Abstract
Background
Digital mammography is the dominant modality for breast cancer screening in the US. No previous studies have investigated how introducing digital mammography affects downstream breast-related care.
Objective
Compare breast-related health care use following a screening mammogram before and after introduction of digital mammography.
Research design and subjects
Longitudinal study of screening mammograms from 14 radiology facilities contributing data to the Breast Cancer Surveillance Consortium performed in the one year before and four years after each facility introduced digital mammography, along with linked Medicare claims. We included 30,211 mammograms for women age 66 years and older without breast cancer.
Measures
Rates of false-positive recall and short-interval follow-up based on radiologists’ assessments and recommendations; rates of follow-up mammography, ultrasound, and breast biopsy use based on Medicare claims.
Results
False-positive recall rates increased following the introduction of digital mammography. Follow-up mammography use was significantly higher across all four years after a facility began using digital compared to the year before (year one odds ratio [OR] = 1.7, 95% confidence interval [CI]: 1.4, 2.1). Among women with false-positive mammography results, use of ultrasound decreased significantly in the second through fourth years after digital mammography began (year two OR = 0.4, 95% confidence interval [CI]: 0.3, 0.6).
Conclusions
Introduction of a new technology led to changes in health care use that persisted for at least four years. Comparative effectiveness research on new technologies should consider not only diagnostic performance but also downstream utilization attributable to this apparent learning curve.
Keywords: cancer screening, comparative effectiveness, digital mammography, health technology, learning curve, mammography
Introduction
Digital mammography has rapidly replaced film-screen mammography as the modality of choice for screening and diagnosis of breast cancer. Of the 12,333 accredited mammography machines as of March 1, 2012 in the United States, 10,383 (84%) were full-field digital units (1). Although previous studies have compared the accuracy and cost-effectiveness of digital and film-screen mammography (2-5), a broader picture of the effects of digital mammography on downstream health care use is necessary to fully understand the implications of this new technology.
Diffusion of new technology, such as digital mammography, may result in altered patterns of downstream health care utilization via several mechanisms. First, changes in breast-related health care use might result from differences in recall rates for film-screen and digital mammography. Higher recall rates will lead to more women receiving subsequent diagnostic mammography and possibly other more invasive procedures. However, studies from European screening programs that have introduced digital mammography have found mixed results with increased recall for digital relative to film in some programs (6-8) and decreased recall in others (9, 10).
Digital mammography may also alter the use of diagnostic services following a positive screening mammogram. For instance, digital screening mammography might be more or less likely to be followed by an ultrasound or one or more diagnostic mammograms before a biopsy is recommended. This could also change over time, as radiologists gain experience with the new technology. A previous study showed that radiologists’ interpretive performance improves across the first 3 years of clinical practice (11). If a similar phenomenon occurs after the introduction of digital mammography, this could result in increased use of follow-up mammography or ultrasound during the first few years of digital that might not reflect long-term patterns.
To investigate these hypotheses, this report estimates changes in breast-related health care use after screening mammography among Medicare beneficiaries following the introduction of digital mammography in US community practice. We used data from the Breast Cancer Surveillance Consortium (BCSC) and linked Medicare claims. We sought to determine whether there were increases in rates of recall or recommendation for short-interval follow-up, whether there were changes in downstream health care use, and whether any changes in use were transient or persisted for at least four years following the introduction of digital mammography.
Methods
Data Sources
Data were obtained from three BCSC mammography registries (http://breastscreening.cancer.gov) (12) participating in linkage of BCSC records and Medicare claims data: New Hampshire Mammography Network, San Francisco Mammography Registry, and Vermont Breast Cancer Surveillance System. Registries collected data from community radiology facilities, including patient characteristics and clinical information at each mammogram. Radiologists’ assessments and recommendations were based on the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS®)(13). Breast cancer diagnoses were obtained by linking BCSC data to hospital-based pathology services, regional Surveillance, Epidemiology, and End Results (SEER) programs, and state tumor registries. Data were pooled at a central Statistical Coordinating Center. Registries and the Coordinating Center received Institutional Review Board approval for active or passive consenting processes or a waiver of consent to enroll participants, link data, and perform analysis. All research procedures were Health Insurance Portability and Accountability Act compliant, and registries and the Coordinating Center received a Federal Certificate of Confidentiality and other protections for the identities of women, physicians, and facilities.
For women who were enrolled in Medicare between 1998 and 2006 and received mammograms at one of the three registries, information on health services use was obtained through a link to the Centers for Medicare and Medicaid Services’ Medicare Program Master Enrollment file using unique Medicare identifiers such as name, date of birth, and social security number. The majority (87%) of BCSC women age 65 and older were successfully linked to Medicare claims data. Medicare eligibility and enrollment information for this period as well as all claims for Medicare-covered services were included in the database.
Participants
We included all film-screen index mammograms performed in the one-year period before a facility's first use of digital mammography and all digital index mammograms performed in subsequent years. The modality of the mammogram (film-screen vs. digital) was reported to the BCSC by radiology facilities for each mammogram. Women receiving a screening mammogram recorded by one of the three participating BCSC registries were included if they met the following criteria: age 66 years or older at the time of the screening mammogram; received at least one prior mammogram; continuously enrolled in Medicare Parts A and B and not enrolled in a Medicare managed care plan for one year before and one year after the screening mammogram; had no prior history of breast cancer and no new diagnosis of invasive carcinoma or ductal carcinoma in situ within one year of the screening mammogram; and received the screening mammogram at a mammography facility that began performing digital mammograms by December 31, 2005. This screening mammogram was defined to be the “index” mammogram. We required age over 66 years at the time of the index mammogram and one year of continuous Medicare enrollment before and after the index mammogram to ensure one full year of complete claims data on either side of the index mammogram. We excluded women enrolled in a Medicare managed care plan because these plans are not required to submit itemized claims to the Center for Medicare and Medicaid Services. Capture of services for these women in our database is therefore expected to be incomplete. We excluded mammograms in the BCSC database if a corresponding Medicare claim for a mammogram could not be found within 7 days before or after the exam date recorded in the database. The number of index screening mammograms included in our study after applying each exclusion criterion is summarized in Supplemental Digital Content 1.
Screening mammograms were identified based on the radiologist's indication (14). To avoid misclassifying diagnostic mammograms as screening, we excluded index mammograms that were unilateral or were preceded by a breast-imaging examination in the previous nine months.
Measures and definitions
A mammogram was considered a false-positive if the BI-RADS assessment following the initial screening mammogram (initial assessment) was: 0 (needs additional imaging evaluation); 4 (suspicious abnormality); 5 (highly suggestive of malignancy); or 3 (probably benign finding) with a recommendation for immediate follow-up. Mammograms were also stratified based on the presence or absence of a radiologist's recommendation for short-interval follow-up.
At each mammogram, patients completed a self-administered survey that included age, race/ethnicity, personal history of breast cancer, family history of breast cancer in a first-degree relative, and previous mammography. Breast density was assigned by the interpreting radiologist at each screening mammogram using the BI-RADS scale (13).
The date at which a facility first introduced digital mammography into regular clinical practice was defined as the earlier of the first report for using digital mammography in response to a questionnaire completed by all BCSC sites, or the first day of the month in which the BCSC captured at least 100 digital mammograms performed at the facility. For facilities where questionnaire information was available, reported dates differed from the first day of the month in which at least 100 digital mammograms were performed by one month or less. A facility's baseline year was defined as the one-year period prior to the date that digital mammography was introduced.
We defined breast-related health care services as mammography, ultrasound, or breast biopsy/other invasive breast procedure. Claims for these services were identified based on International Classification of Disease (ICD)-9 procedure codes, Health Care Common Procedure Coding System (HCPCS) codes, and diagnosis-related groups (DRG) (see Appendix A). For each procedure class, we identified the total number of unique days on which claims for one or more services occurred in the inpatient, outpatient, or physician/carrier Medicare files in the one year before and one year after each index screening mammogram.
Our study design is summarized in Figure 1. The timing of each index mammogram was first determined relative to the date at which digital mammography was introduced at the facility where the mammogram was performed. Utilization was then evaluated in the one year period after the index mammogram was performed and the one year period prior to the index mammogram. Because data were only available through 2005, only mammograms performed at facilities introducing digital mammography by 2001 could contribute to the analysis of utilization 4 years after the introduction of digital mammography.
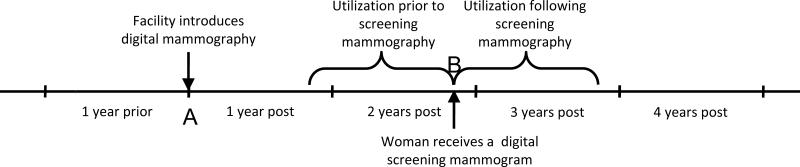
Figure 1.
Study design overview. The arrow at A represents the date at which a facility introduced digital mammography. Mammograms performed at this facility were then classified according to the one year period relative to this date during which they were performed. For instance, the mammogram at B represents a screening examination performed in the period two years after the introduction of digital mammography. For this mammogram, utilization was then evaluated in the one year interval before and one year interval after the mammogram was performed.
Statistical Analysis
We estimated the distribution of patient- and exam-level characteristics for index mammograms performed in the year before digital exams were first performed and the subsequent one-year period. We computed the proportion of mammograms with true-negative results, false-positive results, and short interval follow-up recommendations stratified by year relative to the date at which a facility introduced digital mammography. We compared rates of true-negative results and false-positive results following the introduction of digital mammography to baseline using chi-squared tests. Comparisons for short interval follow-up rates were made using Fisher's exact tests due to small cell sizes. We then computed the count and proportion of index mammograms with subsequent procedure utilization, overall and separately by year relative to the date at which the facility first began using digital mammography. These statistics were also computed stratified by mammogram result (positive or negative) and by presence of a recommendation for short-interval follow-up. We tested differences in the proportion of mammograms with subsequent utilization following the introduction of digital mammography compared to baseline using chi-squared tests.
We used logistic regression estimated via Generalized Estimating Equations with independence working correlation structure to model the odds of at least one subsequent procedure of each type and odds of any subsequent procedures as a function of the modality and year of the index screening exam relative to the date digital mammography was introduced, adjusting for possible confounding factors and clustering within women and radiologists. Separate models were estimated for each class of procedures of interest (mammography, ultrasound, biopsy, any procedure) and for all index mammograms, negative index mammograms, and index mammograms that were positive or had a recommendation for short interval follow-up. All years of follow-up were included in each model. Multivariable models were adjusted for calendar year, prior utilization, age, race (categorized as white vs. non-white), screening interval (categorized as one year vs. other), and mammography facility. We report estimated odds ratios (ORs) for the odds of downstream health care use in the years following digital mammography introduction relative to the baseline year, with 95% confidence intervals (CIs) for these estimates.
Statistical significance was evaluated at the alpha = 0.05 level. Analyses were performed using R 2.13.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of facilities and women
We identified 30,211 screening mammograms from 14 facilities meeting the study inclusion criteria. Among the 14 facilities that began using digital mammography during the study period, 4 (29%) began using digital mammography in 2001, 2 (14%) in 2002, 4 (29%) in 2003, 3 (21%) in 2004, and 1 (7%) in 2005. Characteristics of women and film-screen mammograms performed in the year prior to the introduction of digital mammography and characteristics of women and digital mammograms in the year after digital were largely similar (Table 1), with the exception of breast density. Among mammograms with non-missing breast density, compared to film-screen, a smaller proportion of digital mammograms were interpreted as BI-RADS 2 breast density (54% vs. 61%) and a larger proportion were interpreted as BI-RADS 3 breast density (37% vs. 29%). Characteristics of women included in analyses from the year prior through four years after the introduction of digital mammography are provided in Supplemental Digital Content 2.
Table 1.
Woman and mammogram characteristics for screening mammograms with no subsequent cancer diagnosis before (film) or after (digital) introduction of digital mammography.
| Year Before Digital (N = 8450) N (%) | Year After Digital (N = 6327) N (%) | |
|---|---|---|
| Age | ||
| 66-74 | 4877 (57.7) | 3653 (57.7) |
| 75-84 | 3133 (37.1) | 2303 (36.4) |
| 85+ | 440 (5.2) | 371 (5.9) |
| Race/ethnicity* | ||
| White, non-Hispanic | 7253 (91.6) | 5401 (91.1) |
| Black, non-Hispanic | 99 (1.3) | 57 (1.0) |
| Asian | 370 (4.7) | 316 (5.3) |
| Hispanic | 179 (2.3) | 151 (2.5) |
| Missing/other | 549 (6.5) | 402 (6.4) |
| First degree family history of breast cancer* | ||
| Yes | 1439 (18.9) | 1062 (18.8) |
| No | 6159 (81.1) | 4598 (81.2) |
| Missing | 852 (10.1) | 667 (10.5) |
| BI-RADS Breast density* | ||
| 1: Almost entirely fat | 546 (7.8) | 384 (6.9) |
| 2: Scattered fibroglandular densities | 4221 (60.6) | 3023 (54.2) |
| 3: Heterogeneously dense | 2021 (29) | 2049 (36.7) |
| 4: Extremely dense | 181 (2.6) | 123 (2.2) |
| Missing | 1481 (17.5) | 748 (11.8) |
| Months since previous mammogram* | ||
| 9 - 18 | 6540 (80.2) | 4961 (80.1) |
| 19 - 30 | 1151 (14.1) | 792 (12.8) |
| 31 - 42 | 265 (3.2) | 227 (3.7) |
| >42 | 199 (2.4) | 212 (3.4) |
| Missing | 295 (3.5) | 135 (2.1) |
| Exam year | ||
| 2000 | 1114 (13.2) | -- |
| 2001 | 1639 (19.4) | 729 (11.5) |
| 2002 | 1953 (23.1) | 1428 (22.6) |
| 2003 | 1625 (19.2) | 1660 (26.2) |
| 2004 | 1264 (15.0) | 1229 (19.4) |
| 2005 | 855 (10.1) | 1281 (20.2) |
| Any breast procedure in prior year | ||
| No | 7971 (94.3) | 5948 (94.0) |
| Yes | 479 (5.7) | 379 (6.0) |
Proportions computed from among non-missing values.
Rates of false-positive recall and short interval follow-up
In the year following digital mammography introduction, the proportion of mammograms with a false-positive result increased by about 3% (p < 0.001) (Table 2). The proportion of false-positives decreased in subsequent years; within about four years, it had returned to a level similar to baseline (p = 0.91 at year 4). Overall, rates of short-interval follow-up recommendation were low (<1.0%). However, they increased in the year following the introduction of digital mammography (p < 0.001 at year 1) and then declined to baseline in subsequent years (p = 0.62 at year 4).
Table 2.
Mammogram results among women without breast cancer before and after introduction of digital mammography
| Year relative to digital introduction (N) | False-positive N* (%) | Short interval follow-up N* (%) | Negative N* (%) |
|---|---|---|---|
| One year before (8369) | 572 (6.8) | 15 (0.2) | 7782 (93.0) |
| One year after (6293) | 621 (9.9) | 37 (0.6) | 5635 (89.5) |
| Two years after (6492) | 593 (9.1) | 35 (0.5) | 5864 (90.3) |
| Three years after (5364) | 436 (8.1) | 24 (0.4) | 4904 (91.4) |
| Four years after (3257) | 220 (6.8) | 4 (0.1) | 3033 (93.1) |
Mammograms with missing results or recommendations (1.4%) were excluded from analysis.
Unadjusted rates of subsequent utilization
Subsequent mammography use increased by 3% and 4% in years one and two after baseline (p < 0.001 in both years)(Table 3). Changes in rates of use of any follow-up procedures were similar to those for mammography. Ultrasounds also increased in the first year after digital began compared to baseline (p < 0.001). Subsequent breast-related health care use was generally low among women with negative mammograms (<8% received additional mammography across all years) and high among women with false-positives or recommendations for short-interval follow-up (>70% received additional mammography across all years). Among women with negative mammograms, use of additional mammography increased in the first two years after baseline (p = 0.15 in year 1, p < 0.001 in year 2). Ultrasound use did not change relative to baseline, and biopsy use decreased slightly in this group (p > 0.1 for all comparisons). For women with false-positive screening mammograms or recommendations for short-interval follow-up, subsequent mammography use increased in the year following baseline (p = 0.68), while rates of ultrasound and biopsy use decreased (p > 0.1 for ultrasound and biopsy).
Table 3.
Subsequent use of mammography, ultrasound, biopsy/invasive procedures, or any follow-up in the years before and after introduction of digital mammography, overall and stratified by initial screening examination result.
| Year Before Digital N (%) | 1 Year After Digital N (%) | 2 Years After Digital N (%) | 3 Years After Digital N (%) | 4 Years After Digital N (%) | |
|---|---|---|---|---|---|
| All, N | 8450 | 6327 | 6652 | 5522 | 3260 |
| Mammography | 827 (9.8) | 830 (13.1) | 923 (13.9) | 597 (10.8) | 275 (8.4) |
| Ultrasound | 325 (3.8) | 324 (5.1) | 273 (4.1) | 217 (3.9) | 129 (4.0) |
| Biopsy | 131 (1.6) | 103 (1.6) | 84 (1.3) | 69 (1.2) | 39 (1.2) |
| Any follow-up | 947 (11.2) | 936 (14.8) | 1007 (15.1) | 688 (12.5) | 316 (9.7) |
| Negative*, N | 7765 | 5590 | 5787 | 4837 | 3025 |
| Mammography | 318 (4.1) | 249 (4.5) | 413 (7.1) | 208 (4.3) | 70 (2.3) |
| Ultrasound | 90 (1.2) | 68 (1.2) | 59 (1.0) | 56 (1.2) | 37 (1.2) |
| Biopsy | 51 (0.7) | 25 (0.4) | 28 (0.5) | 25 (0.5) | 18 (0.6) |
| Any follow-up | 384 (4.9) | 294 (5.3) | 453 (7.8) | 257 (5.3) | 101 (3.3) |
| False-positive or short interval follow-up*, N | 595 | 674 | 695 | 528 | 229 |
| Mammography | 500 (84.0) | 575 (85.3) | 499 (71.8) | 377 (71.4) | 205 (89.5) |
| Ultrasound | 231 (38.8) | 254 (37.7) | 211 (30.4) | 160 (30.3) | 92 (40.2) |
| Biopsy | 78 (13.1) | 76 (11.3) | 54 (7.8) | 43 (8.1) | 21 (9.2) |
| Any follow-up | 553 (92.9) | 635 (94.2) | 541 (77.8) | 417 (79) | 215 (93.9) |
Mammograms with missing results or recommendations (1.4%) were excluded from analysis.
Adjusted rates of subsequent utilization
Multivariable logistic regression models indicated significantly higher odds of follow-up mammography use in the first four years of digital mammography (year one OR = 1.68, 95% CI: 1.36, 2.09; year four OR = 1.82, 95% CI: 1.22, 2.72) compared to baseline (Table 4). Ultrasound use significantly increased in the first year after the introduction of digital mammography (OR = 1.31, 95% CI: 1.02, 1.67) but had returned to baseline by the third year (OR = 0.99, 95% CI: 0.62, 1.58). Use of any follow-up procedures was significantly elevated across all four years relative to baseline. There were no significant differences in biopsy use in any year following the introduction of digital mammography relative to baseline.
Table 4.
Adjusted odds ratios for breast-related health care use after introduction of digital mammography relative to the year before.
| Year Before Digital | 1 Year After Digital OR* (95%CI) | 2 Years After Digital OR (95%CI) | 3 Years After Digital OR (95%CI) | 4 Years After Digital OR (95%CI) | |
|---|---|---|---|---|---|
| All mammograms | |||||
| Mammography | ref | 1.68 (1.36,2.09) | 2.08 (1.65,2.62) | 1.84 (1.34,2.51) | 1.82 (1.22,2.72) |
| Ultrasound | ref | 1.31 (1.02,1.67) | 1.12 (0.74,1.71) | 0.99 (0.62,1.58) | 0.92 (0.53,1.62) |
| Biopsy | ref | 1.15 (0.88,1.52) | 1.33 (0.83,2.12) | 1.36 (0.75,2.49) | 1.84 (0.78,4.34) |
| Any follow-up | ref | 1.56 (1.27,1.91) | 1.81 (1.41,2.34) | 1.62 (1.17,2.22) | 1.52 (1.05,2.19) |
| Negative† | |||||
| Mammography | ref | 1.96 (1.44,2.65) | 3.34 (2.29,4.87) | 3.09 (1.87,5.11) | 2.78 (1.53,5.05) |
| Ultrasound | ref | 1.37 (0.77,2.45) | 1.41 (0.60,3.27) | 1.58 (0.49,5.10) | 1.81 (0.39,8.34) |
| Biopsy | ref | 0.70 (0.32,1.56) | 0.75 (0.30,1.87) | 0.84 (0.25,2.78) | 0.89 (0.18,4.48) |
| Any follow-up | ref | 1.63 (1.20,2.22) | 2.43 (1.67,3.54) | 2.18 (1.34,3.56) | 1.96 (1.14,3.39) |
| False-positive or short interval follow-up† | |||||
| Mammography | ref | 1.01 (0.65,1.55) | 0.78 (0.36,1.66) | 0.65 (0.26,1.65) | 0.99 (0.31,3.19) |
| Ultrasound | ref | 0.72 (0.50,1.04) | 0.41 (0.27,0.64) | 0.29 (0.16,0.53) | 0.21 (0.10,0.47) |
| Biopsy | ref | 0.97 (0.66,1.42) | 1.15 (0.54,2.46) | 1.17 (0.45,3.01) | 2.03 (0.56,7.33) |
| Any follow-up | ref | 0.77 (0.37,1.62) | 0.31 (0.09,1.05) | 0.22 (0.05,0.95) | 0.20 (0.03,1.58) |
Odds ratios were adjusted for calendar year, prior utilization, age, race (categorized as white vs. non-white), screening interval (categorized as one year vs. other), and mammography facility.
Mammograms with missing results or recommendations (1.4%) were excluded from analysis.
Among women with a negative mammography result and no recommendation for short-interval follow-up, follow-up mammography use and use of any follow-up procedures were significantly higher following introduction of digital mammography relative to baseline (Table 4). However, ultrasound and biopsy use were not significantly different relative to baseline. Women with a false-positive mammography result or recommendation for short-interval follow-up had significantly decreased use of ultrasound relative to baseline in the second through fourth years after the introduction of digital mammography.
Conclusions
Immediately following the transition to a new technology, performance and downstream health care use might differ from what would be observed once providers have adapted to the new technology. In the case of digital mammography, twelve-month downstream mammography use increased for at least four years after introduction of digital mammography. Among women with false-positive results or recommendations for short interval follow-up, use of ultrasound decreased for at least four years after digital began. This indicates that multiple years of follow-up subsequent to the introduction of this technology are necessary to fully evaluate its effects. The broader implications for public health planning and accurate comparative effectiveness evaluation are that the duration of the learning curve must be estimated and its effects accounted for when projecting the impact of a transition to a new technology.
Several prior studies have examined performance metrics such as recall rates following the adoption of digital mammography. These studies reported a mix of increased and decreased recall rates relative to rates for film-screen mammography (6-10). We found that rates of false-positive recall and recommendation for short-interval follow-up increased in the first few years after a facility adopted digital mammography. Use of follow-up mammography significantly increased after digital was introduced. Increased use of follow-up mammography following the introduction of digital appears to have arisen from a combination of increases in the proportion of women recommended for additional imaging and short-interval follow-up and increases in the proportion of women with negative mammograms who received follow-up mammography.
Observed changes in recall and utilization might be attributable to differences in the population of women receiving digital mammography compared to those receiving film-screen. Specifically, a larger proportion of digital mammograms were interpreted as BI-RADS 3 breast density compared to film-screen mammograms. Women with denser breasts have higher rates of recall (15) that might have contributed to the overall increases in recall rates observed for digital compared to film-screen. Breast density is interpreted as lower on digital compared to film-screen mammograms (16), so the observed higher breast density of digital mammograms is not attributable to differences in radiologists’ interpretation of density.
Our study has several limitations. Because this was an observational study we might not have controlled for all confounding person- and facility-level characteristics. For instance, only facilities that began using digital mammography in 2001 were represented in our health care use estimates at four years after the first use of digital mammography. Because of possible between-facility differences, our adjusted models included mammography facility, allowing us to make within-facility comparisons of utilization. This approach allowed us to account for possible confounding by facility characteristics. Our analysis was also limited to mammograms from three mammography registries within the BCSC. Women captured by the BCSC are similar to women receiving mammography nationally in their distribution of race/ethnicity, education, economic status, and rurality (17). Results from our study are therefore likely to be generalizable to mammography facilities in community practice in the U.S. Sample sizes at four years after the introduction of digital mammography were also small, leading to imprecise odds ratio estimates for some procedures. Additionally, we do not have information on the specific models of digital mammography machines and software used by each facility. As is common with new technology, machines and software are continually being refined. Therefore, digital machines used in 2001 are not necessarily fully comparable to those used in later years. However, we believe the effect of these changes on our results is likely to be small because we have accounted for calendar year in adjusted analyses.
Our analyses of health care use were also limited to Medicare data. We may have missed some health care services if claims were not submitted to Medicare. They also do not include participants in Medicare managed care plans because utilization data were incomplete for these women. Use of Medicare claims also precluded examining some services, such as breast MRI, which are not covered for women who are cancer free. Our analysis may therefore have missed some breast-related healthcare utilization. Finally, our analyses were limited to women age 66 years and older. The specificity of digital mammography compared to film-screen has been shown to be somewhat lower for women age 40-49 years (5). Increases in utilization following the introduction of digital mammography might therefore be more marked in a younger age group.
This study provides an assessment of the downstream effects of changes in health care use following adoption of new medical technology. Our results indicate that introduction of a new screening modality led to increases in recall rates that produced increased breast imaging utilization in the first few years of digital mammography use. Although increases in utilization may attenuate over time, the cycle of innovation and diffusion results in frequent change to screening technologies that will likely restart this cycle. The benefits of technology change should be evaluated relative to these ongoing costs. In the case of digital mammography, this new screening modality has improved sensitivity for some subgroups of women, specifically younger women and those with dense breasts (3, 5).
The impact of new technology on the health care system, providers, and patients is found in the direct costs of acquiring and implementing the technology but also through changes in the use of other health care services. Our study based on digital mammography performance and subsequent health care use in US community practice identified increases in health care use following the introduction of digital mammography. This is consistent with previous research demonstrating that radiologists experience a learning curve during their first few years of clinical practice (11). Our results suggest that digital mammography introduction may initially lead to increased recall as radiologists gain familiarity with the new technology.
Estimating the effects of new technology on health care use is challenging. Longitudinal information from multiple facilities is needed to minimize possible confounding by differences between early- and late-adopters. Capture of clinical measures such as the indication for and result of the exam are needed, in addition to measurement of subsequent use of health services. Studies of the comparative effectiveness of new technologies must account for these additional dimensions if they are to encapsulate the complete spectrum of health and health care effects produced by introducing a new medical technology.
Supplementary Material
Acknowledgments
We thank the participating women, mammography facilities, and radiologists for the data they have provided for this study. A list of BCSC investigators and procedures for requesting BCSC data for research purposes are at: http://breastscreening.cancer.gov/.
Sources of Support
This work was supported by the National Cancer Institute-funded Breast Cancer Surveillance Consortium (U01CA63740, U01CA86076, U01CA86082, U01CA63736, U01CA70013, U01CA69976, U01CA63731, U01CA70040, HHSN261201100031C) and National Cancer Institute grant RC2CA148577. Dr. Hubbard was also supported in part by a grant from GE Healthcare. The collection of cancer data used in this study was supported in part by several state public health departments and cancer registries throughout the U.S. For a full description of these sources, please see: http://www.breastscreening.cancer.gov/work/acknowledgement.html. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Appendix
Appendix A.
Definitions of classes of breast-related procedures.
| HCPCS* | ICD-9 | DRG | |
|---|---|---|---|
| Mammography | 76082 | 87.37 | |
| 76083 | |||
| 76085 | |||
| 76090 | |||
| 76091 | |||
| 76092 | |||
| G0202 | |||
| G0203 | |||
| G0204 | |||
| G0205 | |||
| G0206 | |||
| G0207 | |||
| S8075 | |||
| Ultrasound | 76645 | 88.73 | |
| Biopsy/other invasive breast procedure | 10021 | 85.0 | 261 |
| 10022 | 85.1 | 262 | |
| 19000 | 85.11 | ||
| 19001 | 85.12 | ||
| 19030 | 85.19 | ||
| 19100 | 85.2 | ||
| 19101 | 85.20 | ||
| 19102 | 85.21 | ||
| 19103 | 85.22 | ||
| 19110 | 85.23 | ||
| 19112 | 85.24 | ||
| 19120 | 85.25 | ||
| 19121 | 85.91 | ||
| 19122 | |||
| 19123 | |||
| 19124 | |||
| 19125 | |||
| 19126 | |||
| 19260 | |||
| 19271 | |||
| 19272 | |||
| 19290 | |||
| 19291 | |||
| 19292 | |||
| 19293 | |||
| 19294 | |||
| 19295 | |||
| 76095 | |||
| 76096 | |||
| 76097 | |||
| 76098 |
HCPCS, Health Care Common Procedure Coding System; ICD-9, International Classification of Disease; DRG, diagnosis-related group
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.FDA [March 26, 2012];Radiation-Emitting Products: MQSA National Statistics. Available from: http://www.fda.gov/Radiation-EmittingProducts/MammographyQualityStandardsActandProgram/FacilityScorecard/ucm113858.htm.
- 2.Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773–1783. doi: 10.1056/NEJMoa052911. [DOI] [PubMed] [Google Scholar]
- 3.Pisano ED, Hendrick RE, Yaffe MJ, et al. Diagnostic accuracy of digital versus film mammography: exploratory analysis of selected population subgroups in DMIST. Radiology. 2008;246:376–383. doi: 10.1148/radiol.2461070200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tosteson AN, Stout NK, Fryback DG, et al. Cost-effectiveness of digital mammography breast cancer screening. Ann Intern Med. 2008;148:1–10. doi: 10.7326/0003-4819-148-1-200801010-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerlikowske K, Hubbard RA, Miglioretti DL, et al. Comparative-effectiveness of digital vs. film-screen mammography in community practice in the United States. Ann Intern Med. 2011 doi: 10.7326/0003-4819-155-8-201110180-00005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bluekens AM, Karssemeijer N, Beijerinck D, et al. Consequences of digital mammography in population-based breast cancer screening: initial changes and long-term impact on referral rates. Eur Radiol. 2010;20:2067–2073. doi: 10.1007/s00330-010-1786-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karssemeijer N, Bluekens AM, Beijerinck D, et al. Breast cancer screening results 5 years after introduction of digital mammography in a population-based screening program. Radiology. 2009;253:353–358. doi: 10.1148/radiol.2532090225. [DOI] [PubMed] [Google Scholar]
- 8.Hambly NM, McNicholas MM, Phelan N, et al. Comparison of digital mammography and screen-film mammography in breast cancer screening: a review in the Irish breast screening program. AJR Am J Roentgenol. 2009;193:1010–1018. doi: 10.2214/AJR.08.2157. [DOI] [PubMed] [Google Scholar]
- 9.Sala M, Salas D, Belvis F, et al. Reduction in false-positive results after introduction of digital mammography: analysis from four population-based breast cancer screening programs in Spain. Radiology. 2011;258:388–395. doi: 10.1148/radiol.10100874. [DOI] [PubMed] [Google Scholar]
- 10.Vinnicombe S, Pinto Pereira SM, McCormack VA, et al. Full-field digital versus screen-film mammography: comparison within the UK breast screening program and systematic review of published data. Radiology. 2009;251:347–358. doi: 10.1148/radiol.2512081235. [DOI] [PubMed] [Google Scholar]
- 11.Miglioretti DL, Gard CC, Carney PA, et al. When radiologists perform best: the learning curve in screening mammogram interpretation. Radiology. 2009;253:632–640. doi: 10.1148/radiol.2533090070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballard-Barbash R, Taplin SH, Yankaskas BC, et al. Breast Cancer Surveillance Consortium: a national mammography screening and outcomes database. American Journal of Roentgenology. 1997;169:1001–1008. doi: 10.2214/ajr.169.4.9308451. [DOI] [PubMed] [Google Scholar]
- 13.American College of Radiology . Breast Imaging Reporting and Data System (BI-RADS) Breast Imaging Atlas. American College of Radiology; Reston, VA: 2003. [Google Scholar]
- 14.Breast Cancer Surveillance Consortium [March 26, 2012];BCSC Glossary of Terms. 2010 Available from: http://breastscreening.cancer.gov/data/bcsc_data_definitions.pdf.
- 15.Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138:168–175. doi: 10.7326/0003-4819-138-3-200302040-00008. [DOI] [PubMed] [Google Scholar]
- 16.Harvey JA. Quantitative assessment of percent breast density: analog versus digital acquisition. Technol Cancer Res Treat. 2004;3:611–616. doi: 10.1177/153303460400300611. [DOI] [PubMed] [Google Scholar]
- 17.Sickles EA, Miglioretti DL, Ballard-Barbash R, et al. Performance benchmarks for diagnostic mammography. Radiology. 2005;235:775–790. doi: 10.1148/radiol.2353040738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.