Abstract
Rationale
Activation of peroxisome proliferator-activated receptor-γ (PPARγ) by thiazolidinediones lowers blood pressure, whereas PPARγ mutations cause hypertension. Previous studies suggest these effects may be mediated through the vasculature, but the underlying mechanisms remain unclear.
Objective
To identify PPARγ mechanisms and transcriptional targets in vascular smooth muscle and their role in regulating resistance artery tone.
Methods and Results
We studied mesenteric artery (MA) from transgenic mice expressing dominant negative (DN) mutant PPARγ driven by a smooth muscle cell (SMC)-specific promoter. MA from transgenic mice exhibited a robust increase in myogenic tone. Patch clamp analysis revealed a reduced large conductance Ca2+-activated K+ (BKCa) current in freshly dissociated SMC from transgenic MA. Inhibition of protein kinase C (PKC) corrected both enhanced myogenic constriction and impaired BKCa channel function. Gene expression profiling revealed a marked loss of the regulator of G protein signaling 5 (RGS5) mRNA in transgenic MA, which was accompanied by a substantial increase in angiotensin II-induced constriction in MA. RGS5 siRNA caused augmented myogenic tone in intact mesenteric arteries and increased activation of PKC in SMC cultures. PPARγ and PPARδ each bind to a PPAR response element close to the RGS5 promoter. RGS5 expression in non-transgenic MA was induced following activation of either PPARγ or PPARδ, an effect that was markedly blunted by DN PPARγ.
Conclusions
We conclude that RGS5 in smooth muscle is a PPARγ and PPARδ target, which when activated blunts angiotensin-II-mediated activation of PKC, preserves BKCa channel activity, thus providing tight control of myogenic tone in the microcirculation.
Keywords: PPARγ, PKC, myogenic tone, hypertension, RGS5
INTRODUCTION
Peroxisome proliferator-activated receptorγ (PPARγ) is a ligand-activated transcription factor of the nuclear hormone receptor superfamily. It regulates target gene expression by forming a heterodimeric complex with the retinoid X receptor (RXR) at the regulatory region (PPAR response elements, PPRE) of target genes1. Ligand-mediated activation of PPARγ leads to release of a corepressor complex followed by the recruitment of transcriptional co-activators leading to induction of target genes. Although the role of PPARγ is well-recognized in adipogenesis and fatty acid metabolism, substantial evidence from both humans and animal models indicates that PPARγ may also participate in blood pressure regulation and vascular function, consistent with its expression in vascular tissues 2. Thiazolidinediones (TZD), high affinity synthetic ligands of PPARγ, lower blood pressure and prevent vascular disease. However, these cardiovascular benefits may be due to secondary effects of improved insulin sensitivity and glycemic control. Clinical studies have uncovered adverse events associated with TZD treatment, including increased risk of heart failure, myocardial infarction, and bladder cancer, thus markedly lowering their clinical usefulness 3;4. It remains unclear whether these adverse events stem from a direct activation of PPARγ or from off target effects. Consequently, uncovering the mechanisms and target genes activated by PPARγ is essential to maximize therapeutic potential of PPARγ treatment.
The significance of PPARγ in the regulation of blood pressure is underscored by the observation that patients carrying heterozygous mutations in the ligand-binding domain of PPARγ develop early onset hypertension along with insulin resistance and diabetes 5. Knock-in mice carrying the mouse equivalent of one of the human mutations, developed hypertension, but not insulin resistance suggesting that the vascular and metabolic actions of PPARγ are not inextricably linked 6. Although metabolic anomalies could be unmasked in a genetic background of leptin deficiency 7, the data suggest PPARγ may play an independent role in regulating blood pressure. We hypothesize that PPARγ regulates blood pressure by regulating gene expression within the endothelium and vascular muscle of the arterial wall promoting an anti-oxidant and vasodilatory state. To test this, we generated a mouse model expressing dominant-negative PPARγ (human PPARγ P467L) specifically in smooth muscle cells, under the control of the smooth muscle myosin heavy chain (SMMHC) promoter (termed S-P467L)8. We previously demonstrated that the S-P467L mice exhibited a modest but significant increase in systolic blood pressure during the daytime (121±1.6 mmHg in NT and 128±2.0 mmHg in S-P467L, p<0.05) and nighttime hours (135±1.5 mmHg in NT and 141±2.3 mmHg in S-P467L, p<0.05), increased heart rate (560±11 in NT vs 627±12 in S-P467L, p<0.05), impaired nitric oxide-induced relaxation in aorta, remodeling and hypertrophy in the cerebral microcirculation, but exhibited normal fasting glucose and insulin 8. These findings led us to conclude that smooth muscle PPARγ plays an important role in the regulation of vascular structure and function and blood pressure, independent of systemic changes in metabolism, but the genetic and physiological mechanism responsible remain undefined.
Vascular tone in small arteries is the net result of responses to several stimuli including mechanical forces (e.g. pressure) and vasoactive agents (e.g. angiotensin II, Ang II) 9. Pressure-induced constriction, also known as the myogenic tone, is a characteristic of resistance blood vessels and plays an essential role in control of systemic vascular resistance. Increased myogenic constriction has been reported in different hypertensive models and is associated with vascular diseases. We therefore hypothesized that PPARγ is required for appropriate regulation of resistance vessel tone. Using the S-P467L mouse model, we investigated small artery function using a pressurized myograph, performed gene expression profiling to identify molecular mechanisms underlying the actions of PPARγ, and linked transcriptional activity of PPARγ to the control of vascular tone. We demonstrate that interference with smooth muscle PPARγ causes a marked increase in myogenic tone in the mesenteric artery. The molecular mechanism of the enhanced myogenic tone is through a robust down-regulation of regulator of G protein signaling 5 (RGS5), which causes over-activation of the protein kinase C (PKC) pathway and inhibition of the large conductance calcium-activated potassium channel (BKCa). Moreover, siRNA-mediated RGS5 knockdown in intact mesenteric artery increased myogenic tone. Further studies indicate that RGS5 is a direct gene target of both PPARγ and PPARδ, and that dominant negative PPARγ inhibits PPARγ-mediated gene expression but only some PPARδ target genes. Our findings shed light on a previously unknown molecular target of vascular PPARγ and provide further evidence that impaired smooth muscle PPARγ function is sufficient to interrupt normal vascular homeostasis.
METHODS
An expanded methods section is available in the Online Supplement.
Animals
Six-month-old male and female mice, carrying the dominant negative PPARγ P467L mutation under the control of the smooth muscle myosin heavy chain promoter (S-P467L) as described previously were used8. Care of the mice met the standards set forth by the National Institutes of Health (NIH) guidelines for the care and use of experimental animals. All procedures were approved by The University of Iowa Animal Care and Use Committee.
Vascular function
Second order mesenteric arteries were studied using a pressurized myograph system (DMT). Studies were performed to assess myogenic tone, the role of the endothelium, role of BKCa channel, PKC and Rho kinase and constrictor responses to agonists as described in detail in the Online Supplement.
Transfection of intact mesenteric arteries
The primary branch of the mesenteric artery was transfected with specific siRNA targeting RGS5 (and non-targeting control siRNA) using the 4D-Nucleofector Y unit (Lonza) as described in detail in the Online Supplement. The efficiency of transfection was determined with confocal microscopy (Zeiss S10) using a dye-labeled oligo control and by measuring RGS5 mRNA.
Electrophysiology
Voltage clamp studies were performed on single freshly isolated smooth muscle cells (SMC) derived from the secondary branch of the mesenteric artery as detailed in the Online Supplement. Studies were performed under baseline conditions and in response to inhibitors of BKCa channels, Kv channels, PKC.
Statistical Analysis
All data are expressed as mean ± SEM. Vascular function data were analyzed with 2-way repeated-measures analysis of variance (ANOVA) using Bonferroni post-hoc analyses with multiple-comparisons procedures. Student's t test was used where required. Gene expression fold changes were calculated using the Livak method10. P<0.05 was considered statistically significant. Data were analyzed by use of SigmaStat (Systat Software).
Quantitative real time PCR, western blotting, microarray analysis, electrophoretic mobility shift assays (EMSAs) and ex vivo gene expression were performed as detailed in the Online Supplement.
RESULTS
Transgene expression in S-P467L mice
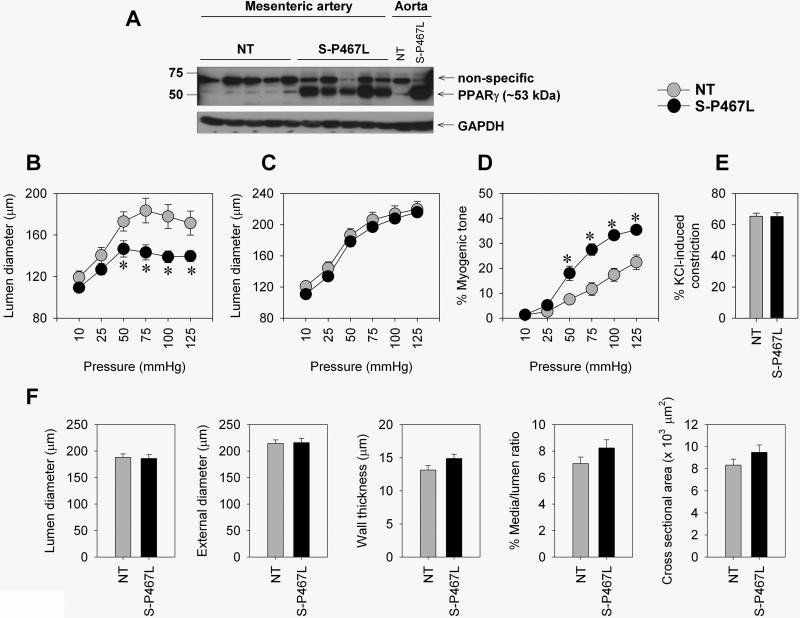
We first verified that the transgene was expressed in small mesenteric arteries of S-P467L mice. Using qRT-PCR, we detected human PPARγ mRNA in mesenteric arteries in transgenic mice (CT values ranging from 21-25) but not non-transgenic (NT) mice (CT was undetectable). Consistently, protein expression of total PPARγ was markedly higher in S-P467L mesenteric arteries compared to those from NT mice (Figure 1A).
Figure 1. Enhanced myogenic tone in S-P467L.
A) Protein expression of total PPARγ in mesenteric arteries (n=5). B-D) Pressure-diameter relationship under active conditions (B), passive diameter curve determined in a Ca2+-free solution (C), % myogenic tone (D) is shown (NT, n=12; S-P467L, n=11). E) Vasoconstriction in response to 100 mmol/L KCl (n=9). F) Structural parameters in Ca2+-free condition (at 75 mmHg) (NT, n=11; S-P467L, n=12). * p<0.05 compared to NT. All data are mean±SEM
Enhanced myogenic tone in S-P467L mice
We determined whether interference with PPARγ altered myogenic constriction in the mesenteric artery. Using a pressurized myograph, we demonstrated that the mesenteric artery from S-P467L exhibited a significant decrease in lumen diameter in response to an increase in luminal pressure (Figure 1B). In contrast, the passive diameter curve, which is determined during Ca2+-free conditions, was similar between the two groups (Figure 1C). Thus, S-P467L mesenteric artery exhibited a marked increase in myogenic tone compared to NT mice (Figure 1D). This augmented myogenic tone in S-P467L mice was apparent in both male and female S-P467L mice. The increase in vasoconstriction is not a result of a generalized increase in contractile activity since 100 mmol/L KCl induced a similar constriction in the two groups (Figure 1E). Structural analysis of mesenteric artery revealed that there were no differences in the lumen or external diameter, wall thickness, percent media/lumen ratio, or cross sectional area (Figure 1F). Therefore, the increase in myogenic tone in S-P467L is not caused by an alteration in vessel structure.
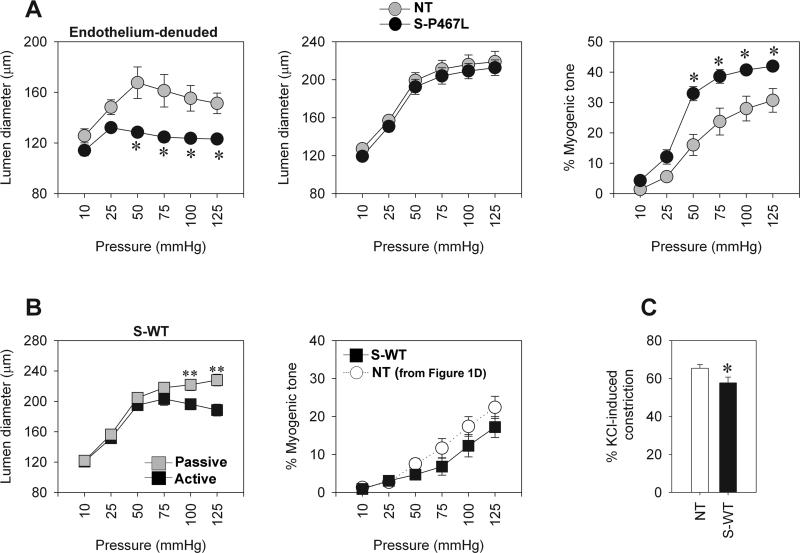
Removal of the endothelium did not alleviate the difference in myogenic tone between the groups suggesting the genetic defect is specifically localized in the smooth muscle (Figure 2A). To test whether the enhanced myogenic tone in S-P467L could be attributed to an overexpression of PPARγ as opposed to the dominant negative actions of the P467L mutation, we measured myogenic constriction in another line of transgenic mice expressing wild-type human PPARγ (at a similar level to P467L mutant PPARγ) specifically targeted to smooth muscle (denoted S-WT) 8. Mesenteric artery from S-WT mice exhibited normal, and perhaps even decreased myogenic tone (Figure 2B), and a moderate decrease in contractility to KCl compared with NT mice (Figure 2C). We conclude that increased myogenic tone is due to interference with PPARγ in smooth muscle cells caused by dominant negative mutant PPARγ and not due to generalized PPARγ over-expression.
Figure 2. Myogenic responses to endothelial removal and wild type PPARγ.
A) Myogenic tone studies in endothelium denuded vessels. Pressure-diameter relationship during active condition, passive diameter curve determined in a Ca-free solution, and % myogenic tone are shown (NT, n=3; S-P467L, n=6). B) Myogenic tone studies in transgenic mice expressing WT PPARγ in smooth muscle (S-WT). Pressure-diameter relationship during active condition (black square) and during Ca2+-free condition (grey square), % myogenic tone compared with the NT mice used in Figure 1 (S-WT, n=7). C) Vasoconstriction in response to 100 mmol/L KCl (S-WT, n=7). * p<0.05 compared to NT. ** p<0.05 compared to active diameter. All data are mean±SEM
Functionally impaired BKCa channel in S-P467L mesenteric artery
To explore the mechanism causing enhanced myogenic tone we first measured expression of TRPC1, TRPC6 and TRPM4, proposed mechanosensors in the vasculature 9. There were no differences in expression of any of genes tested (Online Figure I), suggesting that changes in mechanosensor gene expression do not account for the increased myogenic tone in S-P467L. Similarly, increased oxidative stress is not the cause of increased myogenic tone, as there was no change in the myogenic response to tempol, a potent antioxidant (Online Figure II).
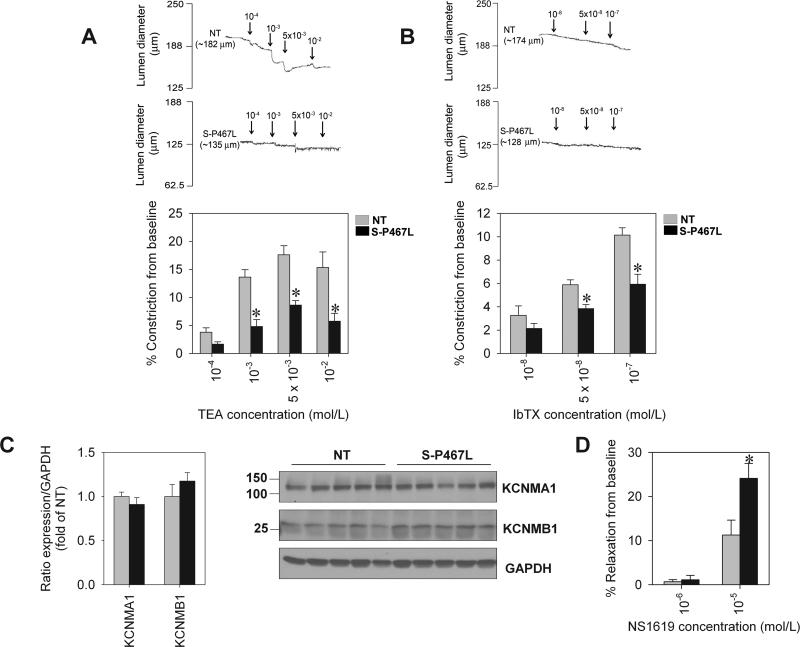
The opening of K+ channels has been shown to be an important mechanism opposing myogenic constriction in different vessel beds, including those in the mesenteric circulation 11. Intraluminal pressure was raised to 75 mmHg to induce myogenic tone in both NT and S-P467L mesenteric artery, and once myogenic response was stably established, a K+ channel inhibitor was added directly into the superfusate. TEA induced vasoconstriction in a dose-dependent manner in NT artery while producing an attenuated response in S-P467L artery (Figure 3A). These effects were evident at 1 mmol/L, a concentration where TEA has been shown to be a BKCa-selective inhibitor 12. Similarly, IbTX, a highly selective BKCa channel blocker, caused constriction of pressurized mesenteric artery from NT but the constriction was markedly blunted in S-P467L (Figure 3B). These findings suggest that the BKCa channel is functionally impaired in S-P467L mesenteric artery. We determined that this impairment is not attributable to altered BKCa subunit expression. First, microarray analysis showed that the level of expression of α and β1 subunit mRNAs encoding the BKCa channel was unchanged in mesenteric artery from S-P467L mice (Online Table I). Second, the level of α and β1 protein subunits did not differ between groups (Figure 3C). In addition, we tested the effect of the BKCa channel opener, NS1619 on vascular reactivity. Myogenic constriction was induced by increased luminal pressure to 100 mmHg and the vasodilatory effect was recorded following addition of NS1619. We observed that 10-6 mol/L NS1619 had a minimal effect on vessel tone in both NT and S-P467L (Figure 3D). However, there was a significantly larger vasodilation response to 10-5 mol/L NS1619 in S-P467L mesenteric artery compared to NT (p<0.05). These findings suggested that the BKCa channel in S-P467L is functional. Taken together with its normal expression, we hypothesized that other mechanisms (i.e. post-translational modification) likely account for the impairment of BKCa activity in S-P467L in response to increased intraluminal pressure.
Figure 3. Impaired response to TEA and IbTX-induced constriction.
A) Representative recording of diameter (upper panel) and summary of data determined by % inhibition from baseline (lower panel) in response to TEA (NT, n=6; S-P467L, n=6). B) Representative recording of diameter (upper panel) and summary of data determined by % inhibition from baseline (lower panel) in response to IbTX (NT, n=6; S-P467L, n=5). C) Protein expression and quantitative analysis of KCNMA1 and KCNMB1 in mesenteric artery (NT, n=5; S-P467L, n=5). D) Vasodilation in response to NS1619 (NT, n=5; S-P467L, n=4). * p<0.05 compared to NT. All data are mean±SEM
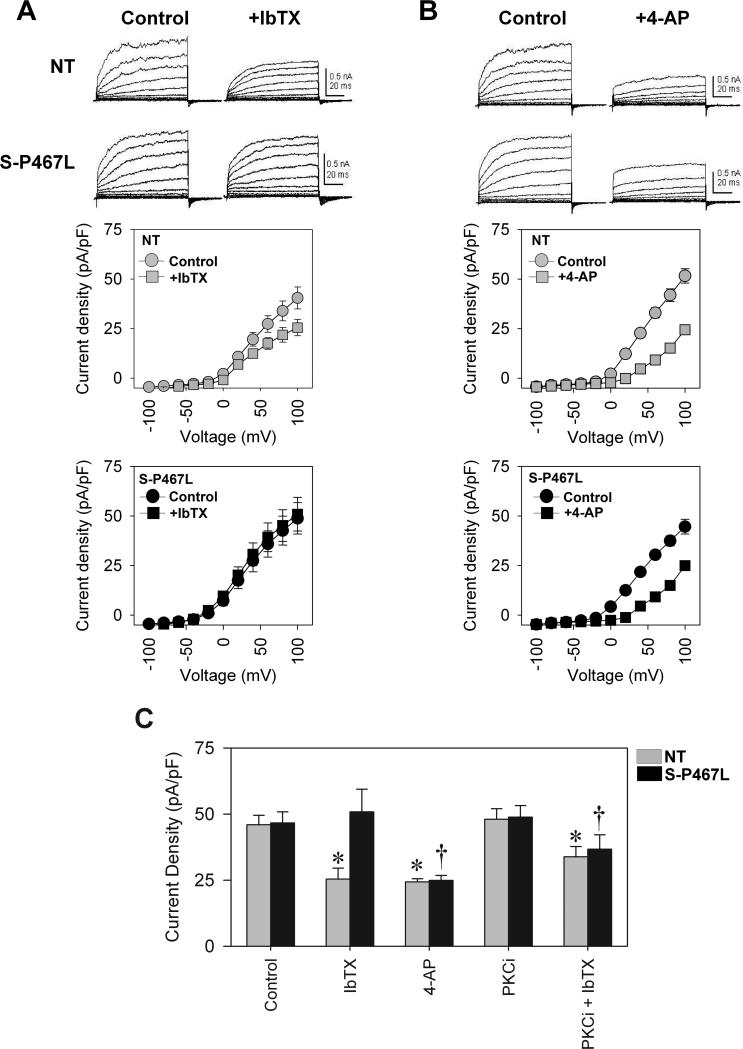
We next measured whole-cell K+ currents using voltage clamp in freshly dissociated smooth muscle cells (SMC) isolated from small mesenteric arteries to investigate the electrophysiological function of the BKCa channel. There was no difference in membrane capacitance between NT and S-P467L SMC (NT=30.35±1.02 pS, n=21 and S-P467L=28.87±0.45 pS, n=18, p>0.05). Total whole-cell K+ current was similar between NT and S-P467L (Figure 4A). Consistent with the vascular reactivity results, addition of the BKCa channel blocker IbTX significantly inhibited the K+ current density in NT SMC, but had no effect in S-P467L SMC (Figure 4A, summarized in panel C). Interestingly, 4-AP, a Kv channel inhibitor blocked whole cell K+ current similarly between NT and S-P467L SMC (Figure 4B, summarized in panel C), suggesting a preservation of Kv channel activity in both groups. Therefore, our observations indicate that S-P467L mice have a selective inhibition of BKCa channel function. Since, the activity of BKCa can be regulated by PKC-mediated phosphorylation 13, we next measured whole cell K+ current in the presence of a PKC inhibitor (chelerythrine chloride). PKC inhibition did not alter total K+ current compared to baseline, and addition of IbTX significantly attenuated K+ currents in NT SMC (Figure 4D). Interestingly, in the presence of the PKC inhibitor, IbTX now attenuated the BKCa current in transgenic SMC (compare the IbTX-response in Figure 4C), strongly suggesting that the impairment of BKCa channel activity in S-P467L mesenteric SMC is PKC-dependent.
Figure 4. Electrophysiological data showed a reduced BKCa current in S-P467L SMC, which is dependent on PKC.
A) Total K+ current before and after IbTX treatment (6 and 7 cells from 3 to 4 animals of each group). B) Total K+ current before and after 4-AP treatment (6 and 7 cells from 4 animals of each group). C) Summary of data at 100 mV from baseline, IbTX, and 4-AP treatment (first three pairs of bars). Last two pairs of bars data from cells treated with 100 nmol/L chelerythrine chloride, a PKC inhibitor (PKCi), and PKCi+IbTX. (6 and 7 cells from 4 animals of each group). * p<0.05 compared to Control or PKCi within NT group, † p<0.05 compared to Control or PKCi within S-P467L group. All data are mean±SEM
Diminished myogenic tone by PKC inhibition
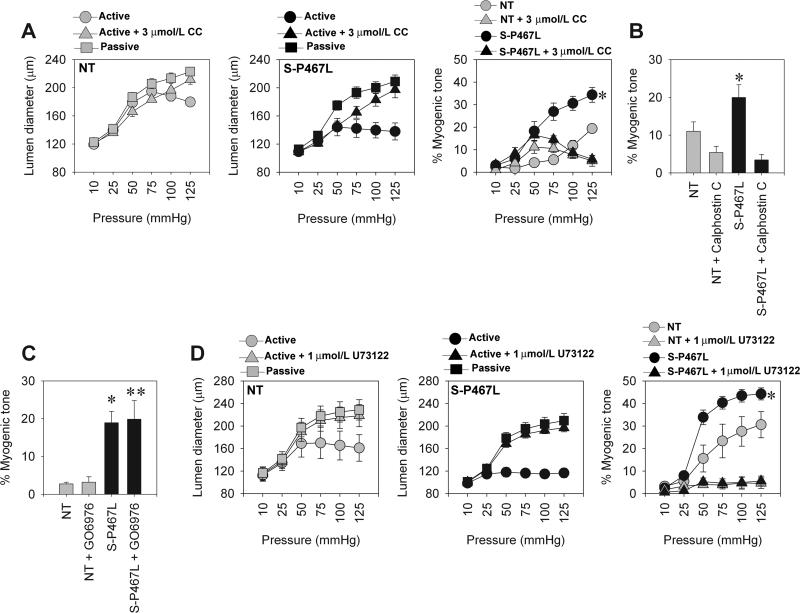
Since PKC-inhibition restored BKCa activity in isolated SMC, we asked if it would also blunt the myogenic response in S-P467L mesenteric artery. PKC inhibition robustly decreased myogenic constriction in S-P467L mesenteric artery at pressures above 50 mmHg, but only blunted myogenic constriction in NT artery at 125 mmHg (Figure 5A). The effect was so pronounced that PKC inhibition abolished the difference between the transgenic and NT groups (see combined data in panel 3). To eliminate the possibility of a non-specific effect of chelerythrine chloride, we utilized Calphostin C, another highly specific PKC inhibitor that acts through a different mechanism 14. Similar to chelerythrine chloride, myogenic tone in S-P467L was markedly decreased after Calphostin C (Figure 5B). Additionally, we used the Ca2+-dependent PKC inhibitor, GO6976, to determine the class of PKC isoforms that might be responsible for these effects. GO6976 had minimal effects on myogenic constriction in both NT and S-P467L (Figure 5C). These data suggest that the Ca2+-independent PKC isoforms are involved in myogenic tone in mesenteric artery, consistent with prior studies 15;16. As it is well established that PKC is directly downstream of Phospholipase C (PLC) signaling, we asked whether the myogenic tone in mesenteric artery is blunted by PLC inhibition. Pre-incubation with the PLC inhibitor, U73122 markedly inhibited the myogenic constriction in both NT and S-P467L mice, and like PKC inhibition, eliminated the difference between groups (Figure 5C).
Figure 5. Myogenic tone after PKC inhibition.
A) Pressure-diameter relationship and % myogenic tone in NT and S-P467L before and after pre-incubation of the artery with 3 μmol/L chelerythrine chloride (CC) (NT, n=7; S-P467L, n=6). B) Summary of % myogenic tone data at P75 mmHg before and after pre-incubation with 10 nmol/L Calphostin C (NT, n=6; S-P467L, n=5). C) Summary of % myogenic tone data at P75 mmHg before and after pre-incubation with 1μmol/L GO6976 (NT, n=4; S-P467L, n=4). D) Pressure-diameter relationship and % myogenic tone in NT and S-P467L before and after pre-incubation of the artery with 1 μmol/L U73122 (NT, n=4; S-P467L, n=5). * p<0.05 compared to NT. ** p<0.05 compared to S-P467L. All data are mean±SEM
Previous studies have reported that Rho kinase can modulate myogenic tone in mesenteric artery17, and our previous finding indicate that the hypercontractility in the aorta, a conduit artery, in S-P467L is dependent on Rho kinase 8. Therefore, we determined the contribution of RhoA/Rho kinase signaling in mesenteric artery. Pre-incubation with the Rho kinase inhibitor, Y27632 attenuated the myogenic response similarly in both NT and S-P467L artery, and myogenic tone in transgenic mice remained significantly higher than that in NT (Online Figure IIIA). The expression of RhoA in mesenteric artery did not differ between groups (Online Figure IIIB). Taken together, our findings demonstrate that the enhanced myogenic constriction in S-P467L is PLC and PKC, but not Rho kinase dependent.
Mechanistic role of RGS5
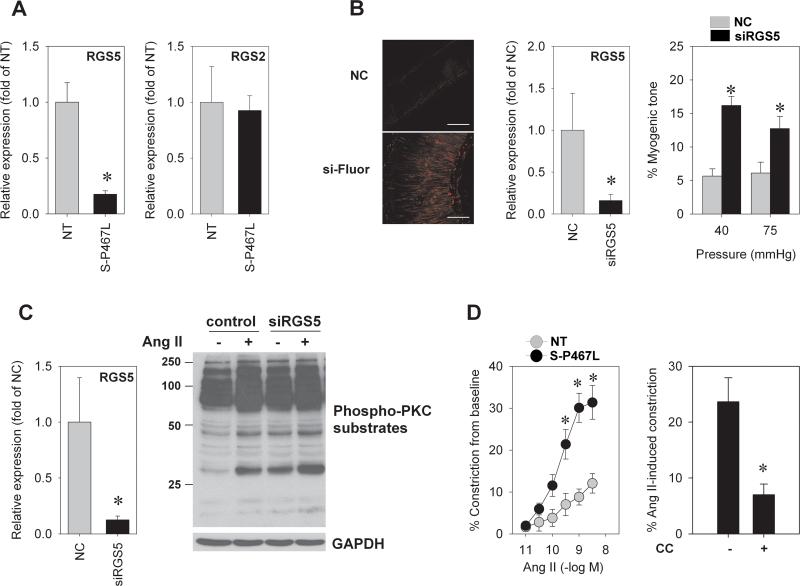
To identify molecular mechanisms underlying the enhanced constriction in S-P467L mesenteric artery, we performed gene expression analysis using exon arrays (array platform, GPL6096; series accession, GSE36482) with RNA isolated from mesenteric arteries of NT (n=4) and S-P467L (n=3) mice. We focused our examination on genes likely to be directly interacting with, or regulating the PLC/PKC signaling pathway. We queried the human protein reference database (http://hprd.org) for proteins known to interact with the Gαq class of G-proteins (i.e., GNAQ, GNA11, GNA14, and GNA15), which robustly activate PLC. Of the 64 genes examined (Online Table II), only one gene, Regulator of G-Protein Signaling-5 (RGS5), was significantly altered (threshold p<0.01), exhibiting a greater than 2-fold reduction in S-P467L mice. Expression of other RGS family members (e.g., RGS2 and RGS4) was similar between the two groups. Consistently, we found a 5-fold reduction in RGS5 mRNA expression in S-P467L mesenteric arteries compared to NT by qRT-PCR (p<0.01), while RGS2 expression was unchanged (Figure 6A). To directly test the hypothesis that loss of RGS5 is sufficient to cause increased myogenic tone in mouse mesenteric arteries, siRNA targeting RGS5 or negative control (NC) siRNA was transfected into small intact mesenteric arteries using electroporation. Confocal microscopy showed a robust fluorescent signal from smooth muscle layers of arteries transfected with siRNA labeling dye (Online Figure IV). Following 30 hr of transfection, the endogenous RGS5 mRNA expression was significantly reduced compared to NC (Figure 6B). There was no significant difference in KCl-induced constriction between NC and siRGS5 transfected vessels (NC = 45±6% and siRGS5 = 35±4%, n=5-6, p=0.2). On the contrary, myogenic constriction was 2- to -3-fold increased in siRGS5 treated group compared to NC (Figure 6B). This finding establishes a direct mechanistic relationship between RGS5 deficiency and enhanced vessel tone.
Figure 6. Role of RGS5.
A) qRT-PCR of RGS5 and RGS2 expression in mesenteric artery (NT, n=10; S-P467L, n=12). B) Fluorescence images from mesenteric artery transfected with negative control (NC) siRNA or with dye-labeled oligo control (si-Fluor), qRT-PCR of RGS5 transcript (n=8) and % myogenic tone (n=5-6) after 30 hr of NC siRNA or siRGS5 transfection in intact mesenteric arteries (* p<0.05 compared to NC). C) RGS5 mRNA expression after siRNA-mediated knockdown in smooth muscle cultures. RGS5 mRNA was measured by qRT-PCR after 48 hr of transfection (* p<0.05 compared to NC) and western blot analysis measuring the level of phosphorylated PKC substrate in response to Ang II and RGS5 siRNA (n=3). D) Concentration response curve of Ang II-induced vasoconstriction (NT, n=7; S-P467L, n=9) and the effect of PKC inhibitor, 3 μmol/L chelerythrine chloride (CC) on 30 nmol/L Ang II-mediated constriction in S-P467L arteries (n=4). * p<0.05 compared to NT. All data are mean±SEM
It is well established that RGS proteins terminate Gα signaling by accelerating its intrinsic GTPase activity; and RGS5 is known to act as a negative regulator of Gαq, whose downstream signaling includes activation of PLC/PKC pathway 18. We therefore tested the hypothesis that decreased expression of RGS5 in S-P467L artery directly contributes to the increase in PKC activity. Rat aortic smooth muscle cells (RASMC) were transfected with either siRNA against RGS5 or negative control siRNA and assays were performed 48 hr later. We observed a >90% decrease in RGS5 mRNA in response to the specific siRNA (Figure 6C). Importantly, we observed an increase in PKC activation, measured by phosphorylation of PKC substrates in vitro, in response to RGS5 siRNA that was comparable to the phosphorylation caused by Ang II (Figure 6C). Prior studies have reported that Ang II signaling was enhanced following RGS5 knockdown in SMC cultures 19. Consistent with this, there was a markedly enhanced contractile response to Ang II in S-P467L (Figure 6D). Pre-incubation of the artery for 30 minutes with the AT1 receptor antagonist losartan completely abolished Ang II-induced vasoconstriction, indicating that this effect is dependent on activation of AT1 receptor (Online Figure VA). The lack of Ang II-induced constriction following losartan treatment is not due to the desensitization of small arteries to Ang II because there was no significant difference between the first and the second application of Ang II (First application = 27.1±5.1% vs. Second application = 20.5±1.6%, n=3, p=0.284). Neither AT1a nor AT1b expression was different between the groups (Online Figure VB), suggesting that changes in receptor expression do not account for the increased Ang II response in S-P467L mesenteric artery further implicating a post-receptor mechanism. Furthermore, the Ang II-induced constriction in S-P467L was dependent on PKC (Figure 6D), similar to that for myogenic constriction. Importantly, vasoconstriction to other agonists (endothelin-1, U-46619, and phenylephrine) was similar in the two strains (Online Figure VC), suggesting a specific over-activation of Ang II signaling.
Since Ang II-induced constriction was markedly increased in S-P467L mesenteric arteries, we next examined the hemodynamic response to infusion of various doses of Ang II in anesthetized mice. As we previously reported, baseline blood pressure was slightly increased in S-P467L mice compared to NT8. Despite the marked increased in Ang II-mediated constriction, infusion of Ang II increased blood pressure in a dose dependent manner in both groups. Surprisingly, there was no difference in pressor responses to Ang II between NT and S-P467L mice. The tachycardia phenotype previously reported in S-P467L mice remained intact at baseline and during the Ang II infusion in mice under anesthesia (Online Figure VI).
Mechanism for blunted RGS5 expression
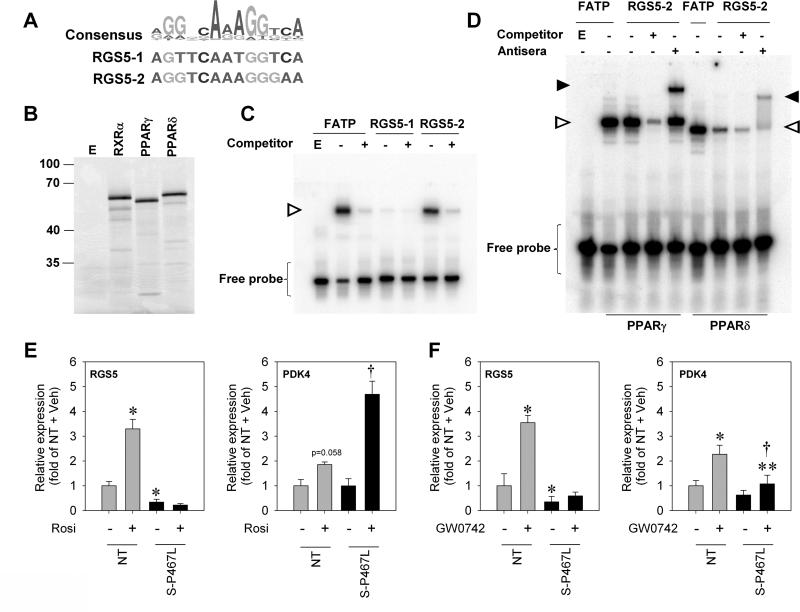
To identify DNA sequences near the RGS5 gene capable of binding PPARγ, we used a sequence-based model generated from experimentally validated PPARγ binding sites 20 and identified two potential PPREs in the proximity of the RGS5 promoter region. The first (RGS5-1) is located 3 kb upstream of the transcriptional start site and the second (RGS5-2) is located in the first intron of RGS5 near a region of high evolutionary sequence conservation (Figure 7A). Electrophoretic mobility shift assays (EMSAs) were used to examine the ability of PPARγ to bind to these potential PPREs using in vitro transcribed/translated proteins (Figure 7B). Reticulocyte lysate programmed with empty vector and incubated with a control probe carrying the PPRE from the known PPARγ target gene FATP indicated that the binding was specific to the PPARγ/RXRα programmed lysates (Figure 7C). We found that PPARγ/RXRα produced a strong shift complex with the RGS5-2 probe but not the RGS5-1 probe (Figure 7C). The RGS5-2 shift complex corresponded to that seen when PPARγ/RXRα was incubated with the FATP probe. PPARγ/RXRα also produced a complex that was supershifted with the addition of PPARγ antibody (Figure 7D). Because prior studies showed that RGS5 expression could be induced by PPARδ stimulation 21;22, we investigated if PPARδ could also bind to the RGS5-2 site. PPARδ formed a complex with the RGS5-2 probe that was supershifted by the anti-epitope antibody present in the PPARδ fusion protein (Figure 7D). These findings demonstrate that both PPARγ and PPARδ can bind to a PPRE sequence located in the first intron of RGS5.
Figure 7. RGS5 is a PPARγ and PPARδ target gene.
A) Top: A position weighted matrix of known PPAR gamma binding sites (Accession Number: MA0065.2) obtained from JASPAR (http://jaspar.genereg.net/), an open source database of experimentally determined transcription factor binding sites. The position-weighted matrix was visualized using sequences logos (created with WebLogo 3 at http://weblogo.threeplusone.com/). B) In vitro transcription/translation of proteins used for EMSA. An aliquot of the reaction used for EMSA was labeled with S35-methionine and separated by SDS-PAGE. C-D) EMSA using the indicated probe and in vitro transcribed/translated PPARγ (with RXRα) or PPARδ (with RXRα). The presence of the shift and supershift products is indicated by open and closed triangles, respectively. E-F) Ex vivo gene expression in mesenteric arteries-treated with either vehicle (DMSO), rosiglitazone (Rosi) or GW0742 (NT, n=5-6; S-P467L, n=5-6). * p<0.05 compared to NT + Veh, † p<0.05 compared to S-P467L + Veh. All data are mean±SEM
We next tested the hypothesis that the reduction in RGS5 expression in S-P467L was a result of dominant-negative interference with ligand-activated PPARγ. As before, there was a substantial decrease in RGS5 mRNA expression in S-P467L mesenteric artery indicating that the gene expression phenotype of these vessels was preserved after a 6-hour incubation ex vivo (Figure 7E). Strikingly, induction of PPARγ with rosiglitazone significantly increased RGS5 mRNA in NT, but had no effect in S-P467L arteries (Figure 7E). These data support our hypothesis that downregulation of RGS5 in S-P467L mice could be attributable, at least in part, to active repression and loss of agonist-mediated induction by dominant negative PPARγ. It is interesting to note however that expression of PDK4 (Figure 7E) and FABP4 (data not shown), classic targets of PPARγ were significantly induced by rosiglitazone in S-P467L. This observation was unexpected but may be consistent with previous studies reporting that saturating concentrations of TZD can potentially override dominant negative effects23. Our data suggests that this effect may be selective for some genes, perhaps genes with particularly strong PPARγ binding sites. Indeed, several genome wide ChIP studies have identified PDK4 and FABP4 to have functional PPRE sequences 24;25. Only one of these studies identified a functional PPRE in RGS5 26. It is important to note this may be more of a reflection of the restricted cell-specific expression of RGS5 than strength of the PPARγ binding.
Since our EMSA result indicated that PPARδ can also bind to a PPRE sequence near the RGS5 promoter, we investigated whether PPARδ-mediated RGS5 gene expression was affected by dominant negative PPARγ. RGS5 mRNA was markedly induced in NT by 6 hr incubation of mesenteric artery with PPARδ agonist, GW0742 (Figure 7F). This induction was abrogated in S-P467L arteries. To examine whether the inhibitory effect of this mutant receptor extends to other gene targets of PPARδ, we evaluated the expression of PDK4. At baseline, there was no difference in PDK4 expression in NT and S-P467L (Figure 7F). Activation of PPARδ by GW0742 induced PDK4 mRNA in both groups although the induction was blunted in S-P467L (Figure 7F). We therefore determined if dominant negative PPARγ resulted in general interference with all PPARδ-regulated gene expression. We queried a dataset for PPARδ target genes identified using a combination of Chip-Seq and expression microarrays in human myofibroblasts 26. We mapped 100 of these genes to their mouse counterparts on the Affymetrix Mouse Exon 1.0 ST Array. Of these, 6 genes were removed from the analysis as their expression level in mesenteric artery was below the threshold for detection using the DABG (Detection Above Background Level) algorithm implemented in the Affymetrix Powertools software. Whereas two genes (TIMP4 and CPT1A) exhibited a modest but statistically significant decrease (p<0.01) in expression in S-P467L mesenteric artery, no other genes exhibited a significant change (Online Table III). Thus the effects of dominant negative PPARγ appear to be selective for certain PPARδ target genes.
DISCUSSION
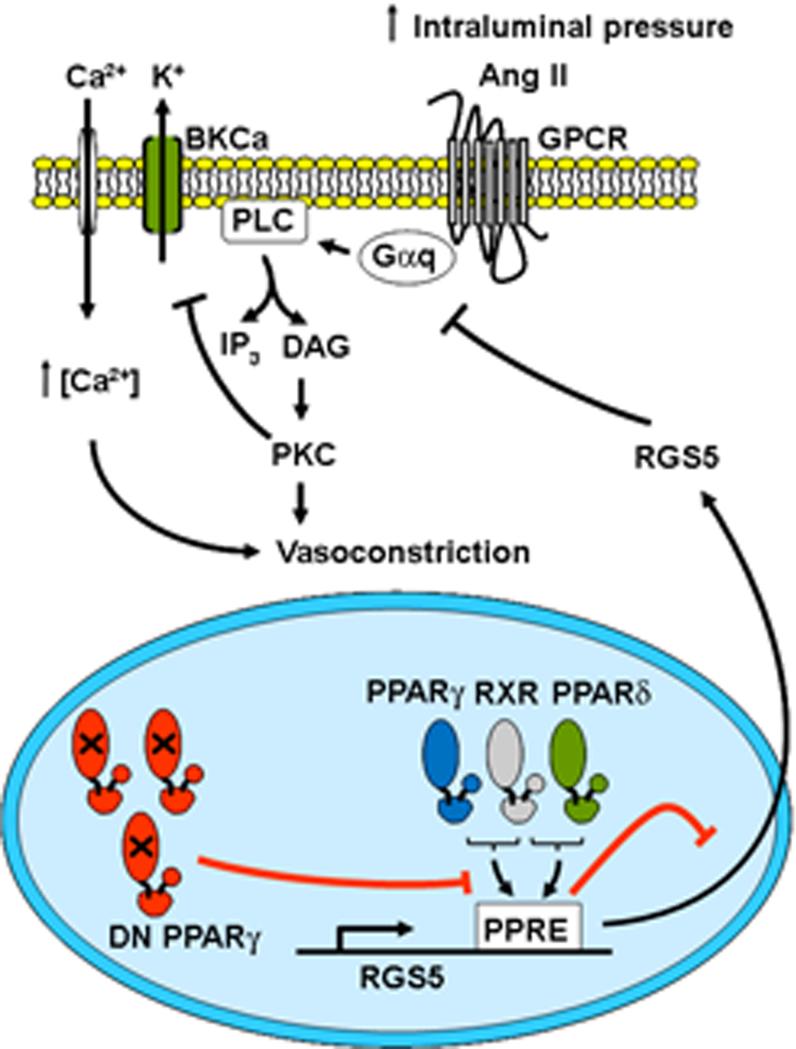
Data showing that patients carrying dominant negative PPARγ develop severe hypertension and clinical studies reporting a blood pressure lowering effect of TZDs have raised the possibility that PPARγ plays an important role in the regulation of blood pressure 2. Although multiple studies support the direct action of TZD in vasculature, it remains unclear whether this effect occurs through PPARγ and what target genes and pathways are engaged. We conclude that smooth muscle dominant negative PPARγ causes downregulation of RGS5, a novel PPARγ and PPARδ target gene, resulting in a marked increase in myogenic tone and Ang II-induced constriction of the mesenteric artery via a mechanism dependent upon increased PKC activity with consequent inhibition of the BKCa channel (Figure 8).
Figure 8. Summary of findings.
Dominant negative (DN) PPARγ leads to downregulation of RGS5, a novel PPARγ and PPARδ target, resulting in an increase myogenic tone and Ang II-induced constriction. The hypercontraction is dependent upon increased PKC activity with consequent inhibition of the BKCa channel.
Vascular tone in small arteries and arterioles is a major determinant of resistance in the circulation controlled by various stimuli including neurohumoral and myogenic components 9. Myogenic constriction is essential in the regulation of microcirculation blood flow and provides the basal tone in resistance artery. During myogenic constriction, an elevation of intraluminal pressure results in membrane depolarization and calcium entry through L-type Ca2+ channels, followed by activation of the contractile apparatus 17. As the response ensues, constriction without additional Ca2+ influx is achieved by Ca2+ sensitization, which involves activation of Rho-kinase and PKC-mediated inhibition of myosin light chain phosphatase, leading to sustained vasoconstriction. As a counter regulatory mechanism, opening of the BKCa channel is stimulated by Ca2+ sparks leading to a hyperpolarizing outward current to oppose myogenic constriction. Our studies highlight a contribution of BKCa channel function during increased intraluminal pressure. A reduction in BKCa channel activity in S-P467L SMC is likely attributable from a loss of smooth muscle PPARγ function since BKCa channel activity was reported to be enhanced in other hypertensive models 12.
Evidence that the function of the BKCa channel is impaired in mesenteric vessels from S-P467L transgenic mice is two-fold. First, we showed that vasoconstriction to BKCa channel inhibition was greatly reduced. This was not due to an inability of the vessel to further constrict because vasoconstriction to KCl was observed even after addition of TEA or IbTX. Second, K+ currents generated in SMC from S-P467L were not dampened after pharmacological inhibition of the BKCa channel. It is notable that despite the compromised activity of BKCa channels in S-P467L, total K+ currents were unexpectedly similar to those in NT. Hence, it is possible that other voltage-dependent K+ channels in S-P467L SMC compensate for loss of the BKCa current. Importantly however, there was no evidence for changes in the expression of voltage-gated, inward rectifying, two pore, or calcium activated channels (Online Table I). Other K+ channels such as Kv have been shown to influence myogenic tone 27. We showed that 4-AP sensitive Kv channel activity is preserved and equivalent in isolated SMC from S-P467L compared with NT mice making it unlikely that increased Kv activity underlies this compensation. Notably, neither a preserved activity of the 4-AP sensitive Kv channels nor an upregulation of other K+ channels activity is sufficient to normalize myogenic tone in S-P467L.
Since there was no change in BKCa subunit expression and BKCa channels in S-P467L can be activated by NS1619, we speculate that altered BKCa channel function in S-P467L might be due to other mechanisms. Dynamic regulation of BKCa channel activity has been suggested to occur at multiple levels. Post-translational modification as well as uncoupling to Ca2+ sparks plays a critical role in the determination of BKCa channel activity. It was reported that PKC inhibits the open-state probability of the BKCa channel by phosphorylating α-subunits at distinct serine residues in smooth muscle cells 13. Alternatively, activation of PKC has been reported to decrease Ca2+ spark frequency and BK channel activity in SMC from cerebral vessels 28. In accordance with these data, inhibition of PKC in S-P467L SMC significantly restored IbTX-sensitive currents and blunted myogenic constriction, providing evidence that exaggerated PKC activation in transgenic artery underlies hypercontraction in resistance artery. Our data also suggest that the BKCa channel is not irreversibly attenuated, but may be more responsive to PLC/PKC activity.
As myogenic constriction in transgenic vessel was dependent upon activation of PLC/PKC pathway, this let us to hypothesize that the increased resistance of small artery tone in S-P467L is probably related to aberrant Gαq signaling. Activation of Gαq-coupled receptor and its downstream signaling have been implicated in mediating myogenic constriction in different resistance vessel beds 29. RGS5 mRNA was markedly decreased in S-P467L mesenteric artery. Genome-wide linkage and association studies suggest that RGS5 may be a candidate gene for hypertension in humans 30. RGS5 expression is enriched in arterial smooth muscle and is considered a marker of arterial smooth muscle cells and pericytes 31. It functions as a negative regulator of Gαq and Gαi, the G proteins used most often by vasoactive agents such as Ang II 19. It was shown previously that knockdown of RGS5 promotes hypercontractility and enhanced Ang II signaling in smooth muscle cells in culture 19. Our data provide novel evidence that downregulation of RGS5 was sufficient to cause enhanced myogenic constriction in intact resistance arteries and over-activation of PKC in smooth muscle cells. Thus, we conclude that the increase in vasomotor tone in mesenteric artery of S-P467L is attributable to a loss of RGS5 transcript, which we hypothesize is due to a direct action of dominant negative PPARγ.
We have previously validated the mechanisms of dominant negative PPARγ activity by comparing gene expression in aorta from mice treated with rosiglitazone with knock-in mice globally expressing dominant negative PPARγ. Expression of genes in aorta induced by TZD were suppressed in aorta from P465L, and were enriched for functionally validated PPARγ binding sites 20.
Given that RGS5 was markedly upregulated following TZD treatment in NT while attenuated in S-P467L artery, we hypothesized that RGS5 might be a direct PPARγ target. In support of this, we observed that PPARγ can bind to a highly conserved PPRE located in the first intron of the gene. These findings are consistent with previous reports that PPARγ can activate other target genes by binding to PPRE sequences in introns 32. In addition to PPARγ, we demonstrated that PPARδ can also bind to the same PPRE gene and that PPARδ agonist induces RGS5 expression. RGS5 might be a direct PPARδ target and the loss of PPARδ-mediated induction of RGS5 in S-P467L artery suggests that dominant negative PPARγ may also interfere with PPARδ signaling. This raises the possibility that this mutant not only competes with the function of endogenous PPARγ, but it may also preclude PPARα-mediated transactivation. Crosstalk between a different dominant negative mutation in PPARγ with PPARα has been reported 33. Although this questions the selectivity of the S-P467L model, our data showing that only 2% of known PPARδ targets exhibited significantly altered expression in mesenteric artery from S-P467L mice suggest that this crosstalk is highly gene selective. It is not clear what mechanisms determine the selectivity of PPAR action when they share a similar PPRE 25;26. It is possible that 1) flanking regions of PPREs might be distinct for each PPAR isoform, thereby allowing the regulation of specific transcription, 2) different PPARs might selectively use particular co-factors for activation of the same gene, 3) changes in endogenous ligand in different cell types in response to different physiological cues might influence which PPAR is activated, and 4) the phosphorylated state of each PPAR might dictate which form is active. The latter is supported by a recent study demonstrating that phosphorylation of PPARγ at serine 273 affects only a group of genes involved in insulin-sensitizing effects but not those related to adipogenesis 34.
Given that S-P467L mesenteric arteries exhibited an augmented contractile response to Ang II, it is surprising the increase in blood pressure induced by Ang II infusion was similar to that in NT mice. As we previously reported, the S-P467L mice exhibited an increase in arterial pressure despite a robust tachycardia 8, it is possible that the aberration of autonomic function in these mice has an impact on overall blood pressure which masks the response to acute Ang II. Despite unaltered pressor response to Ang II in S-P467L, augmented Ang II signaling may have a deleterious impact on the local control of blood flow and vascular structure, both of which are critical factors determining cardiovascular complications. It was previously reported that Ang II-induced vascular hypertrophy or vascular damage partly occurs independently of increased blood pressure 35. Activation of signaling pathways including ERK1/2, JAK/STAT and NADPH oxidase-derived reactive oxygen species are widely accepted to affect Ang II-induced vascular hypertrophy and inflammation 36. We speculate that an augmented Ang II signaling and vasomotor tone in S-P467L likely exacerbate the vascular complications in the long term. Although we did not observe vascular remodeling in mesenteric arteries of transgenic mice at baseline, microscopic examination of vascular wall components (i.e. collagen, elastin and other extracellular matrix components) is an important area of future inquiry.
In conclusion, we demonstrated that RGS5 gene expression can be regulated by both PPARγ and PPARδ. Specific overexpression of dominant negative PPARγ in smooth muscle leads to a marked decrease of RGS5 expression leading to enhanced post-receptor signaling. Loss of RGS5 resulted in augmented myogenic tone in resistance artery through increased PKC signaling and decreased BKCa channel activity. The molecular mechanism responsible for downregulation of RGS5 is due to the direct effect of dominant negative PPARγ on smooth muscle where it prevents endogenous PPARγ and PPARδ from regulating RGS5 gene expression. Our studies uncover a novel target of the PPAR family of transcription factors in the vasculature and specifically address the role of PPARγ in resistance artery.
Supplementary Material
Novelty and Significance.
What Is Known?
Anti-diabetes medications which target peroxisome proliferator-activated receptor (PPAR)-γ lower blood pressure, whereas mutations in PPARγ cause high blood pressure.
Mice with PPARγ mutations targeted to the blood vessel exhibit high blood pressure as a result of severe vascular dysfunction.
What New Information Does This Article Contribute?
Mice expressing mutant PPARγ in vascular smooth muscle exhibit increased myogenic tone of small resistance arteries and increased constriction to angiotensin-II.
Enhanced myogenic tone is due to decreased large conductance Ca2+-activated K+ (BKCa) channel activity, which can be restored by inhibition of protein kinase C (PKC).
The enhanced vasoconstriction is due to a decrease in expression of the PPARγ target gene Regulator of G Protein Signaling 5 (RGS5) in mesenteric artery as a result of the mutation in PPARγ.
Clinical studies have shown that thiazolidinediones, previously used to treat insulin resistance in type 2 diabetes, act as PPARγ agonists and lower blood pressure. Genetic data suggest that some mutations in PPARγ cause hypertension. We hypothesize that some beneficial cardiovascular effects of PPARγ may be mediated through the vasculature, but the underlying mechanisms remain unclear. Here, we demonstrate that interfering with PPARγ in vascular smooth muscle causes a marked increase in myogenic tone and angiotensin II-induced constriction in the mesenteric artery. The molecular mechanism involves a robust down-regulation of RGS5, a novel PPARγ target gene, which causes over-activation of the PKC pathway and inhibition of the BKCa channel. Expression of RGS5 is impaired in response to mutant PPARγ. These findings uncover a previously unknown molecular target of vascular PPARγ, specifically addresses how PPARγ regulates resistance artery function, and explains why drugs which activate PPARγ can have beneficial effects on the cardiovascular system in diabetes. It will be important to determine the effect on this pathway of new PPARγ-activating drugs as they are developed to combat the epidemic of type 2 diabetes.
ACKNOWLEDGMENTS
Transgenic mice were generated at the University of Iowa Transgenic Animal Facility supported by grants from the NIH and from the Carver College of Medicine. We thank Norma Sinclair, JoAnne Schwarting, and Patricia Yarolem for genotyping mice and Deborah R. Davis for blood pressure measurement. The authors also would like to thank Dr. Jason A. Scott (University of Iowa), Dr. Amy Banes-Berceli (Oakland University, MI), Dr. Eric Belin de Chantemele (Georgia Health Sciences University, GA) and Dr. Matthew J. Socha (University of Missouri-Columbia, MO) for their helps and suggestions.
SOURCES OF FUNDING
This work was supported through research grants from the NIH to CDS (HL084207, HL048058, HL061446, HL062984 and NS024621), FMF (HL038901, HL062984, and NS024621), SKE (HD037831), and JLG (HL098276) and from the American Heart Association to PK (11POST5720021) and CJP (10PRE3740029). The authors also gratefully acknowledge the generous research support of the Roy J. Carver Trust.
Non-standard Abbreviations
- 4-AP
4-aminopyridine
- Ang II
angiotensin II
- AT1
angiotensin II receptor, type 1
- BKCa
the large conductance Ca2+-activated K+ channel
- EGTA
ethylene glycol tetraacetic acid
- EMSAs
electrophoretic mobility shift assays
- FABP4
fatty acid binding protein 4
- FATP
fatty acid transporter protein
- Fluor
fluorescence
- IbTX
iberiotoxin
- Kv
the voltage-dependent K+ channel
- NT
non-transgenic
- NC
negative control
- PDK4
pyruvate dehydrogenase kinase 4
- PKC
protein Kinase C
- PLC
phospholipase C
- PPARγ
peroxisome proliferator-activated receptor γ
- PPARδ
peroxisome proliferator-activated receptor δ
- PPRE PPAR
response elements
- RGS5
the regulator of G protein signaling 5
- RXR
retinoid X receptor
- SMC
smooth muscle cells
- SNP
sodium nitroprusside
- TEA
tetraethylammonium
- TRPC1
transient receptor potential cation channel, subfamily C, member 1
- TRPC6
transient receptor potential cation channel, subfamily C, member 6
- TRPM4
transient receptor potential cation channel, subfamily M, member 4
- TZD
thiazolidinediones
Footnotes
DISCLOSURES
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ricote M, Glass CK. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta. 2007;1771:926–935. doi: 10.1016/j.bbalip.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ketsawatsomkron P, Pelham CJ, Groh S, Keen HL, Faraci FM, Sigmund CD. Does peroxisome proliferator-activated receptor-gamma (PPAR gamma) protect from hypertension directly through effects in the vasculature? J Biol Chem. 2010;285:9311–9316. doi: 10.1074/jbc.R109.025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 4.Govindan J, Evans M. Pioglitazone in clinical practice: where are we now? Diabetes Ther. 2012;3:1–8. doi: 10.1007/s13300-012-0001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O'Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 6.Tsai YS, Kim HJ, Takahashi N, Kim HS, Hagaman JR, Kim JK, Maeda N. Hypertension and abnormal fat distribution but not insulin resistance in mice with P465L PPARgamma. J Clin Invest. 2004;114:240–249. doi: 10.1172/JCI20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray SL, Nora ED, Grosse J, Manieri M, Stoeger T, Medina-Gomez G, Burling K, Wattler S, Russ A, Yeo GS, Chatterjee VK, O'Rahilly S, Voshol PJ, Cinti S, Vidal-Puig A. Leptin deficiency unmasks the deleterious effects of impaired peroxisome proliferator-activated receptor gamma function (P465L PPARgamma) in mice. Diabetes. 2006;55:2669–2677. doi: 10.2337/db06-0389. [DOI] [PubMed] [Google Scholar]
- 8.Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM, Sigmund CD. Interference with PPAR gamma function in smooth muscle causes vascular dysfunction and hypertension. Cell Metab. 2008;7:215–226. doi: 10.1016/j.cmet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill MA, Meininger GA, Davis MJ, Laher I. Therapeutic potential of pharmacologically targeting arteriolar myogenic tone. Trends Pharmacol Sci. 2009;30:363–374. doi: 10.1016/j.tips.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 11.Jackson-Weaver O, Paredes DA, Gonzalez Bosc LV, Walker BR, Kanagy NL. Intermittent hypoxia in rats increases myogenic tone through loss of hydrogen sulfide activation of large-conductance Ca(2+)-activated potassium channels. Circ Res. 2011;108:1439–1447. doi: 10.1161/CIRCRESAHA.110.228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paterno R, Heistad DD, Faraci FM. Functional activity of Ca2+-dependent K+ channels is increased in basilar artery during chronic hypertension. Am J Physiol. 1997;272:H1287–H1291. doi: 10.1152/ajpheart.1997.272.3.H1287. [DOI] [PubMed] [Google Scholar]
- 13.Zhou XB, Wulfsen I, Utku E, Sausbier U, Sausbier M, Wieland T, Ruth P, Korth M. Dual role of protein kinase C on BK channel regulation. Proc Natl Acad Sci U S A. 2010;107:8005–8010. doi: 10.1073/pnas.0912029107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laher I, Zhang JH. Protein kinase C and cerebral vasospasm. J Cereb Blood Flow Metab. 2001;21:887–906. doi: 10.1097/00004647-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Loufrani L, Lehoux S, Tedgui A, Levy BI, Henrion D. Stretch induces mitogen-activated protein kinase activation and myogenic tone through 2 distinct pathways. Arterioscler Thromb Vasc Biol. 1999;19:2878–2883. doi: 10.1161/01.atv.19.12.2878. [DOI] [PubMed] [Google Scholar]
- 16.Baek EB, Jin C, Park SJ, Park KS, Yoo HY, Jeon JH, Earm YE, Kim SJ. Differential recruitment of mechanisms for myogenic responses according to luminal pressure and arterial types. Pflugers Arch. 2010;460:19–29. doi: 10.1007/s00424-010-0791-7. [DOI] [PubMed] [Google Scholar]
- 17.Schubert R, Lidington D, Bolz SS. The emerging role of Ca2+ sensitivity regulation in promoting myogenic vasoconstriction. Cardiovasc Res. 2008;77:8–18. doi: 10.1016/j.cardiores.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Manzur M, Ganss R. Regulator of G protein signaling 5: a new player in vascular remodeling. Trends Cardiovasc Med. 2009;19:26–30. doi: 10.1016/j.tcm.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Liu M, Mullah B, Siderovski DP, Neubig RR. Receptor-selective effects of endogenous RGS3 and RGS5 to regulate mitogen-activated protein kinase activation in rat vascular smooth muscle cells. J Biol Chem. 2002;277:24949–24958. doi: 10.1074/jbc.M203802200. [DOI] [PubMed] [Google Scholar]
- 20.Keen HL, Halabi CM, Beyer AM, de Lange WJ, Liu X, Maeda N, Faraci FM, Casavant TL, Sigmund CD. Bioinformatic analysis of gene sets regulated by ligand-activated and dominant-negative peroxisome proliferator-activated receptor gamma in mouse aorta. Arterioscler Thromb Vasc Biol. 2010;30:518–525. doi: 10.1161/ATVBAHA.109.200733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zarzuelo MJ, Jimenez R, Galindo P, Sanchez M, Nieto A, Romero M, Quintela AM, Lopez-Sepulveda R, Gomez-Guzman M, Bailon E, Rodriguez-Gomez I, Zarzuelo A, Galvez J, Tamargo J, Perez-Vizcaino F, Duarte J. Antihypertensive effects of peroxisome proliferator-activated receptor-beta activation in spontaneously hypertensive rats. Hypertension. 2011;58:733–743. doi: 10.1161/HYPERTENSIONAHA.111.174490. [DOI] [PubMed] [Google Scholar]
- 22.Takata Y, Liu J, Yin F, Collins AR, Lyon CJ, Lee CH, Atkins AR, Downes M, Barish GD, Evans RM, Hsueh WA, Tangirala RK. PPARdelta-mediated antiinflammatory mechanisms inhibit angiotensin II-accelerated atherosclerosis. Proc Natl Acad Sci U S A. 2008;105:4277–4282. doi: 10.1073/pnas.0708647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li G, Leff T. Altered promoter recycling rates contribute to dominant-negative activity of human peroxisome proliferator-activated receptor-gamma mutations associated with diabetes. Mol Endocrinol. 2007;21:857–864. doi: 10.1210/me.2006-0401. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen R, Pedersen TA, Hagenbeek D, Moulos P, Siersbaek R, Megens E, Denissov S, Borgesen M, Francoijs KJ, Mandrup S, Stunnenberg HG. Genome-wide profiling of PPARgamma:RXR and RNA polymerase II occupancy reveals temporal activation of distinct metabolic pathways and changes in RXR dimer composition during adipogenesis. Genes Dev. 2008;22:2953–2967. doi: 10.1101/gad.501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefterova MI, Steger DJ, Zhuo D, Qatanani M, Mullican SE, Tuteja G, Manduchi E, Grant GR, Lazar MA. Cell-specific determinants of peroxisome proliferator-activated receptor gamma function in adipocytes and macrophages. Mol Cell Biol. 2010;30:2078–2089. doi: 10.1128/MCB.01651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adhikary T, Kaddatz K, Finkernagel F, Schonbauer A, Meissner W, Scharfe M, Jarek M, Blocker H, Muller-Brusselbach S, Muller R. Genomewide analyses define different modes of transcriptional regulation by peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta). PLoS One. 2011;6:e16344. doi: 10.1371/journal.pone.0016344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen TT, Luykenaar KD, Walsh EJ, Walsh MP, Cole WC. Key role of Kv1 channels in vasoregulation. Circ Res. 2006;99:53–60. doi: 10.1161/01.RES.0000229654.45090.57. [DOI] [PubMed] [Google Scholar]
- 28.Bonev AD, Jaggar JH, Rubart M, Nelson MT. Activators of protein kinase C decrease Ca2+ spark frequency in smooth muscle cells from cerebral arteries. Am J Physiol. 1997;273:C2090–C2095. doi: 10.1152/ajpcell.1997.273.6.C2090. [DOI] [PubMed] [Google Scholar]
- 29.Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008;27:3092–3103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang YP, Liu X, Kim JD, Ikeda MA, Layton MR, Weder AB, Cooper RS, Kardia SL, Rao DC, Hunt SC, Luke A, Boerwinkle E, Chakravarti A. Multiple genes for essential-hypertension susceptibility on chromosome 1q. Am J Hum Genet. 2007;80:253–264. doi: 10.1086/510918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams LD, Geary RL, McManus B, Schwartz SM. A comparison of aorta and vena cava medial message expression by cDNA array analysis identifies a set of 68 consistently differentially expressed genes, all in aortic media. Circ Res. 2000;87:623–631. doi: 10.1161/01.res.87.7.623. [DOI] [PubMed] [Google Scholar]
- 32.Helledie T, Grontved L, Jensen SS, Kiilerich P, Rietveld L, Albrektsen T, Boysen MS, Nohr J, Larsen LK, Fleckner J, Stunnenberg HG, Kristiansen K, Mandrup S. The gene encoding the Acyl-CoA-binding protein is activated by peroxisome proliferator-activated receptor gamma through an intronic response element functionally conserved between humans and rodents. J Biol Chem. 2002;277:26821–26830. doi: 10.1074/jbc.M111295200. [DOI] [PubMed] [Google Scholar]
- 33.Semple RK, Meirhaeghe A, Vidal-Puig AJ, Schwabe JW, Wiggins D, Gibbons GF, Gurnell M, Chatterjee VK, O'Rahilly S. A dominant negative human peroxisome proliferator-activated receptor (PPAR){alpha} is a constitutive transcriptional corepressor and inhibits signaling through all PPAR isoforms. Endocrinology. 2005;146:1871–1882. doi: 10.1210/en.2004-1405. [DOI] [PubMed] [Google Scholar]
- 34.Choi JH, Banks AS, Estall JL, Kajimura S, Bostrom P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Bluher M, Griffin PR, Spiegelman BM. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466:451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffin SA, Brown WC, MacPherson F, McGrath JC, Wilson VG, Korsgaard N, Mulvany MJ, Lever AF. Angiotensin II causes vascular hypertrophy in part by a non-pressor mechanism. Hypertension. 1991;17:626–635. doi: 10.1161/01.hyp.17.5.626. [DOI] [PubMed] [Google Scholar]
- 36.Heeneman S, Sluimer JC, Daemen MJ. Angiotensin-converting enzyme and vascular remodeling. Circ Res. 2007;101:441–454. doi: 10.1161/CIRCRESAHA.107.148338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.