Briand LA, Flagel SB, Seeman P, Robinson TE. Cocaine self-administration produces a persistent increase in dopamine D2 High receptors. Eur Neuropsychopharmacol. 2008;18:551–556. doi: 10.1016/j.euroneuro.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG. Nucleus accumbens cell firing during maintenance, extinction, and reinstatement of cocaine self-administration behavior in rats. Brain Res. 2000;866:44–54. doi: 10.1016/s0006-8993(00)02217-4. [DOI] [PubMed] [Google Scholar]
- Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P. Dopaminergic control of synaptic plasticity in the dorsal striatum. Eur J Neurosci. 2001;13:1071–1077. doi: 10.1046/j.0953-816x.2001.01485.x. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriele A, See RE. Lesions and reversible inactivation of the dorsolateral caudate-putamen impair cocaine-primed reinstatement to cocaine-seeking in rats. Brain Res. 2011;1417:27–35. doi: 10.1016/j.brainres.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Gratton A, Wise RA. Drug- and behavior-associated changes in dopamine-related electrochemical signals during intravenous cocaine self-administration in rats. J Neurosci. 1994;14:4130–4146. doi: 10.1523/JNEUROSCI.14-07-04130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Hearing MC, Schwendt M, McGinty JF. Suppression of activity-regulated cytoskeleton-associated gene expression in the dorsal striatum attenuates extinction of cocaine-seeking. International Journal of Neuropsychopharmacology. 2011;14:784–795. doi: 10.1017/S1461145710001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Horman B, Tang W. Differential regulation of ionotropic glutamate receptor subunits following cocaine self-administration. Brain Res. 2005;1064:75–82. doi: 10.1016/j.brainres.2005.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14:1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The structure of the caudate nucleus of the cat: light and electron microscopy. Philos Trans R Soc Lond B Biol Sci. 1971;262:383–401. doi: 10.1098/rstb.1971.0102. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Stein EA. Fluctuations in nucleus accumbens dopamine during cocaine self-administration behavior: an in vivo electrochemical study. Neuroscience. 1995;64:599–617. doi: 10.1016/0306-4522(94)00436-9. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr Opin Pharmacol. 2009;9:59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30:126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Kreek MJ. Acute tolerance to the dopamine response induced by a binge pattern of cocaine administration in male rats: an in vivo microdialysis study. J Pharmacol Exp Ther. 1994;268:916–921. [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee BL, Meshul CK. Time-dependent changes in extracellular glutamate in the rat dorsolateral striatum following a single cocaine injection. Neuroscience. 2005;133:605–613. doi: 10.1016/j.neuroscience.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Miguens M, Del Olmo N, Higuera-Matas A, Torres I, Garcia-Lecumberri C, Ambrosio E. Glutamate and aspartate levels in the nucleus accumbens during cocaine self-administration and extinction: a time course microdialysis study. Psychopharmacology. 2008;196:303–313. doi: 10.1007/s00213-007-0958-x. [DOI] [PubMed] [Google Scholar]
- Owesson-White CA, Ariansen J, Stuber GD, Cleaveland NA, Cheer JF, Wightman RM, et al. Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell. Eur J Neurosci. 2009;30:1117–1127. doi: 10.1111/j.1460-9568.2009.06916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacchioni AM, Gabriele A, See RE. Dorsal striatum mediation of cocaine-seeking after withdrawal from short or long daily access cocaine self-administration in rats. Behav Brain Res. 2011;218:296–300. doi: 10.1016/j.bbr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. New York: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Justice JB., Jr Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacol Biochem Behav. 1989;34:899–904. doi: 10.1016/0091-3057(89)90291-8. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebec GV, Christensen JR, Guerra C, Bardo MT. Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res. 1997;776:61–67. doi: 10.1016/s0006-8993(97)01004-4. [DOI] [PubMed] [Google Scholar]
- Ren Z, Sun WL, Jiao H, Zhang D, Kong H, Wang X, et al. Dopamine D1 and N-methyl-D-aspartate receptors and extracellular signal-regulated kinase mediate neuronal morphological changes induced by repeated cocaine administration. Neuroscience. 2010;168:48–60. doi: 10.1016/j.neuroscience.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Jurson PA, Bennett JA, Bentgen KM. Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by prior experience with (+)-amphetamine: a microdialysis study in freely moving rats. Brain Res. 1988;462:211–222. doi: 10.1016/0006-8993(88)90549-5. [DOI] [PubMed] [Google Scholar]
- Saigusa T, Tuinstra T, Koshikawa N, Cools AR. High and low responders to novelty: effects of a catecholamine synthesis inhibitor on novelty-induced changes in behaviour and release of accumbal dopamine. Neuroscience. 1999;88:1153–1163. doi: 10.1016/s0306-4522(98)00275-9. [DOI] [PubMed] [Google Scholar]
- Samuvel DJ, Jayanthi LD, Manohar S, Kaliyaperumal K, See RE, Ramamoorthy S. Dysregulation of dopamine transporter trafficking and function after abstinence from cocaine self-administration in rats: evidence for differential regulation in caudate putamen and nucleus accumbens. J Pharmacol Exp Ther. 2008;325:293–301. doi: 10.1124/jpet.107.130534. [DOI] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology. 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Suto N, Ecke LE, You ZB, Wise RA. Extracellular fluctuations of dopamine and glutamate in the nucleus accumbens core and shell associated with lever-pressing during cocaine self-administration, extinction, and yoked cocaine administration. Psychopharmacology. 2010;211:267–275. doi: 10.1007/s00213-010-1890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Wise RA, Vezina P. Dorsal as well as ventral striatal lesions affect levels of intravenous cocaine and morphine self-administration in rats. Neurosci Lett. 2011;493:29–32. doi: 10.1016/j.neulet.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Wesley M, Freeman WM, Liang B, Hemby SE. Alterations in ionotropic glutamate receptor subunits during binge cocaine self-administration and withdrawal in rats. J Neurochem. 2004;89:1021–1033. doi: 10.1111/j.1471-4159.2004.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeneman MM, Broekhoven MH, Damsteegt R, Vanderschuren LJ. Distinct contributions of dopamine in the dorsolateral striatum and nucleus accumbens shell to the reinforcing properties of cocaine. Neuropsychopharmacology. 2012;37:487–498. doi: 10.1038/npp.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM. Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. J Comp Neurol. 1980;194:599–615. doi: 10.1002/cne.901940308. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Nobrega JN, Carroll ME, Niznik HB, Shannak K, Lac ST, et al. Heterogeneous subregional binding patterns of 3H-WIN 35,428 and 3H-GBR 12,935 are differentially regulated by chronic cocaine self-administration. J Neurosci. 1994;14:2966–2979. doi: 10.1523/JNEUROSCI.14-05-02966.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]