Summary
Most vertebrates process visual information using elaborately structured photosensory tissues including the eyes and pineal. However there is strong evidence that other tissues can detect and respond to photic stimuli [1, 2, 3]. Many reports suggest that photosensitive elements exist within the brain itself and influence physiology and behavior, however a long standing puzzle has been the identity of the neurons and photoreceptor molecules involved [4, 5]. We tested whether light cues influence behavior in zebrafish larvae through deep brain photosensors. We found that larvae lacking eyes and pineal perform a simple light-seeking behavior triggered by loss of illumination (`dark photokinesis'). Neuroanatomical considerations prompted us to test orthopedia (otpa) deficient fish which showed a profound reduction in dark photokinesis. Using targeted genetic ablations, we narrowed the photosensitive region to neurons in the preoptic area. Neurons in this region express several photoreceptive molecules, but expression of the melanopsin opn4a is selectively lost in otpa mutants, suggesting that opn4a mediates dark photokinesis. Our findings shed light on the identity and function of deep brain photoreceptors and suggest that otpa specifies an ancient population of sensory neurons that mediate behavioral responses to light.
Results
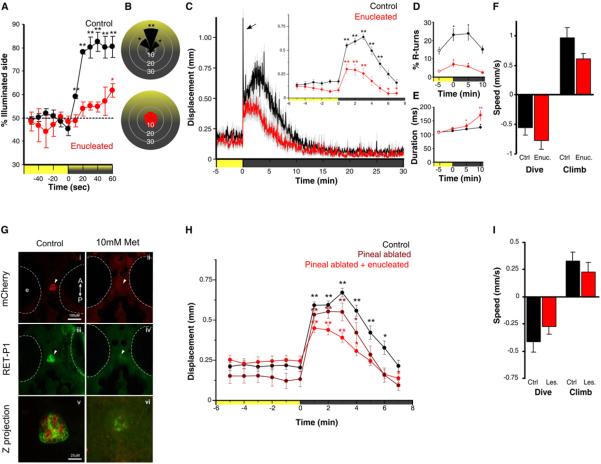
Evidence that deep brain photoreception influences physiology and behavior has accumulated in a variety of non-mammalian vertebrates including fish [1, 6, 7, 8]. We therefore looked for light-driven behaviors in larval zebrafish that persist after removal of the eyes. Responses to simple light flashes and optomotor stimuli were completely absent after enucleation (data not shown), however during phototaxis experiments [9], larvae showed a reduced but significant tendency to swim toward a weak light stimulus (Figure 1A). Light-seeking behavior differed from normal fish as it occurred without larvae orienting their body axis toward the illuminated area (Figure 1B), suggesting a different navigational strategy. Non-directional `photokineses' have been described in many organisms, requiring only heightened locomotor activity in darkness [10]. Heightened locomotor activity drives individuals in a non-directional, stochastic fashion out of dark zones, and `traps' them in brighter zones due to reduced activity. Indeed upon loss of illumination, zebrafish larvae are known to show a transient period of hyperactivity before settling into a state of low baseline activity [11, 12, 13]. This hyperactive state has been termed the visual motor response (VMR) [14]. Zebrafish larvae enucleated at 5 dpf showed a robust VMR at 7 dpf as assessed by gross locomotor activity (Figure 1C). We obtained similar results from three independent genetic experiments rendering blind fish (Figure S1A–C). Kinematic analysis of swimming during the VMR showed specific defects in enucleated larvae. Control larvae initially perform an O-bend (large angle turn) in the first few seconds following abrupt loss of illumination then deploy routine turn (R-turn) maneuvers at high frequency for around 5 min (Figure S1 E and F). During subsequent dark adaptation, the magnitude and duration of swim bouts gradually increases (Figure S1G–H) while the frequency of R-turn initiations decreases after 10 min (Figure S1F). Enucleated fish lose O-bends (Figure 1C and S1E), indicating that these responses are mediated by the retina. However, consistent with photokinesis behavior, enucleated fish continue to show increased R-turns and longer swim bouts (Figure 1D and 1E). In blind cavefish larvae light extinction provokes a vertical swimming `shadow response'[15]. We therefore assessed vertical movement during the VMR. Under baseline conditions, larvae swim near the top of the tank. On loss of illumination, larvae rapidly swim downwards, then quickly return to the surface when the lights are switched back on (Figure S1K). This dive response was also intact after enucleation (Figure 1F), suggesting that the VMR and dive response occur as a part of a simple, extra-ocular driven strategy for photokinesis that relies on elevated locomotor activity in the dark. To exclude a contribution from the photoreceptive pineal gland, we used the transgenic line Tg(tph2:NfsB-mCherry)y227 that expresses nitroreductase in pineal (Figure 1G, S1D). Metronidazole treatment ablated pineal photoreceptors but did not eliminate the VMR (Figure 1H) or dive response (F1,6= 1.62, p=0.25). VMR and dive responses also persisted after combined pineal ablation and enucleation (Figure 1H and 1I), suggesting that a deep brain photosensor mediates photokinesis.
Figure 1. Light-driven behavior in larval zebrafish without eyes or pineal.
(A) Attraction of control and enucleated larvae to a phototaxis stimulus, measured by the percent of larvae observed on the illuminated side of the testing arena over time. Enucleated larvae exhibit a gradual shift to the illuminated side of the arena (symbols show one sample t-test to 50%; N=4 groups of 15 larvae). Larval positions were recorded every second and then averaged over 10 s for each time point. Color along X-axis indicates light condition.
(B) Larval body orientation during exposure to a phototaxis stimulus. A significant proportion of control larvae exhibit a `head-on' orientation towards the spotlight (one way ANOVA; F7, 24=51.21, p<0.001; comparisons are Tukey post-hoc), whereas enucleated larvae show no bias in body orientation (ANOVA; F7, 24=1.73, p=0.15; N=4 groups of 15 fish). Data represents mean proportion of larvae oriented relative to the target light over 1 min.
(C) Locomotor activity during dark-induced VMR. Arrow indicates O-bend spike only observed in controls. Inset: enucleated larvae significantly increase activity following light extinction (repeated measures ANOVA; F2.5, 88.7=16.57, p<0.001) (N=36 larvae). Data represents the mean activity for the preceding minute. Color along X-axis indicates light condition. Pairwise comparisons are to the baseline time point at -5 min.
(D and E) Kinematic analysis of VMR. Enucleated larvae (red) retain elevated (D) R-turn initiation frequency (repeated measures ANOVA; F2.1, 56.6=4.63, p=0.013) and (E) swim bout duration (repeated measures ANOVA; F3, 40=6.41, p=0.001) as seen in controls. Data represents the mean of observations during the first 16 s following each time point. Pairwise comparisons are to the baseline measurement at -5 min (empty circles)(control: N=18 groups of 10 larvae; enucleated: N=28 groups of 10 larvae).
(F) Diving and climbing speed during VMR. In either response, enucleated larvae were not significantly different from controls (dive: t-test, p=0.27; climb: t-test, p=0.07) (N=6 groups of 5 larvae). Additionally, speed in all conditions is significantly different from 0 (one sample t-test, p<0.005). Data represents mean vertical swim speed over the first 20s of dive and ascent.
(G) Nitroreductase mediated ablation of the pineal. Epifluorescent image of dorsal view of pineal (arrow) in untreated and metronidazole (Met) treated Tg(tph2:NfsB-mCherry)y227 larvae with (i-ii) anti-mCherry (red) and (iii-iv) anti-RET-P1 (green) (6 dpf). Scale bar is 100 μm. (v) Confocal z projection (mCherry + RET-P1) showing concurrent nitroreductase and opsin expression in the pineal. Scale bar is 25 μm.
(H) VMR in enucleated, pineal-ablated larvae. Both pineal ablated and pineal ablated-enucleated larvae show a robust VMR following light extinction (repeated measures ANOVA; F3.8, 131.3 =32.44, p<0.001) (control and double lesioned: N=36 larvae; pineal ablated: N=26 larvae). Data represents mean activity for the preceding minute. Pairwise comparisons are to baseline time point -5 min.
(I) Diving and climbing speed of enucleated, pineal-ablated larvae during VMR. In either response, lesioned fish were not significantly different from controls (t-test: dive: p=0.26; climb: p=0.42). Mean speed of dive and ascent for both groups is significantly different from zero (one sample t-test, p<0.005) (N=14 groups of 5 larvae). Data represents mean swim speed over the first 20 s of dive and ascent. For all panels, error bars show SEM and * p<0.05, ** p<0.01.
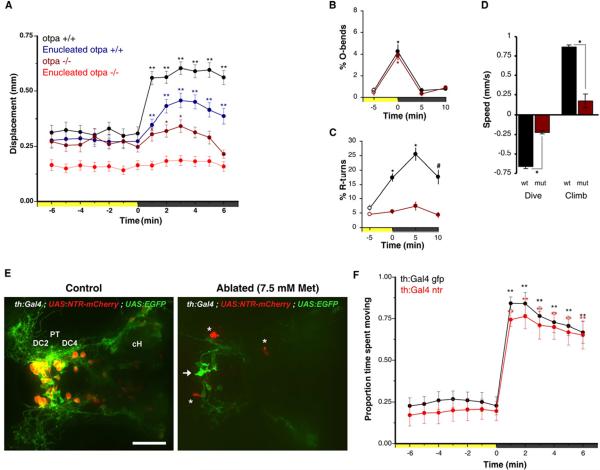
Several “non-visual” opsins are expressed in the zebrafish brain [16, 17, 18]. Evidence linking deep brain photoreception to physiological responses is strongest for the hypothalamus [1, 3]. Intriguingly, at least two opsins, multiple tissue opsin a (tmtopsa) and melanopsin 4a (opn4a) have been reported to be expressed within a domain in the hypothalamus specified by the Orthopedia transcription factor [18, 19]. We therefore tested the VMR and dive responses of otpa mutant larvae [20]. otpa homozygotes exhibit no obvious morphological abnormalities, possess normal baseline movement under constant illumination (Figure 2A), and normal retina mediated photic responses, evidenced by robust O-bends to the loss of illumination (Figure 2B). However mutants are severely affected in the VMR, failing to increase R-turn movements after loss of illumination (Figure 2A and 2C). Following enucleation, mutants completely lose the VMR response (Figure 2A), excluding a potential contribution from other photoreceptive areas. Nitroreductase ablation of orthopedia neurons in Tg(otpb.A:Gal4)zc67; Tg(UAS:Nfsb-mCherry)c294 [21, 22] double transgenic larvae confirmed the deficit (Figure S2A and S2C). otpa mutants also showed an impaired dive response (Figure 2D). For larvae with mutations in both the otpa and otpb paralogous genes we found the VMR deficit not significantly distinct from otpa single mutants (data not shown). These results suggest that these extra-ocular light-driven behaviors are mediated by neurons within the otp expression domain. Mouse Otp specifies corticotropin-releasing hormone (CRH), somatostatin (SST), oxytocin/isotocin (OT/IT) and other neuroendocrine neurons in the hypothalamus [23]. However loss of otpa alone does not lead to a clear reduction in the number of neuroendocrine neurons in the zebrafish hypothalamus (Figure S3B; for crh see [24]). otpa mutants show reduced differentiation of A11 type ventral diencephalic dopaminergic (DA) neurons [20] suggesting that reduced DA modulation of locomotor activity may contribute to the photokinesis phenotype. Nitroreductase ablation of DA neurons using Tg(BAC th:Gal4VP16) m1233 ; Tg(UAS:EGFPCAAX) m1230; Tg(UAS-E1b:NfsB-mCherry)c294 triple transgenic larvae did not produce a VMR deficit (Figure 2E–F), despite loss of more than 70% of A11 neurons in most larvae (Figure S2D,E).Therefore the VMR defect in otpa mutants is independent of DA signaling and is likely due to loss of neurons in the more rostral group of preoptic neurons in the otpa domain which do not co-express TH (Figure 3A).
Figure 2. Reduction of VMR in otpa mutants and lack of dopaminergic contribution.
(A) Locomotor activity during dark-induced VMR of intact and enucleated otpa mutants and sibling larvae. Intact mutants show a response to light extinction (repeated measures ANOVA; F3.0,175=8.5, p<0.01; N=59 larvae) that is greatly reduced relative to controls (intact siblings: N=47 larvae; enucleated siblings: N=97 larvae). Without eyes, mutants lose any response to light extinction (repeated measures ANOVA; F3.2,317 =1.72, p=0.16; N=101 larvae). Data represents mean activity for the preceding minute. Color along X-axis indicates light condition. Pairwise comparisons to baseline time point 0 min: *p<0.05, **p<0.01.
(B and C) Kinematic analysis of photokinesis in intact otpa mutants. Otpa mutants retain O-bend responses to light extinction (B) (repeated measures ANOVA ; F3,9=53.7 ; p<0.001 ; N=4 groups of 10 larvae) but do not show characteristic increases in R-turn initiation (C ; F3,9=2.1; p=0.17 ; N=4 groups of 10 larvae). # p<0.05, * p<0.01 for pairwise comparisons to baseline at -5 min (empty circles). Data represents the mean and SEM of observations during the first 16 s following each time point.
(D) Diving and climbing speed of intact otpa mutant larvae. Compared to siblings, otpa mutants exhibit significantly reduced diving speed during and climbing speed following a 60 s dark flash (t-test: dive: p<0.001; climb: p<0.005 ; N=3 groups of 8 larvae). Data represents mean and SEM swim speed over first 20 s of dive and ascent.
(E) Nitroreductase mediated ablation of dopaminergic (DA) neurons in Tg(BACth:Gal4VP16) m1233; Tg(UAS:EGFPCAAX); Tg(UAS-E1b:NfsB-mCherry) triple transgenic larvae. The asterisk indicates mCherry aggregates remaining from ablated cells. The arrow indicates GFP expressing non-ablated cells. PT-posterior tuberculum; DC2, DC4 - Otp-dependent dopaminergic groups 2 and 4; cH - caudal hypothalamus. Dorsal view. Scale bar is 50 μm.
(F) VMR in DA neuron ablated larvae. Control Tg(th:Gal4VP16); Tg(UAS:EGFPCAAX) (black line) and DA neuron ablated larvae Tg(th:Gal4VP16); Tg(UAS-E1b:NfsB-mCherry) (red line) show similar, robust VMR following light extinction (repeated measures ANOVA; F12, 564 =148.29, p<0.001) (N=48 larvae). Data represents mean and SEM activity as in A. Pairwise comparisons to baseline time point at -5 min *p<0.05, **p<0.01.
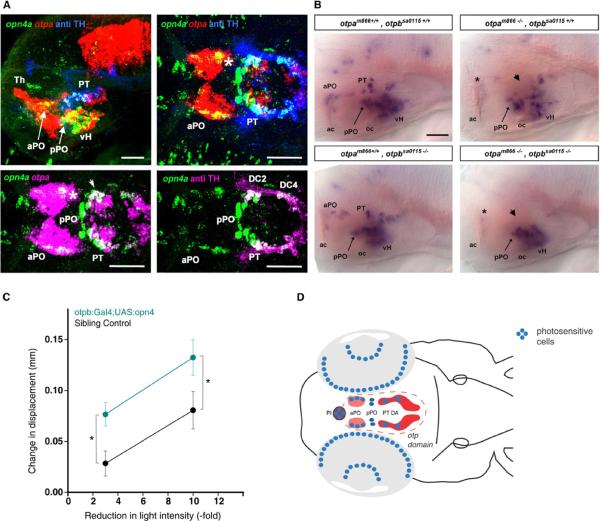
Figure 3. opn4a expression depends on Otp activity in areas of co-expression with otpa.
(A) Analysis of expression domains of opn4a, otpa, and TH (anti-TH) in 3 dpf wild type larvae. Top row shows z-projections of combined channels (see labels) of confocal stacks recorded from lateral (left) and dorsal (right) views of the brain. Anterior at left, dorsal at top for lateral view. Bottom row shows z-projections of channel combinations of a confocal stack showing dorsal views of the brain. Scale bars are 50 μm. opn4a is co-expressed with otpa in the anterior preoptic area (aPO) (asterisk) and posterior tuberculum (PT)(arrowhead).
(B) Expression of opn4a in otpa and otpb mutants. Whole-mount in situ hybridization reveals loss of opn4a expression in the aPO (arrow) and PT (arrowhead) of otpa and otpa, otpb double mutants (3 dpf). otpb mutants alone did not significantly affect opn4a expression, which is in line with the previously reported compensation of otpb knockdown by otpa activity in A11 DA neuron differentiation [20]..Anterior at left, dorsal up. Scale bar is 50 μm.
(C) Increase in activity following decrements in light intensity in enucleated Tg(otpb.A:Gal4)zc67; Tg(UAS:GFP-v2a-opn4)y233 larvae. Data shows the difference in mean activity between 2 min after light change and 1 min prior to light change (mean displacement at t2 – mean displacement at t-1). Enucleated opn4 overexpressing larvae show an increased response to decrements in light intensity (repeated measures, 2 way ANOVA; F1, 100 =8.84, p<0.005) (non-expressing control: 28 larvae; GFP positive in otpb domain: N=42 larvae). * p<0.001
(D) Schematic summarizing our results suggesting preoptic opn4a expressing neurons are deep brain photoreceptor driving dark photokinesis. We eliminated all depicted photoreceptive regions except the PO and found the VMR response remained intact. As otpa mutants lack aPO but not pPO opn4a expression, and as mutants without eyes do not show a VMR, the behavior must originate in the opn4a positive cells in the aPO (pinkfill). The illustrated DA domain (dark red color fill) only comprises the diencephalic groups 2–6 in the posterior tuberculum (PT DA).
We next examined opsin genes as candidates to mediate the otpa photokinesis phenotype. Expression of several opsins was not affected in otpa mutants (Figure S3C; for tmtopsa see also Figure S3D,E). In contrast, opn4a was robustly expressed in the anterior and posterior preoptic area (aPO, pPO), posterior tuberculum (PT) and ventral hypothalamus (vH) (Figure 3A and 3B). We detected opn4a and otpa co-expression in PT and aPO but not in vH or pPO (Figure 3A). Surprisingly, we found that the Otp-dependent A11-type DA neurons designated groups DC2 and DC4 in the zebrafish PT also strongly coexpress opn4a (Figure 3A) suggesting that A11 DA neurons have the potential to sense light. However, our nitroreductase mediated ablation of these neurons did not eliminate the VMR response, thus A11 DA dependent light-driven behaviors remain to be identified. Consistent with otpa and opn4a coexpression, in otpa mutants, or after nitroreductase mediated ablation of Otp neurons, opn4a expression was lost in aPO and PT but remained intact in vH and pPO (Figure 3B and Figure S2B). Next we tested whether double transgenic Tg(otpb.A:Gal4)zc67 ; Tg(UAS:GFP-v2a-opn4)y233 larvae, which overexpress melanopsin in Otp neurons, are sensitized to the VMR. We determined that 3-fold and 10-fold reductions in light intensity normally produce only a small VMR in enucleated larvae (Figure S3F). Enucleated larvae overexpressing opn4 in the Otp domain showed a significantly enhanced response to small light decrements as compared to enucleated non-expressing siblings (Figure 3C). Our experiments suggest that opn4a neurons in aPO or PT mediate deep brain photoreception. The PT domain does not appear to contribute to the VMR as the response persisted after ablation of PT DA opn4a coexpressing neurons. In summary, our data reveals that photokinesis requires opn4a expression specifically in neurons of the preoptic region.
Discussion
We show that a simple light-seeking behavior in zebrafish in response to loss of illumination is mediated by deep brain photoreceptors, and not by the retina or pineal. Our findings strongly implicate otpa neurons of the preoptic region of the hypothalamus as photoreceptors for dark photokinesis (Figure 3D). Dark photokinesis is distinct from zebrafish phototaxis which is a robust directional navigation toward or away from target light cues that relies on retinal vision [9]. Instead, dark photokinesis is an undirected hyperactivity in darkness, which results in the aggregation of organisms into a lit area. This behavior may allow larvae to swim out of dark zones when they cannot visually detect illuminated areas of their environment. Our findings explain the puzzling observation that the VMR is robustly induced in 7 dpf noir mutants despite the absence of a detectable electroretinogram signal [25]. It has been reported that changes in illumination do not produce a VMR in eyeless chokh mutants [13]. In contrast, we observed a robust VMR in chokh mutants (Figure S1C). The difference may result from the longer duration of our VMR assay, or from our efforts to presort mutants for behavioral responsiveness (startle response) prior to VMR testing. In chokh mutants opn4a cells are lost in the vH (not shown), but present in both PT and aPO (Figure S3G), likely explaining why mutants retain the VMR response.
The teleost hypothalamus was first implicated as a site of extra-ocular photoreception in a classic study by von Frisch examining pigmentation control [1]. We show here that hypothalamic otp dependent cells in the preoptic region which express the photopigment melanopsin, may mediate dark photokinesis. Extra-ocular photoreception in other organisms has also been linked to acute behavioral responses. Loss of illumination triggers a pineal mediated vertical swim response in cavefish embryos [15]. Embryonic zebrafish respond to intense light by 30 hpf [26] which is before retinal ganglion cell axons have exited the retina. Intense light also elicits motor responses through deep brain photoreceptors in adult eels, suggesting that encephalic photoreception is not a phenomenon confined to early development [7]. In birds, photoperiodic gonadal growth is regulated by deep brain photoreceptors and recent reports have shown that vertebrate ancient opsin and opsin5 are both expressed in the hypothalamus [27, 28]. In adult teleosts, reptiles and birds as much as 10% of short wavelength visible light reaches the hypothalamus [29]. However deep brain photoreception may also regulate behavior in neonatal mammals where significant amounts of light penetrate into the brain [30]. Extra-ocular sensors drive negative phototaxis in neonatal rats [31]and a recent report has shown that opn4 is required for this behavior in newborn mice [32]. Melanopsin has been detected in the preoptic region in mice, but it is not clear whether this is due to intrinsic expression or derives from melanopsin positive retinal ganglion cells which are known to project to the hypothalamus [33]. An intriguing possibility is thus that hypothalamic melanopsin expressing neurons also drive locomotor responses to light in newborn mammals.
The hyperactivity that drives dark photokinesis is homeostatic, as it enables larvae to return to an illuminated environment. Interestingly a recent study has shown that otpa also regulates hormonal responses to homeostatic challenge [24]. Moreover, the similarities in projection behaviors of neuroendocrine and A11-type DA neurons led to the hypothesis that the Otp-dependent DA system may be involved in setting basic behavioral states [34]. Our findings strengthen the notion that a fundamental role of the Orthopedia system is to drive a coordinated physiological response to environmental challenges.
Experimental Procedures
Behavioral testing
Behavioral tests were carried out at 5–7 dpf. Danio rerio strain Tuebingen long fin (TL) and AB were used as wild type for photokinesis and VMR characterization. Two different apparatus were used to measure VMR, resulting in different measures of locomotor activity (displacement and proportion of time moving).
Ablations
Ablations were accomplished using nitroreductase mediated cell ablation. Nitroreductase is a bacterial enzyme that converts the pro-drug metronidazole (Sigma, St. Louis, MO) into a cell-impermeable cytotoxin enabling cell specific conditional ablation [35, 36].
Statistical analysis
For all experiments, repeated measures ANOVA with simple main effects contrasts were used for within subject pairwise comparisons between baseline and `off' stimulus time points. Analyses were performed using SPSS (ver 17.0) or Microsoft Office Excel.
Supplementary Material
Highlights
Larvae aggregate in the light even after loss of retinal and pineal photoreception.
Dark driven photokinesis is absent in eyeless otpa mutant larvae.
The melanopsin opn4a is co-expressed with otpa and selectively lost in otp mutants.
otpa neurons of the preoptic area are deep brain photoreceptors for dark photokinesis.
Acknowledgements
We thank the Sanger Zebrafish Mutation Project for the otpbsa0115 allele. We thank Dr. Alida Filippi for opn4a histological sections, Dr. Jochen Wittbrodt for chokhs399 fish, the European Zebrafish Resource Center and Dr. J. Maier for chokht25327 fish, Dr. Josh Bonkowsky for Tg(otpb.A:Gal4)zc67, Erin Beddows and Mattanja Sonn for excellent technical support, the zebrafish community for sharing reagents, and H. Codore and S. Götter for expert zebrafish care. This work was supported by the Intramural Research Program of the National Institute for Child Health and Human Development (HB), the German Research Foundation (DFG-SFB780-B6 to WD) and by the European Commission (FP7, DOPAMINET, to WD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information Supplemental Information includes three figures and detailed Experimental Procedures and can be found with this article online.
References
- 1.Frisch K.v. Beitrage zur Physiologie der Pigmentzellen in der Fischhaut. Pflugers Archiv European Journal of Physiology. 1911;138:319–387. [Google Scholar]
- 2.Adler K. Extraocular photoreception in amphibians. Photophysiology. 1976;23:275–298. [PubMed] [Google Scholar]
- 3.Yokoyama K, Oksche A, Darden TR, Farner DS. The sites of encephalic photoreception in photoperiodic induction of the growth of the testes in the white-crowned sparrow, Zonotrichia leucophrys gambelii. Cell and tissue research. 1978;189:441–467. doi: 10.1007/BF00209132. [DOI] [PubMed] [Google Scholar]
- 4.Groos G. The comparative physiology of extraocular photoreception. Experientia. 1982;38:989–991. doi: 10.1007/BF01955340. [DOI] [PubMed] [Google Scholar]
- 5.Silver R, Witkovsky P, Horvath P, Alones V, Barnstable CJ, Lehman MN. Coexpression of opsin- and VIP-like-immunoreactivity in CSF-contacting neurons of the avian brain. Cell and tissue research. 1988;253:189–198. doi: 10.1007/BF00221754. [DOI] [PubMed] [Google Scholar]
- 6.Scharrer E. Die Lichtempfindlichkeit Blinder Elritzen. (Untersuchungen Über das Zwischenhirn der Fische I.) Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 1928;7:1–38. [Google Scholar]
- 7.Van Veen T, Hartwig HG, Muller K. Light-dependent motor activity and photonegative behavior in the eel (Anguilla anguilla L.) J. Comp. Physiol. 1976;111:209–219. [Google Scholar]
- 8.Tabata M, Minh-Nyo M, Oguri M. Thresholds of retinal and extraretinal photoreceptors measured by photobehavioral response in catfish, Silurus asotus. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 1989;164:797–803. [Google Scholar]
- 9.Burgess HA, Schoch H, Granato M. Distinct Retinal Pathways Drive Spatial Orientation Behaviors in Zebrafish Navigation. Current Biology. 2010;20:381–386. doi: 10.1016/j.cub.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraenkel GS, Gunn DL. The Orientation of Animals, Kineses, Taxes and Compass Reactions. Dover Publications; New York: 1961. [Google Scholar]
- 11.Prober DA, Rihel J, Onah AA, Sung R-J, Schier AF. Hypocretin/Orexin Overexpression Induces An Insomnia-Like Phenotype in Zebrafish. J. Neurosci. 2006;26:13400–13410. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burgess H, Granato M. Modulation of locomotor activity in larval zebrafish during light adaptation. Journal of Experimental Biology. 2007;210:2526–2539. doi: 10.1242/jeb.003939. [DOI] [PubMed] [Google Scholar]
- 13.Emran F, Rihel J, Adolph AR, Wong KY, Kraves S, Dowling JE. OFF ganglion cells cannot drive the optokinetic reflex in zebrafish. Proc Natl Acad Sci U S A. 2007;104:19126–19131. doi: 10.1073/pnas.0709337104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emran F, Rihel J, Adolph AR, Dowling JE. Zebrafish larvae lose vision at night. Proc Natl Acad Sci U S A. 2009;107:6034–6039. doi: 10.1073/pnas.0914718107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshizawa M, Jeffery WR. Shadow response in the blind cavefish Astyanax reveals conservation of a functional pineal eye. J Exp Biol. 2008;211:292–299. doi: 10.1242/jeb.012864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mano H, Kojima D, Fukada Y. Exo-rhodopsin: a novel rhodopsin expressed in the zebrafish pineal gland. Molecular Brain Research. 1999;73:110–118. doi: 10.1016/s0169-328x(99)00242-9. [DOI] [PubMed] [Google Scholar]
- 17.Kojima D, Torii M, Fukada Y, Dowling JE. Differential expression of duplicated VAL-opsin genes in the developing zebrafish. J Neurochem. 2008;104:1364–1371. doi: 10.1111/j.1471-4159.2007.05093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tessmar-Raible K, Raible F, Christodoulou F, Guy K, Rembold M, Hausen H, Arendt D. Conserved sensory-neurosecretory cell types in annelid and fish forebrain: insights into hypothalamus evolution. Cell. 2007;129:1389–1400. doi: 10.1016/j.cell.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 19.Matos-Cruz V, Blasic J, Nickle B, Robinson PR, Hattar S, Halpern ME. Unexpected diversity and photoperiod dependence of the zebrafish melanopsin system. PloS one. 2011;6:e25111. doi: 10.1371/journal.pone.0025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryu S, Mahler J, Acampora D, Holzschuh J, Erhardt S, Omodei D, Simeone A, Driever W. Orthopedia homeodomain protein is essential for diencephalic dopaminergic neuron development. Curr Biol. 2007;17:873–880. doi: 10.1016/j.cub.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Fujimoto E, Stevenson TJ, Chien C-B, Bonkowsky JL. Identification of a dopaminergic enhancer indicates complexity in vertebrate dopamine neuron phenotype specification. Developmental Biology. 2011;352:393–404. doi: 10.1016/j.ydbio.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davison JM, Akitake CM, Goll MG, Rhee JM, Gosse N, Baier H, Halpern ME, Leach SD, Parsons MJ. Transactivation from Gal4-VP16 transgenic insertions for tissue-specific cell labeling and ablation in zebrafish. Developmental Biology. 2007;304:811–824. doi: 10.1016/j.ydbio.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acampora D, Postiglione MP, Avantaggiato V, Di Bonito M, Vaccarino FM, Michaud J, Simeone A. Progressive impairment of developing neuroendocrine cell lineages in the hypothalamus of mice lacking the Orthopedia gene. Genes Dev. 1999;13:2787–2800. doi: 10.1101/gad.13.21.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amir-Zilberstein L, Blechman J, Sztainberg Y, Norton WH, Reuveny A, Borodovsky N, Tahor M, Bonkowsky JL, Bally-Cuif L, Chen A, et al. Homeodomain protein otp and activity-dependent splicing modulate neuronal adaptation to stress. Neuron. 2012;73:279–291. doi: 10.1016/j.neuron.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maurer CM, Schonthaler HB, Mueller KP, Neuhauss SC. Distinct retinal deficits in a zebrafish pyruvate dehydrogenase-deficient mutant. J Neurosci. 2010;30:11962–11972. doi: 10.1523/JNEUROSCI.2848-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kokel D, Bryan J, Laggner C, White R, Cheung CY, Mateus R, Healey D, Kim S, Werdich AA, Haggarty SJ, et al. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nature chemical biology. 2010;6:231–237. doi: 10.1038/nchembio.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halford S, Pires SS, Turton M, Zheng L, González-Menéndez I, Davies WL, Peirson SN, GarcÃ-a-Fernández JM, Hankins MW, Foster RG. VA Opsin-Based Photoreceptors in the Hypothalamus of Birds. Current Biology. 2009;19:1396–1402. doi: 10.1016/j.cub.2009.06.066. [DOI] [PubMed] [Google Scholar]
- 28.Nakane Y, Ikegami K, Ono H, Yamamoto N, Yoshida S, Hirunagi K, Ebihara S, Kubo Y, Yoshimura T. A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proceedings of the National Academy of Sciences. 2010;107:15264–15268. doi: 10.1073/pnas.1006393107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartwig HG, Veen T. Spectral characteristics of visible radiation penetrating into the brain and stimulating extraretinal photoreceptors. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 1979;130:277–282. [Google Scholar]
- 30.Viggiani E, Ciesla M, Russo OL. The shielding power of the rat skull to visible light. Experientia. 1970;26:850–851. doi: 10.1007/BF02114217. [DOI] [PubMed] [Google Scholar]
- 31.Routtenberg A, Strop M, Jerdan J. Response of the infant rat to light prior to eyelid opening: mediation by the superior colliculus. Dev Psychobiol. 1978;11:469–478. doi: 10.1002/dev.420110510. [DOI] [PubMed] [Google Scholar]
- 32.Johnson J, Wu V, Donovan M, Majumdar S, Renteria RC, Porco T, Van Gelder RN, Copenhagen DR. Melanopsin-dependent light avoidance in neonatal mice. Proc Natl Acad Sci U S A. 2010;107:17374–17378. doi: 10.1073/pnas.1008533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen S-K, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-Expressing Retinal Ganglion-Cell Photoreceptors: Cellular Diversity and Role in Pattern Vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tay TL, Ronneberger O, Ryu S, Nitschke R, Driever W. Comprehensive catecholaminergic projectome analysis reveals single-neuron integration of zebrafish ascending and descending dopaminergic systems. Nature communications. 2011;2:171. doi: 10.1038/ncomms1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech Dev. 2007;124:218–229. doi: 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curado S, Anderson RM, Jungblut B, Mumm J, Schroeter E, Stainier DY. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn. 2007;236:1025–1035. doi: 10.1002/dvdy.21100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.