Abstract
Exposure to an immunogen results in a constellation of behavioral changes collectively referred to as “sickness behaviors,” with alterations in cytokine expression previously shown to contribute to this sickness response. Since behaviors observed during ethanol withdrawal are strikingly similar to sickness behaviors, we hypothesized that behavioral manifestations of ethanol withdrawal might be an expression of sickness behaviors induced by ethanol-related changes in peripheral and/or central cytokine expression. Accordingly, behaviors exhibited during a modified social investigation test were first characterized in male rats following an acute injection of lipopolysaccharide (LPS; 100 μg/kg). Subsequently, behavioral changes after either a high (4-g/kg; Experiment 2) or low dose (0.5 g/kg; Experiment 3) of ethanol were also examined in the same social investigation test, as well as in the forced-swim test (FST; Experiment 4). Results from these experiments demonstrated similar reductions in both exploration and social investigatory behavior during acute illness and ethanol withdrawal, while a seemingly paradoxical decrease in immobility was observed in the FST during acute ethanol withdrawal. In follow-up studies, neither indomethacin (Experiment 5) nor interleukin-1 receptor antagonist (Experiment 6) pre-exposure reversed the ethanol withdrawal-induced behavioral changes observed in this social investigation test. Taken together, these studies demonstrate that the behavioral sequelae of acute illness and ethanol withdrawal are similar in nature, while antagonist studies suggest that these behavioral alterations are not reversed by blockade of IL-1 receptors or inhibition of prostaglandin synthesis. Though a direct mechanistic link between cytokines and the expression of acute ethanol withdrawal-related behaviors has yet to be found, future studies examining the involvement of brain cytokines as potential mediators of ethanol effects are greatly needed.
Keywords: Rat, Ethanol, Lipopolysaccharide, Acute Withdrawal, Social Behavior, Exploration, Indomethacin, Interleukin-1 Receptor Antagonist
1. Introduction
Alcohol abuse and dependence are prominent health issues in our society, with national survey data indicating that in 2007 approximately 23% of the population aged 12 years or older had engaged in binge drinking (defined as 5 or more drinks on one occasion) within the past month (SAMHSA, 2008) and in 2006 a reported 7.9% of the population needing treatment for alcohol dependence (SAMHSA, 2007). Development of alcohol dependence has been characterized as spiraling cycle, in which negative consequences following acute intoxication perpetuate future (increased/excessive) use (e.g., for reviews see Koob, 2003; 2011). In particular, the unpleasant signs and symptoms of acute withdrawal are thought to serve as a negative-reinforcing mechanism that causes an individual to re-engage in ethanol consumption in order to alleviate the negative state caused by prior ethanol exposure. Although examination of acute withdrawal from an acute intoxicating dose of ethanol (often termed “hangover”) may be far removed from the negative reinforcement mechanisms driving continued intake in a dependent individual, there is limited evidence to suggest that hangover severity could serve as a marker for alcohol use disorder risk (Piasecki et al., 2010). Therefore, identification of the mechanisms underlying the expression of acute withdrawal symptoms could potentially provide important insight into the contributors to this part of the addiction process, as well as provide potential targets for the development of therapeutic strategies and agents.
Physiological symptoms of acute withdrawal following acute ethanol exposure in humans often include fatigue, headache, nausea, anorexia, diarrhea, and sometimes even tremor (Bogin et al., 1986; McKinney and Coyle, 2006; Smith and Barnes, 1983; Wiese et al., 2000). Furthermore, there appears to be a negative affective component to acute alcohol withdrawal as well, with humans self-reporting anxiety, guilt, and depression (Bogin et al., 1986; Smith and Barnes, 1983; Swift and Davidson, 1998) or a general “decreased mood” (see McKinney, 2010) for review). A similar constellation of behavioral changes can also be observed among rats after a large acute bolus of ethanol. For example, rats experiencing withdrawal from acute ethanol exposure have been reported to be significantly less active (Buck et al., 2011; Doremus-Fitzwater and Spear, 2007; Sinclair and Gustafsson, 1987) and to exhibit reduced social interaction (Doremus-Fitzwater and Spear, 2007; Varlinskaya and Spear, 2004). Additionally, rats have been shown to form a conditioned place aversion to acute ethanol withdrawal (Morse et al., 2000), to exhibit significantly increased levels of anxiety (e.g., Doremus et al., 2003; Gauvin et al., 1997; Zhang et al., 2007) and to demonstrate brain reward deficits (i.e., increased ICSS thresholds) following acute exposure to ethanol (Schulteis and Liu, 2006)—behavioral signs which would indicate a negative affective component to acute ethanol withdrawal in rodents that is similar to that observed among humans.
When considering this constellation of behavioral alterations exhibited during acute ethanol withdrawal, one could note the striking similarities to the behavioral changes observed during a sickness response. When rodents are acutely administered an endotoxin such as lipopolysaccharide (LPS; the gram-negative component of the cell wall of bacteria), they later exhibit typical “sickness behaviors” that include fever, reduced food and water intake, reduced activity, reduced social and sexual behavior, lethargy, and piloerection (Dantzer, 2004). Though in the past the sickness response was thought to be a maladaptive behavioral state, more recently sickness itself has been recognized as a recuperative response that is meant to conserve energy and encourage recovery from an invading pathogen (for reviews see Dantzer, 2004; 2009). Indeed, other negative affective symptoms of the sickness response have been observed that are strikingly similar to the behaviors observed during acute withdrawal, with, for instance, depression cited as a psychological symptom of sickness in humans (Dunn and Swiergiel, 2008; Swift and Davidson, 1998) and decreased brain reward observed among rats exhibiting a sickness response (e.g., Barr et al., 2003).
The initiation and expression of the sickness response has been shown to be coordinated by the activation of various cytokines (for review see Dantzer, 2004), including the pro-inflammatory cytokines IL-1, IL-6 and TNFα. In particular, IL-1 appears to play a prominent role, with, for instance, both peripheral and central administration of IL-1 inducing typical sickness behaviors (e.g., Anforth et al., 1998; Plata-Salaman et al., 1988); for review see Dantzer, 2009). Furthermore, inhibition of IL-1 activity within the brain reportedly blocks the sickness response normally exhibited following immune activation (Kent et al., 1992; Konsman et al., 2008). Mechanistically, several different pathways are seemingly responsible for communicating peripheral cytokine signals to the brain (for review see Dantzer, 2009), resulting in increased expression of pro-inflammatory cytokines within the CNS (e.g., Rivest and Rivier, 1991; Sapolsky et al., 1987).
Given the similarities between behavioral alterations observed during acute ethanol withdrawal and sickness-related behaviors, and recent data in humans showing increased circulating cytokines in withdrawing alcoholics (Gonzalez-Quintela et al., 2000; Kim et al., 2003), it is possible that exposure to acute binge doses of ethanol may result in activation of inflammatory processes in brain that could be responsible for withdrawal-associated changes in behavior. Notably, a marked fever response has also been observed during ethanol withdrawal (Brasser and Spear, 2002; Sinclair and Taira, 1988)—an effect that further implicates the involvement of cytokines in the expression of acute withdrawal symptoms. Additional evidence to suggest that immune activation may be involved in expression of ethanol withdrawal behaviors comes from studies that have used compounds to inhibit prostaglandin synthesis. Prostaglandins are a final common mediator of sickness behaviors, being both the cause and consequence of cytokine induction (Dantzer, 2004; Konsman et al., 2002). Studies have shown that plasma prostaglandins are increased during acute ethanol withdrawal (Kaivola et al., 1983; Parantainen, 1983), and there is limited evidence to suggest that nonsteroidal anti-inflammatory drugs (NSAIDS) may in some instances (Dhir et al., 2005; Hale et al., 1992; Kaivola et al., 1983; Romeo et al., 2000), but not all (e.g., Segarnick et al., 1985), attenuate the negative behavioral alterations of withdrawal.
The purpose of the current experimental series, therefore, was to compare the behavioral alterations induced by a direct immune challenge with those observed following an acute administration of ethanol. In order to stimulate a classic sickness response, rats were first administered LPS (Experiment 1), and then behavior was assessed in a modified social investigation test (Arakawa et al., 2009; Deak et al., 2009). Subsequently, rats were given either a high dose (4-g/kg i.p.; Experiment 2) or a low dose of ethanol (0.5 g/kg i.p.; Experiment 3) and then also tested in the same social investigation test. Experiment 4 then examined depressive-like behavior in the modified rat forced-swim test (FST) following a heavy injection of ethanol. Finally, to directly test the hypothesis that these behavioral consequences of an acute ethanol challenge were due to an immune response induced by pro-inflammatory cytokines, indomethacin (a COX inhibitor; Experiment 5) and IL-1Ra (Experiment 6) were administered prior to ethanol exposure in an attempt to attenuate the behavioral consequences of ethanol exposure.
2. General Methods
2.1 Subjects
Adult male Sprague-Dawley rats (300-350g) were purchased from Harlan Laboratories. All rats were given 2 weeks to acclimate to the colony conditions before the onset of any experimentation. Colony conditions were maintained at 22 ± 1°C with 14:10 light:dark cycle (lights on 06:00h). Animals were pair-housed in standard Plexiglas bins and provided ad libitum access to both food and water. In all experiments, rats were handled briefly for 3-5 minutes for 2 days prior to experimentation. In all experiments, animals were maintained and treated in accordance with the guidelines set forth by the Institute of Laboratory Animal Resources (1996) and protocols approved by the Binghamton University IACUC committee.
2.2. Behavioral Tests
2.2.1. Social investigation and exploratory activity paradigm
In this paradigm, a rat was placed individually into modified breeder tub consisting of a clear Plexiglas box (65 cm × 24 cm × 15 cm) lined with bedding (for more details see Bekkedal et al., 1998; Bekkedal et al., 1999; Deak et al., 2009). In both ends of the apparatus, a 3.2 cm hole was surrounded by photoelectric cells to detect movement of the animal's head into the opening. Two of these testing chambers were placed 10 cm apart (end-to-end), such that each apparatus would have a social (the common hole between the two boxes) and a non-social hole (on the opposite side of the cage where no social interaction was possible). Activity at the social hole permitted an animal to investigate a conspecific and provided a measure of social investigation, whereas activity at the non-social hole provided an index of general exploratory activity. On the day of testing, each animal was transferred from their home cage to the dimly lit experimental room and placed in adjacent boxes for a total of 30 minutes (without an experimenter present). In all experiments, rats were placed in a box such that their social “partner” was a similarly drug-treated, familiar animal, as has been previously done in other tests of social investigation (e.g. File, 2000; Genn et al., 2003). Familiar animals were used as social partners in order to avoid the influence of novelty under these testing circumstances. Given that we have previously demonstrated that sick animals can influence the social behavior of a healthy partner in this social investigation task (e.g., Arakawa et al., 2010), we chose to use like-treated animals as social partners and avoid any potential confound that could have been introduced by the differential influence of the drug-exposed animal on the testing partner. During the test sessions, the frequency and duration of photobeam breaks, indicative of head pokes through a hole, were automatically recorded at both the social and nonsocial holes. Head pokes were scored separately for each animal in the pair and accumulated across the 30-min test session. Since frequency of head poking and duration of head poking were highly correlated, only data for number of head pokes are shown throughout these studies.
2.2.2. Forced swim test
The FST was administered in a similar manner as described elsewhere (Cryan and Lucki, 2000; Deak et al., 2005b; Porsolt et al., 1978). Briefly, rats were transported to a separate treatment room and immediately placed in a cylinder of water (45 cm high, 20 cm diameter) filled to a depth of 30 cm and maintained at 25 ± 1°C. Rats were forced to swim for 15 min, after which they were quickly towel-dried and returned to their home cages. Forty-eight hours later, all rats were once again exposed to the swim apparatus for a 5-min test session. All rats were exposed to the FST in a cylinder that had been freshly rinsed and filled with clean water. All swim sessions were videotaped from a side angle using a Canon ZR 800 digital video camcorder, with behavioral assessments performed by observers that were blind to experimental treatment. Behavioral categorization of immobility (defined as the absence of all movement except that which was necessary to stay afloat) was as previously described (Cryan et al., 2002; Detke et al., 1995), with videotapes were scored on an interval-based system (5 s each). Five-second intervals were indicated by a remote beep governed by a computer. Previous results from our laboratory have validated our FST paradigm, since desipramine was shown to significantly reduce the immobility that is normally seen on the test day (Deak et al., 2005b).
2.2.3. Open field test
Thirty minutes after the test swim, rats were placed individually into an open field apparatus for 10 min in order to detect nonspecific locomotor activity resulting from prior drug administration. Each test chamber (40 cm × 40 cm × 30 cm) consisted of a Plexiglas box equipped with infrared beams. A computer-recorded beam breaks, with total distance moved (cm), vertical activity (counts), and rest time (sec) analyzed.
2.3. Drugs
LPS (from E. coli serotype 0111:B4) was purchased from Sigma Chemical Co. (St. Louis, MO). LPS was initially diluted in sterile, pyrogen free saline (0.9%) and aliquots were stored at -20°C until needed. On the day of experimentation, a frozen aliquot was thawed and diluted to 100 μg/ml in pyrogen-free physiological saline. LPS injections were administered at a dose of 100 μg/kg (i.p.) and all experiments were conducted using aliquots from a single lot of LPS. In all experiments, ethanol (95%) was diluted fresh daily with pyrogen-free physiological saline in order to make a 20% solution (v/v). Ethanol injections were administered at a dose of either 0.5 or 4.0 g/kg (i.p.). Indomethacin (Sigma; i.p. at 10 mg/kg) was mixed fresh daily in 0.1 M NaHCO3 and gently heated prior to administration. IL-1ra (Kinaret) was initially diluted in sterile pyrogen-free saline and stored at 4°C until needed. On the day of experimentation, the smaller dose was diluted further from the concentrated stock with saline in order to maintain a constant volume of administration. IL-1ra was administered ICV (10 or 100 μg) at a volume of 1.34 μl. In all experiments, vehicle-injected controls were given pyrogen-free physiological saline isovolumetric to the largest ethanol (or other drug) dose.
2.4. ICV cannulation and microinjection procedure
Rats were anesthetized with isoflurane (dose to effect) and implanted with a 23-gauge stainless steel guide cannula (AP = –1.0 mm, ML = 0, DV = –6.0, relative to bregma) that terminated 1 mm above the third ventricle. Sterile stainless steel stylettes were inserted into each cannula in order to maintain patency. Each cannula was secured to the skull with dental acrylic and stainless steel screws inserted into the surrounding skull using standard aseptic technique. Rats were given 2–3 weeks of recovery prior to experimentation. IL-1ra was administered with a microinjection pump at a flow rate of 1.34 μl/1.5 min while gently restraining the rat in a towel. Injections were given an additional 1 min to diffuse prior to removal of the microinjection needle. Verification of guide cannula placement was performed within a few days of the conclusion of the experiment via a dipsogenic response to angiotensin (Kitiyakara et al., 2002). Briefly, all rats were microinjected with 30 μg of angiotensin II (Sigma) in sterile saline and returned to their home cages. Water bottles were weighed immediately prior to injection and then again 30 min later to determine the quantity of water consumed. Consumption of 10 g or more of water in the 30-min period was considered a successful placement of the cannula, with no rats failing to achieve this criterion (average intake = 17.73 ± 5.45 g of water in 30 min). There were a few rats whose cannulae were not patent (n = 8 rats, 13.3% of total) for behavioral testing, which then served as procedural controls for the angiotensin test. These rats drank only a few grams of water during the 30-min test period (average consumption = 6.00 ± 2.78g), likely reflecting slight spillage and dripping during bottle placement/removal, as virtually no consummatory behavior was observed in these rats.
2.5. Data Analysis
Before analysis, data sets were first examined for possible outliers (a score of more than 2 standard deviations from the mean of a particular group was considered an outlier), with any animals identified as outliers mentioned within an experiment and removed from all analyses. Data were then analyzed according to the experimental design described in each individual experiment, with Fisher's Least Significant Difference (LSD) post hoc test generally used to explore the locus of significant effects.
3. Experiment 1: Behavioral alterations in the social investigation paradigm following LPS
3. 1. Method
To determine whether the social interaction paradigm would reliably detect sickness behavior induced by a direct immunological challenge, adult male Sprague-Dawley rats (n=4 per group; N=8) were injected (i.p.) with 100 μg/kg LPS or saline and then returned to their home cages. Rats were then placed in the social investigation boxes for a 30-min test session at 3, 6, and 24 hr after injection. Data were analyzed using a 2 (Drug Exposure) × 3 (Testing Time) mixed ANOVA, with post hoc tests performed using Fishers LSD in order to explore the locus of significant main effects and interactions (p ≤ 0.05).
3.2. Results
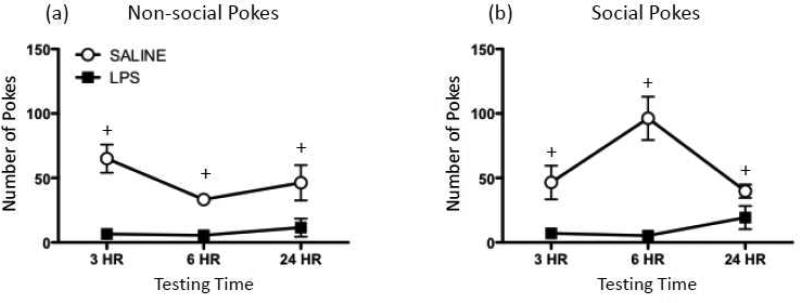
Pretreatment with LPS significantly suppressed investigation at the nonsocial hole [F(1,6) = 31.43, p ≤ 0.01] at all of the time points tested (see Figure 1b). A main effect of Drug Exposure for pokes at the social hole [F(1,6) = 68.23, p ≤ 0.001], as well as a significant interaction between Drug Exposure and Testing Time [F(2,12) = 5.63, p ≤ 0.05] revealed significant reductions in social pokes at all testing time points for LPS-exposed rats compared to controls (Figure 1a). Though social pokes showed a trend towards recovery at the 24 hr test session, post hoc tests demonstrated that the impact of LPS treatment was still significant at this time point.
Figure 1.
Behavioral effects of lipopolysaccharide. (a) Non-social and (b) social pokes exhibited by rats 3 hr, 6 hr, or 24 hr after injection with either 100 μg/kg lipopolysaccharide (LPS; black squares) or saline (white circles). In this figure (as well as in all subsequent figures), data are expressed as mean ± S.E.M. Plus signs (+) indicate a significant difference between saline- and LPS-exposed animals at a given testing time.
4. Experiment 2: Alterations in social behavior and exploratory activity following a 4-g/kg acute ethanol challenge
4.1. Method
Since behavioral alterations typical of an acute immune challenge (Dantzer, 2004) were also observed in this modified social investigation paradigm, including reduced social and exploratory activity, Experiment 2 determined whether similar changes in behavior would be detected during withdrawal from acute ethanol exposure. Therefore, adult male Sprague-Dawley rats (n=8 per group; N=16) were injected (i.p.) with 4-g/kg ethanol or saline and then tested in the social investigation chambers for 30 min both 18 (peak withdrawal) and 42 hrs (recovery period) later. Previous studies have demonstrated that the 18 hr time point represents the approximately 3 hours post-clearance of ethanol challenge, with other withdrawal behaviors readily observable at this time (e.g., Doremus et al., 2003; Doremus-Fitzwater and Spear, 2007; Varlinskaya and Spear, 2004). Additionally, on the day prior to ethanol administration, rats in this study were pre-exposed to the social investigation boxes for a 30-min period of baseline testing. This extra day of baseline testing was included since rats were given a large bolus of fluid and it was unknown as to how such an injection might impact behavior in this paradigm. Data were analyzed using 2 (Drug Exposure) × 3 (Testing Time) mixed ANOVA, with Fisher's LSD selected for post hoc tests.
4.2. Results
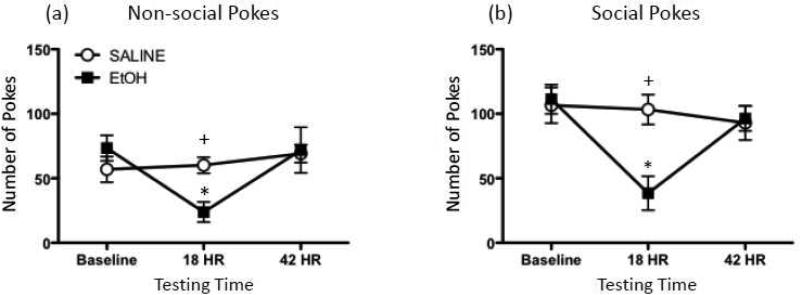
Outlier tests determined that one ethanol-exposed animal met the criterion for exclusion, with this rat not included in any of the analyses for this study. In the analysis of both social (Figure 2a) and non-social pokes (Figure 2b), a significant Drug Exposure × Testing Time interaction was observed [F(2,26) = 8.08, p ≤ 0.01 and F(2,26) = 6.14, p ≤ 0.01, respectively], with this interaction revealing no significant group differences for either social or exploratory behavior at baseline. At the 18 hr time point, however, prior ethanol administration was shown to have significantly reduced both social and non-social hole pokes relative to their baseline levels, as well as in comparison to saline-exposed animals at the same test time. By 42 hr post-drug exposure, behavioral differences between saline- and ethanol-injected animals were no longer apparent.
Figure 2.
Behavioral effects of a 4-g/kg ethanol bolus. (a) Non-social and (b) social pokes exhibited by rats during baseline testing, as well as 18 or 42 hr after injection with either 4-g/kg ethanol (EtOH; black squares) or saline (white circles). Asterisks (*) denote a significant difference within a group for a particular time point relative to baseline, whereas plus signs (+) indicate a significant difference from saline-exposed animals at a given testing time.
5. Experiment 3: Alterations in social behavior and exploratory activity following a 0.5-g/kg acute ethanol challenge
5.1. Method
The results of Experiment 2 demonstrated that an acute 4-g/kg dose of ethanol was sufficient to suppress both social and exploratory-like behaviors during withdrawal in this social investigation test. In order to determine that these behavioral alterations are specific to withdrawal following a heavy ethanol challenge, Experiment 3 was conducted to examine social investigation and exploratory activity at several time points after a dose of ethanol that results in mild intoxicating effects, but fails to produce a robust acute withdrawal response. On the first day of experimentation, all rats (n=8 per group; N=16) were given a 30-min baseline test in the social investigation boxes and were then returned to their home cages. The following day, rats were injected (i.p.) with 0.5 g/kg ethanol (20%) or equivolume saline and tested in the social investigation chambers both 30 min and 4 hr post-injection [i.e. 3 hr post-ethanol clearance]. Data were analyzed using 2 (Drug Exposure) × 3 (Testing Time) mixed ANOVA, with Fisher's LSD selected for post hoc tests.
5.2. Results
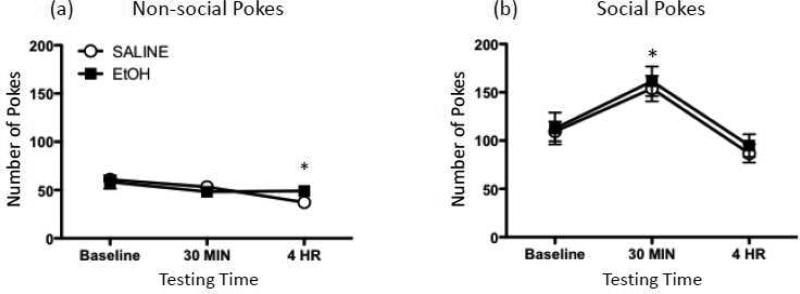
Regardless of time of test, administration of this low dose of ethanol altered neither social nor non-social pokes (Figure 3a and 3b). A significant main effect of Testing Time, however, was observed for both behavioral measures [non-social pokes: F(2,28) = 4.85, p ≤ 0.05; social pokes: F(2,28) = 14.54, p ≤ 0.0001], indicating that, regardless of drug exposure, non-social pokes significantly decreased from baseline to the 4 hr testing time. In contrast, social pokes were significantly increased at the 30 min time point relative to both the baseline and 4 hr test time points.
Figure 3.
Behavioral effects of a 0.5-g/kg ethanol challenge. (a) Non-social and (b) social pokes exhibited by rats during baseline testing, as well as 30 min or 4 hr after injection with either 0.5-g/kg ethanol (EtOH; black squares) or saline (white circles). Asterisks (*) denote a significant difference at a particular time point relative to baseline, collapsed across exposure condition.
6. Experiment 4: Behavioral alterations in the forced swim test following an acute 4-g/kg ethanol challenge
6.1. Method
An acute large (4-g/kg) dose of ethanol was sufficient to induce reductions in exploratory and social investigative behavior during withdrawal in Experiment 2. Since these behaviors parallel the behavioral changes observed following an acute immune challenge in Experiment 1, we hypothesized that ethanol withdrawal-related behavioral symptoms might be an expression of a sickness-like response. Previous studies have reported evidence to suggest that sickness is a motivational state (e.g., Aubert et al., 1997; for review see Dantzer, 2001), which can be overcome by environmentally challenging conditions. If rats are indeed experiencing a sickness response during withdrawal from acute ethanol, it would then be expected that exposure to a threatening context, such as exposure to the forced swim test, would fail to reveal significant behavioral differences between ethanol withdrawn and control rats.
In Experiment 4, therefore, rats (N=24) were injected with either 4-g/kg ethanol (i.p.) or saline and then examined in the FST. As mentioned above, all rats were first given a baseline (15-min) exposure to the FST. Since prior ethanol exposure would likely impact initial experience in the FST, rats were given their first (15-min) exposure to the FST in a drug-naïve state. In order to keep day-1 testing free from possible intoxication/withdrawal effects, we were forced to deviate from the typical design of a 24 interval between baseline and test-day FST sessions. Specifically, for the ethanol-exposed animals (n=16), one group received the ethanol challenge 18 hours prior to the second (5-min) forced-swim test session, while the other ethanol group was administered ethanol 42 hours prior to their second day of testing. In order to control for the possible effects of differences in timing between FST exposures on subsequent behavior, the saline-injected group (n=8) was counterbalanced as to whether they were given their second FST exposure 18 or 42 hours after saline administration. Thirty minutes following the second FST session, animals were exposed to a 10-min open field test (described above).
6.2. Results
6.2.1. Forced-swim test
During the first 15-min FST exposure, immobility was analyzed using a 3 (Time bin: 5-min blocks) × 3 (Exposure group) mixed ANOVA, with Fisher's LSD used as a post hoc test to explore the locus of significant main effects and interactions. Results of this analysis showed that immobility steadily and significantly increased from the first 5 minutes (2.50 ± 0.70; mean ± SEM) to the last 5 minutes of the pretest session (47.48 ± 2.70) [F(2,42) = 177.01, p ≤ 0.00001]. Therefore, no significant differences were observed between groups during this pretest which occurred prior to ethanol exposure.
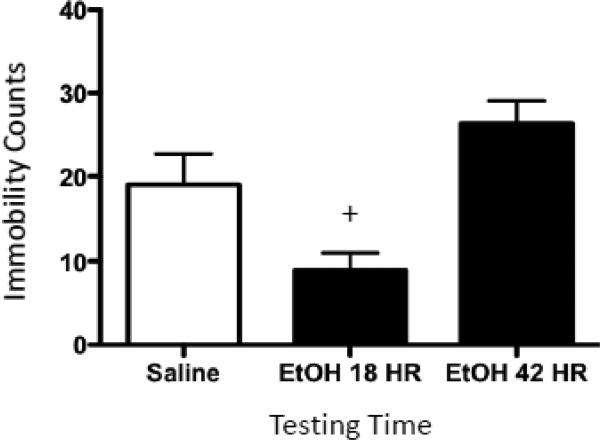
For the second FST exposure (5-min test day), immobility data were analyzed using a one-way ANOVA (i.e. data were not analyzed across time bins) and post hoc tests were performed using Fisher's LSD test. A significant main effect of Drug Exposure in this analysis [F(2,21) = 9.21, p ≤ 0.01] showed that rats receiving ethanol 18 hours prior to the second test exhibited a significant decrease in immobility relative to saline controls and rats tested at the 42 hr time point (Figure 4). By the 42 hours post-ethanol exposure, however, decreases in immobility were no longer apparent when compared to saline-treated controls.
Figure 4.
Forced-swim behavior exhibited following a 4-g/kg ethanol challenge. Immobility counts observed by rats administered either saline (white bar) or ethanol (EtOH) before being tested for 5 min in a forced-swim task. The first black bar represents animals that received 4 g/kg ethanol 18 hr prior to forced-swim testing (EtOH 18 HR), whereas the second black bar represents rats that received 4-g/kg ethanol 42 hr before this forced-swim test (EtOH 42 HR). The plus sign (+) indicates a significant difference from saline-exposed animals.
6.2.2. Open field test
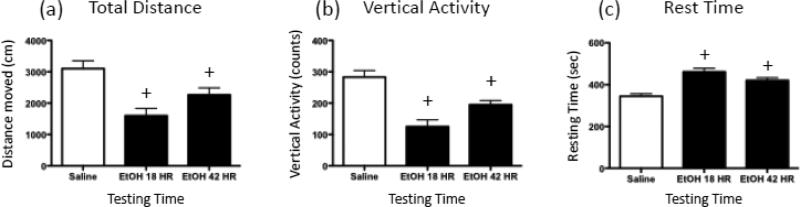
In the open field (Figure 5), total distance moved by ethanol-exposed animals tested at 18 hr and 42 hr was significantly decreased relative to the saline controls [F(2,21) = 10.35, p ≤ 0.001]. When vertical activity was examined, it was similarly observed that ethanol-exposed animals tested at both time points exhibited significantly less vertical activity than the saline controls [F(2,21) = 17.19, p ≤ 0.0001]; and the 18 hr ethanol group demonstrated less vertical activity than the 42 hr ethanol animals. The analysis of rest time revealed that, compared to saline-exposed rats, ethanol-exposed animals tested at both time points showed significantly increased time at rest [F(2,21) = 17.37, p ≤ 0.0001].
Figure 5.
Open field behavior exhibited following a 4-g/kg ethanol bolus. Rats were given either saline (white bar) or ethanol (EtOH) before being tested for 10 min in a novel open field, with (a) total distance traveled (cm), (b) vertical activity (counts), and (c) rest time (sec) during the session recorded and later analyzed. The first black bar represents animals that received 4 g/kg ethanol 18 hr prior to open field testing (EtOH 18 HR), whereas the second black bar represents rats that received 4-g/kg ethanol 42 hr before the open field test (EtOH 42 HR). The plus sign (+) indicates a significant difference from saline-exposed animals.
7. Experiment 5: Effects of Indomethacin on withdrawal-related behaviors following acute ethanol exposure
7.1. Method
Results from the previous experiments have collectively demonstrated that behavioral alterations observed during withdrawal from an acute ethanol challenge share many similarities with a sickness response. Administration of drugs that inhibit prostaglandin synthesis, such as indomethacin (a nonselective COX inhibitor), have been shown to reverse the sickness response induced by injection of inflammatory compounds including LPS (de Paiva et al., 2010; Fishkin and Winslow, 1997; Yirmiya et al., 1997). Furthermore, previous studies have reported that some of the behavioral aspects of ethanol withdrawal can by prevented by prostaglandin inhibition (Dhir et al., 2005; Hale et al., 1992; Kaivola et al., 1983; Romeo et al., 2000). The goal of Experiment 5, therefore, was to determine if disruption of prostaglandin synthesis would attenuate reductions in exploratory activity and social investigation seen 18 hr after an ethanol bolus. In order to minimize potential anxiogenic effects of an unfamiliar testing environment during acute ethanol withdrawal, rats (n=8 per group; N=32) were first familiarized to the social investigation testing situation during three (one per day) 30-min baseline tests. Immediately after their final baseline test, rats were injected (i.p.) with either 4-g/kg ethanol or an isovolumetric dose of saline. Fifteen hours later (3 hr before the 18-hr test session), animals were administered indomethacin (10 mg/kg, i.p.) or vehicle (NaHCO3) and placed back in their home cages. 18 hr after ethanol administration, rats were assessed for expression of withdrawal-related behaviors in the social investigation apparatus, with these animals tested one final time 24 hr later (the 42 hr post-ethanol recovery time point).
7.2. Results
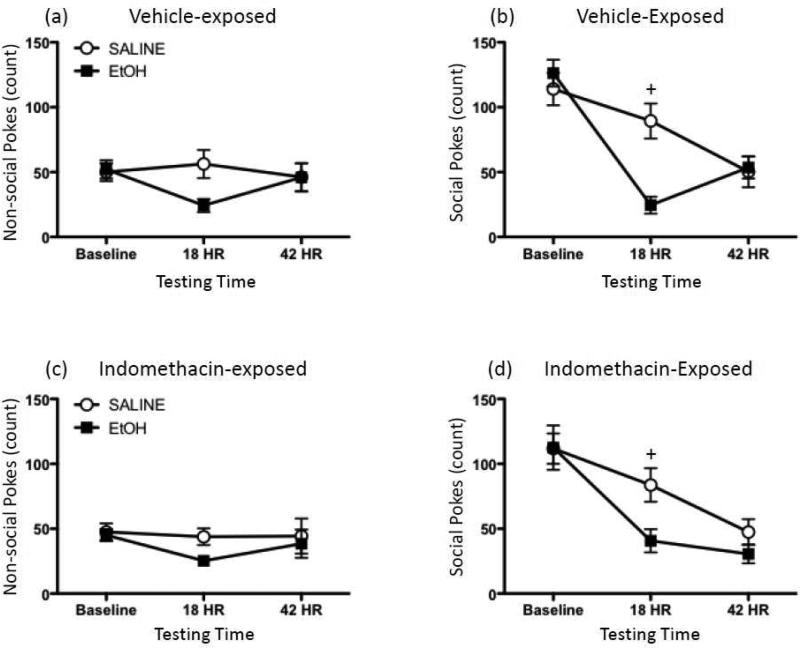
Two animals (one saline- and one ethanol-exposed rat, both treated with indomethacin) were outliers and were therefore excluded from all analyses. Baseline data were averaged across the three consecutive exposures, with data then analyzed using a 2 (Ethanol Exposure) × 2 (Indomethacin Exposure) × 3 (Testing Time) mixed ANOVA, and post hoc tests performed using Fisher's PLSD. In the analysis of non-social pokes (Figure 6a, c), there was a marginally significant interaction between Ethanol Exposure and Test Day (p=0.066), with a pattern of results similar to those observed in Experiment 2: ethanol-exposed animals had a strong tendency to exhibit significant reductions in exploratory activity 18 hours post-injection, with these ethanol-related effects no longer apparent by the 42 hr time point. No significant main effect or interactions involving Indomethacin Exposure were observed in this analysis.
Figure 6.
Effects of indomethacin on ethanol withdrawal-related behavior in the social investigation test. Rats were injected with either 4-g/kg ethanol (EtOH; black squares) or saline (white circles). Fifteen hours later, animals were administered either a vehicle injection or 10 mg/kg indomethacin. 3 hr and 27 hr following this second injection (i.e. 18 and 42 hr after EtOH or saline exposure), animals were then tested in the social investigation test. Non-social pokes exhibited by (a) vehicle- and (c) indomethacin-exposed rats were analyzed, as well as pokes directed at the social hole by (b) vehicle- and (d) indomethacin-treated animals. Plus signs (+) indicate a significant difference between saline- and ethanol-exposed animals at a given testing time, collapsed across indomethacin treatment.
When social pokes were examined (Figure 6b, d), ethanol animals were again found to exhibit similar levels of social investigation at baseline relative to saline-exposed rats, with significant reductions in social investigation for ethanol rats observed at the 18 hr post-exposure time point when compared to their saline-treated controls, regardless of indomethacin treatment (Ethanol Exposure × Testing Time interaction: [F(2,52) = 10.83, p ≤ 0.001). By 42 hr post-exposure, however, ethanol- and saline-exposed animals displayed comparable numbers of social pokes. As with non-social pokes, there was neither a significant main effect nor any interactions involving Indomethacin Exposure observed in the analysis of social pokes.
8. Experiment 6: Effects of IL-1ra on withdrawal-related behaviors following acute ethanol exposure
8.1. Method
We hypothesized that the behaviors exhibited by ethanol withdrawn rats following an acute large ethanol challenge, since similar to sickness behaviors, were related to an ethanol-induced activation of the immune response, and therefore, increased expression of pro-inflammatory cytokines. Administration of a general prostaglandin inhibitor, however, did not successfully reverse the withdrawal-related behavioral alterations we have repeatedly observed following acute ethanol challenge. In other experiments from our lab, it has been shown that central gene expression of the pro-inflammatory cytokine, IL-1, is significantly increased 18 hr following an i.p. 4-g/kg acute ethanol challenge (Deak et al., in prep). Hence, in the current experiment, central injection of the IL-1ra was assessed as a more specific mechanism that would potentially reverse ethanol withdrawal-associated reductions in exploratory and social investigative behaviors.
Rats (n=6-10 per group; N=49) were stereotaxically implanted with a guide cannula into the third ventricle (described above) and given at least 2 weeks of recovery. Importantly, unpublished pilot data from our laboratory have demonstrated that cannulated rats behave similarly to their non-cannulated counterparts in our social investigation paradigm, with animals still capable of poking their heads into both chamber holes. All animals were given three baseline exposures (and these data averaged prior to analysis) to the social investigation apparatus. After the final baseline test, rats were injected (i.p.) with either 4-g/kg ethanol or equivolume saline. Seventeen and a half hours later, they were given a microinjection of either saline, 10, or 100 μg of IL-1ra 30 min prior to behavioral testing at 18 hr post-ethanol exposure, with rats tested a final time 42 hr after ethanol exposure. These doses of IL-1ra were selected based on previous studies in which IL-1ra was able to block stress- (Maier and Watkins, 1995), as well as IL-1-induced (Linthorst et al., 1995), sickness behaviors.
8.2. Results
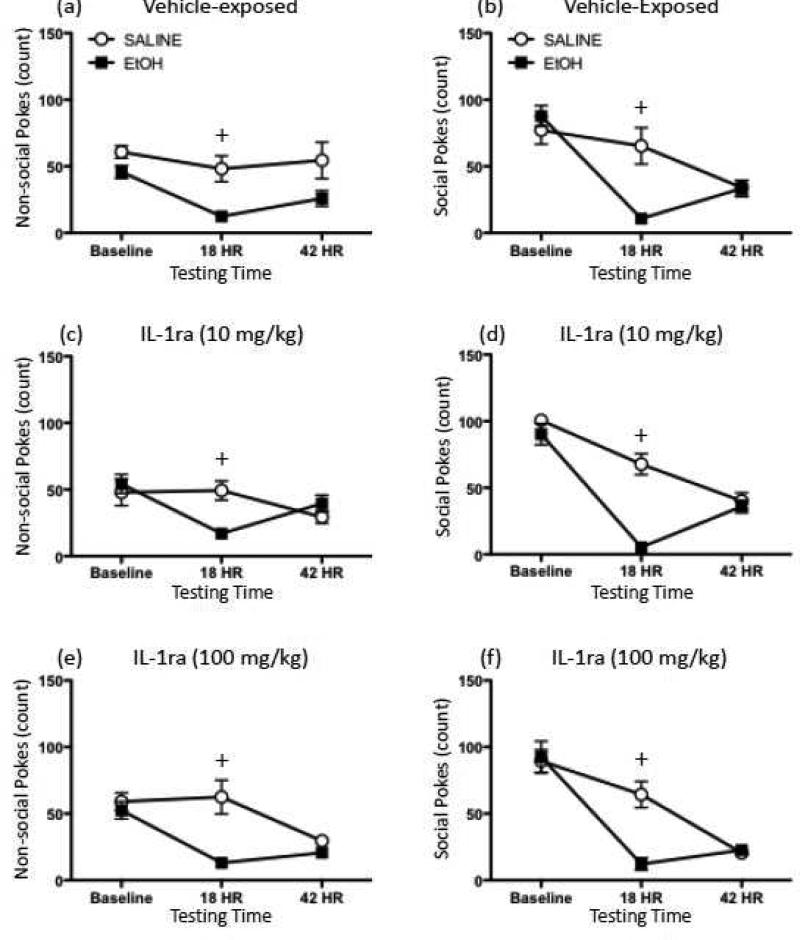
One rat (saline-exposed rat that received the low dose of IL-1ra) was excluded from all analyses as an outlier. Data for both social and non-social pokes were analyzed using a 2 (Ethanol Exposure) × 3 (IL-1ra Treatment) × 3 (Testing Time) repeated measures mixed ANOVA and post hoc tests performed using Fisher's LSD. In the analysis of both nonsocial and social pokes (Figure 7), a significant interaction of Testing Time with Ethanol Exposure was observed [nonsocial pokes: F(2,84) = 17.73, p ≤ 0.00001; social pokes: F(2,84) = 39.76, p ≤ 0.00001]. For both measures, this interaction revealed that poking behavior decreased significantly across tests, with both 18 hr and 42 hr frequency of poking behavior significantly lower than at baseline in both the ethanol- and saline-exposed groups. Additionally, at the 18 hr withdrawal time point, ethanol-exposed animals were found to exhibit significantly fewer social and non-social pokes than their saline-treated counterparts at the same testing time. The effects of ethanol exposure did not significantly interact with IL-1ra Treatment for either measure, although a significant interaction of Testing Time with IL-1ra Treatment [F(4,84) = 2.64, p ≤ 0.05] was observed in the analysis of non-social pokes. Post hocs performed on this interaction showed that for all three groups, non-social pokes significantly decreased across testing days, with non-social pokes exhibited by the three groups comparable on both the baseline and 18 hr testing points and animals receiving the highest dose of IL-1ra tending to poke less than the animals that did not receive IL-1ra (p = 0.071).
Figure 7.
Effects of IL-1ra on ethanol withdrawal-related behaviors exhibited in the social investigation test. Rats were injected with either 4-g/kg ethanol (EtOH; black squares) or saline (white circles). Seventeen and a half hours later, animals were administered either vehicle, 10, or 100 μg of IL-1ra (intracerebroventricularly; ICV) 30 min prior to behavioral testing in the social investigation apparatus. Rats were tested again at a time point equivalent to 42 hr post-ethanol injection. Non-social pokes exhibited by rats given (a) vehicle, (c) 10 μg IL-1ra, or (e) 100 μg IL-1ra; as well as pokes directed at the social hole by (b) vehicle-, (d) 10 μg IL-1ra-, or (f) 100 μg IL-1ra-treated animals were analyzed. Plus signs (+) indicate a significant difference between saline- and ethanol-exposed animals at a given testing time, collapsed across IL-1ra treatment.
9. Discussion
Taken together, these studies demonstrate several significant findings regarding the behavioral and immunological changes observed after withdrawal from an acute dose of ethanol. In Experiment 1, the modified social investigation task utilized in this series of studies was shown to be a valid and sensitive behavioral paradigm for the detection of sickness behavior following an acute immunological challenge (LPS administration), as reflected by reductions in both social investigation and exploratory activity. In subsequent experiments (Experiments 2, 5, and 6), it was then consistently demonstrated that the behaviors observed during withdrawal from a large acute ethanol bolus are similar to those exhibited following LPS administration, as analogous reductions in both social investigation and exploratory activity were found 18 hours after a 4 g/kg injection of ethanol. Experiment 3 revealed that these behavioral changes are unique to a high dose of alcohol since no alterations in social investigation and exploratory activity were observed at any of the time points examined following administration of a low dose (0.5 g/kg) of ethanol. Furthermore, Experiment 4 demonstrated that this hypoactivity is not also manifested as an increase in immobility in the FST, suggesting that these acute withdrawal-related behaviors may reflect ethanol-associated alterations in motivational processes. Finally, although we hypothesize that increased expression of pro-inflammatory cytokines may play a role in the expression of acute withdrawal behaviors, these behaviors were not significantly influenced by peripheral administration indomethacin (Experiment 6) nor central injection of IL-1ra (Experiment 7).
LPS is commonly used to induce a sickness-like state in animals (e.g., Dantzer, 2004; Deak et al., 2005a). Reductions in general activity following exposure to LPS have previously been reported in the literature, with several groups showing significant hypoactivity in the open field (Swiergiel and Dunn, 2007; Yirmiya et al., 1994), home cage (Hayley et al., 2008; Huang et al., 1999), and other behavioral paradigms (e.g., the elevated plus-maze; Swiergiel and Dunn, 2007). Additionally, reduced sexual (Yirmiya, 1996) and social behaviors (Arakawa et al., 2009; Bluthe et al., 1992b; Fishkin and Winslow, 1997) have been consistently observed. The current results demonstrate that these behavioral changes can be seen in yet another behavioral paradigm—one that is entirely automated, thereby minimizing issues regarding inter-rater reliability and observer bias (Deak et al., 2009). When examining behavioral changes in this same modified social investigation task during acute ethanol withdrawal, similar to behavior following LPS exposure, rats demonstrated a significant reduction in both social investigation and general activity, which was most pronounced 18 hr post-ethanol administration but resolved by 42 hr post-ethanol exposure. With respect to the literature regarding acute withdrawal-induced changes in behavior following an acute ethanol exposure, these results are not novel, with previous instances of reduced activity (e.g., Doremus-Fitzwater and Spear, 2007; Slawecki and Roth, 2004) and social behavior (e.g., Varlinskaya and Spear, 2004) reported during withdrawal from acute ethanol administration. These findings do, however, confirm similar results in a new automated behavioral task, while also demonstrating additional similarity in ethanol withdrawal behavior and the sickness response.
Further support for the notion that acute withdrawal-related behavioral changes and sickness behaviors may be mediated via similar mechanisms was obtained when rats were exposed to the FST during acute withdrawal. Although rats acutely given LPS or other illness-inducing agents have been shown to exhibit reduced activity and general lethargy, these same animals will overcome these “deficits” during a potentially life-threatening situation, such as the FST (Deak et al., 2005b). It should be noted, however, that the sensitivity of the FST toward illness-related changes in despair-like behavior may depend on species, strain and/or other subject characteristics, as discrepant results have been reported on the association between illness and depression using this model (Deak et al., 2005b; Dunn and Swiergiel, 2005; Dunn et al., 2005; Swiergiel et al., 1997). When ethanol withdrawn rats were exposed to the FST in Experiment 4 of the current report, however, a somewhat paradoxical decrease was observed in immobility in rats that had received ethanol 18 hr prior to the swim test, with these changes no longer present 42 hr post-exposure. Clearly, the hypoactivity observed in the modified social interaction test during acute ethanol withdrawal does not generalize to this environmentally and physiologically challenging task, suggesting that rats exposed to ethanol are not debilitated, but rather are capable of overcoming withdrawal-related effects.
Classically, immobility expressed during the FST is often used as a marker of despair in the rat that is analogous to depression in humans (Duman, 2010; Lucki, 1997; Porsolt, 1979), since increasing amounts of immobility in the FST with repeated exposures can be attenuated by drugs that are clinically efficacious antidepressants (for review see Cryan et al., 2002; Deak and Larish, 2006). Previously, acute exposure to alcohol has been reported to reduce immobility in the FST (Hirani et al., 2002; Kokare et al., 2008), while ethanol withdrawal has been shown to increase immobility (Getachew et al., 2010; Hirani et al., 2002; Walker et al., 2010). These reports of increased immobility during ethanol withdrawal, however, have been observed following chronic exposure to ethanol. To our knowledge, there have been no studies investigating behavioral alterations in the FST during withdrawal from an acute ethanol bolus. Although it is unclear at this time as to why rats exhibited decreased immobility during withdrawal from acute ethanol exposure, it is possible that the differences in duration of ethanol exposure could account for the opposite pattern of immobility found in the current report. Future studies are needed to corroborate these findings and determine the underlying mechanisms of this behavioral change in the FST during acute withdrawal. Regardless, these results provide an important context for understanding other behavioral changes observed during withdrawal from acute alcohol exposure—they indicate that hypoactivity observed in a novel environment and reduced exploration/interaction in a test of social investigation does not generalize to a behavioral test (FST) involving a significant threat, and which requires behavioral activity for survival. In this way, these novel findings fit well with the general viewpoint that acute illness represents a change in motivational state of the animal in favor of recuperative behaviors, rather than as a sign of debilitation (Dantzer, 2001; Hart, 1988).
In order to further test the hypothesis that acute withdrawal-related behaviors and the sickness response might be mediated via similar mechanisms (i.e. involving endogenous inflammatory mechanisms), anti-inflammatory agents were administered to animals in an attempt to attenuate the behavioral manifestations of acute withdrawal. In previous studies, anti-inflammatory agents such as indomethacin, pentoxifylline (a TNFα synthesis inhibitor), and IL-1ra have been shown to reverse sickness behaviors induced by LPS administration (Pollak et al., 2003), with IL-10 also blocking LPS-induced reductions in social interaction (Bluthe et al., 1999). Furthermore, footshock-induced reductions in social behavior have been observed within our own laboratory, using the same social investigation task used in the current report (Arakawa et al., 2009). Therefore, both indomethacin (Experiment 5) and IL-1ra (Experiment 6) were examined for their ability to potentially reverse acute ethanol withdrawal behaviors. Contrary to what was expected, neither indomethacin nor IL-1ra was able to block the reduction in general activity and social behavior observed 18 hours after a 4-g/kg ethanol injection. Given the previously reported effectiveness of IL-1ra at reversing stress-related increases in anxiogenic behavior in this task (Arakawa et al., 2009), and the general lack of behavioral effects of IL-1ra when administered to control animals (e.g., Avitsur et al., 1997; Bluthe et al., 1995), it seems unlikely that the null effects of IL-1ra in these studies was due to any intrinsic anxiogenic properties of the drug, or a general lack of efficacy. There are many other possible explanations for this outcome, however, the first being that neither COX nor IL-1 mechanisms are involved in the behavioral changes observed in this model. Indeed, there is prior evidence to suggest that, while inhibition of prostaglandin synthesis can reverse some illness- (Avitsur et al., 1999; Bluthe et al., 1992a; De La Garza et al., 2004) and withdrawal-related (Dhir et al., 2005; Hale et al., 1992; Kaivola et al., 1983; Romeo et al., 2000) behaviors, these effects are not always observed (e.g., (Nadjar et al., 2010; Otterness et al., 1991; Segarnick et al., 1985).
Other possible explanations for these results, however, would not entirely preclude the involvement of cytokine signaling in the expression of ethanol-related behaviors. For instance, it is a possibility that the timing of administration of the drugs was not optimal, with perhaps, injection of these anti-inflammatory agents prior to ethanol exposure being more effective. In doing so, changes in inflammatory factors that occurred during ethanol intoxication, such as the changes in IL-6 and TNF observed during acute intoxication (Deak, et al., in prep), may have been prevented, thus attenuating later down-stream alterations in cytokine signaling and ultimately the expression of withdrawal-related behaviors. Alternatively, since animals in the current studies were given a limited range of doses of these drugs, it remains a possibility that the doses administered were not optimal for blocking the behavioral changes observed during acute ethanol withdrawal, though it can be noted that the doses chosen were in the range of effective doses described in other studies. Physiological redundancy may also have contributed to the lack of a reversal. For example, IL-1 may in fact have been responsible for the behaviors we observed, but after blockage by IL-1ra, another cytokine may have compensated and driven these behaviors. Indeed, there is evidence that certain cytokines share multiple receptor subtypes (Ozaki and Leonard, 2002), which would allow for multiple cytokines to be involved in the expression of certain sickness or ethanol withdrawal behaviors.
The likelihood remains that other anti-inflammatory agents may still be able to block these behaviors. For example, IL-10 may be a better candidate in view of the fact that it is a general anti-inflammatory that reduces the expression of multiple pro-inflammatory cytokines including IL-1, IL-6, and TNF (Dantzer, 2004), whereas IL-1ra blocks the IL-1 receptor selectively. Indomethacin is also selective as it specifically blocks COX-dependent expression of prostaglandins. Though no one to our knowledge has attempted to block the behavioral manifestations of withdrawal from acute ethanol exposure with an anti-inflammatory agent, Jung et al. (2006) were able to reverse the social deficits caused by withdrawal using rosiglitazone, a thiazolidinedione used to treat diabetes. Interestingly, rosiglitazone has also been shown to have anti-inflammatory properties by reducing NFκB binding and TNF concentrations (Mohanty et al., 2004). Future studies which utilize a more general anti-inflammatory agent given at various time points relative to the onset of ethanol intoxication are certainly needed before the possibility that cytokine signaling is mechanistically linked to expression of acute ethanol withdrawal behaviors is entirely excluded.
In summary, the present findings confirm and extend prior studies using a novel social investigation paradigm by demonstrating that it is highly sensitive to illness-inducing agents such as LPS. While exposure to a high dose of ethanol (4 g/kg) produced highly similar behavioral outcomes, exposure to a lower dose of ethanol (0.5 g/kg) that should produce little if any signs of alcohol withdrawal, was without effect on this behavioral task. Despite the similarity between the effects of LPS and (high dose) ethanol in this test, administration of anti-inflammatory agents at the time of ethanol clearance was unable to reverse the behavioral changes observed after ethanol exposure. Interestingly, rats exposed to the FST after injection of LPS (Deak et al., 2005b) or during withdrawal from acute alcohol exposure (present studies) did not display the typical reductions in activity associated with these challenges, supporting the notion that neither LPS nor ethanol exposure produce signs of behavioral debilitation in the animals. Taken together, these findings demonstrate some intriguing parallels between acute illness and responses to ethanol, suggesting that additional studies examining the involvement of brain cytokines as potential mediators of ethanol effects are greatly needed. Such findings could provide novel targets for clinical treatment of withdrawal following binge ethanol exposure, and perhaps a therapeutic window in which certain contributors to the negative reinforcement cycle of addiction could be prevented. Additionally, given the role that cytokines have been shown to play in stress-related processes (e.g., see Deak et al., in press for review), growing evidence from our lab (Deak et al., in prep) and others (e.g.,Breese et al., 2008; Crews et al., 2011; Qin et al., 2008) of an involvement of cytokines in ethanol-related intoxication and withdrawal effects could provide another potential mechanism by which the complicated relationship between stress and ethanol abuse/addiction could be explored.
Highlights.
LPS exposure reduced social exploration and activity in a social investigation test
Acute ethanol (4 g/kg) decreased social and non-social behaviors during withdrawal
Ethanol withdrawal was associated with reduced immobility in the forced swim test
Indomethacin (i.p.) did not block expression of acute withdrawal-related behaviors
IL-1ra (i.c.v.) did not block expression of acute withdrawal-related behaviors
Acknowledgements
Supported by NIH grant number R21AA016305-01 to T.D., the Developmental Exposure Alcohol Research Center (DEARC; P50AA017823), and the Center for Development and Behavioral Neuroscience at Binghamton University. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the above stated funding agencies. The authors have no conflicts of interest to declare.
Abbreviations
- ICSS
intracranial self-stimulation
- LPS
lipopolysaccharide
- IL-1
interleukin-1
- IL-6
interleukin-6
- TNFα
tumor necrosis factor-alpha
- NSAID
non-steroidal anti-inflammatory drug
- FST
forced swim test
- COX
cyclooxygenase
- IL-1ra
interleukin-1 receptor antagonist
- ICV
intracerebroventricularly
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anforth HR, Bluthe RM, Bristow A, Hopkins S, Lenczowski MJ, Luheshi G, et al. Biological activity and brain actions of recombinant rat interleukin-1alpha and interleukin-1beta. Eur Cytokine Netw. 1998;9:279–88. [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, Deak T. Sickness-related odor communication signals as determinants of social behavior in rat: a role for inflammatory processes. Horm Behav. 2010;57:330–41. doi: 10.1016/j.yhbeh.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Blandino P, Jr., Deak T. Central infusion of interleukin-1 receptor antagonist blocks the reduction in social behavior produced by prior stressor exposure. Physiol Behav. 2009;98:139–46. doi: 10.1016/j.physbeh.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert A, Goodall G, Dantzer R, Gheusi G. Differential effects of lipopolysaccharide on pup retrieving and nest building in lactating mice. Brain Behav Immun. 1997;11:107–18. doi: 10.1006/brbi.1997.0485. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Pollak Y, Yirmiya R. Different receptor mechanisms mediate the effects of endotoxin and interleukin-1 on female sexual behavior. Brain Res. 1997;773:149–161. doi: 10.1016/s0006-8993(97)00927-x. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Weidenfeld J, Yirmiya R. Cytokines inhibit sexual behavior in female rats: II. Prostaglandins mediate the suppressive effects of interleukin-1beta. Brain Behav Immun. 1999;13:33–45. doi: 10.1006/brbi.1999.0556. [DOI] [PubMed] [Google Scholar]
- Barr AM, Song C, Sawada K, Young CE, Honer WG, Phillips AG. Tolerance to the anhedonic effects of lipopolysaccharide is associated with changes in syntaxin immunoreactivity in the nucleus accumbens. Int J Neuropsychopharmacol. 2003;6:23–34. doi: 10.1017/S146114570200319X. [DOI] [PubMed] [Google Scholar]
- Bekkedal MY, Panksepp J, Rossi J., 3rd Long-term changes in rat social behavior following treatment with trimethylolpropane. Neurotoxicol Teratol. 1998;20:307–16. doi: 10.1016/s0892-0362(97)00089-5. [DOI] [PubMed] [Google Scholar]
- Bekkedal MY, Rossi J, 3rd, Panksepp J. Fetal and neonatal exposure to trimethylolpropane phosphate alters rat social behavior and emotional responsivity. Neurotoxicol Teratol. 1999;21:435–43. doi: 10.1016/s0892-0362(99)00002-1. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Beaudu C, Kelley KW, Dantzer R. Differential effects of IL-1ra on sickness behavior and weight loss induced by IL-1 in rats. Brain Res. 1995;677:171–176. doi: 10.1016/0006-8993(95)00194-u. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Castanon N, Pousset F, Bristow A, Ball C, Lestage J, et al. Central injection of IL-10 antagonizes the behavioural effects of lipopolysaccharide in rats. Psychoneuroendocrinology. 1999;24:301–11. doi: 10.1016/s0306-4530(98)00077-8. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Crestani F, Kelley KW, Dantzer R. Mechanisms of the behavioral effects of interleukin 1. Role of prostaglandins and CRF. Ann N Y Acad Sci. 1992a;650:268–75. doi: 10.1111/j.1749-6632.1992.tb49135.x. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R, Kelley KW. Effects of interleukin-1 receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Brain Res. 1992b;573:318–20. doi: 10.1016/0006-8993(92)90779-9. [DOI] [PubMed] [Google Scholar]
- Bogin RM, Nostrant TT, Young MJ. Propranolol for the treatment of the alcoholic hangover. Am J Drug Alcohol Abuse. 1986;12:279–84. doi: 10.3109/00952998609007397. [DOI] [PubMed] [Google Scholar]
- Brasser SM, Spear NE. Physiological and behavioral effects of acute ethanol hangover in juvenile, adolescent, and adult rats. Behav Neurosci. 2002;116:305–20. doi: 10.1037//0735-7044.116.2.305. [DOI] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH, Navarro M, Wills TA, Angel RA. Repeated lipopolysaccharide (LPS) or cytokine treatments sensitize ethanol withdrawal-induced anxiety-like behavior. Neuropsychopharmacology. 2008;33:867–76. doi: 10.1038/sj.npp.1301468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck HM, Hueston CM, Bishop C, Deak T. Enhancement of the hypothalamic-pituitary-adrenal axis but not cytokine responses to stress challenges imposed during withdrawal from acute alcohol exposure in Sprague-Dawley rats. Psychopharmacology (Berl) 2011;218:203–15. doi: 10.1007/s00213-011-2388-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun. 2011;25(Suppl 1):S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Lucki I. 5-HT4 receptors do not mediate the antidepressant-like behavioral effects of fluoxetine in a modified forced swim test. Eur J Pharmacol. 2000;409:295–9. doi: 10.1016/s0014-2999(00)00858-x. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–45. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am. 2009;29:247–64. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Fabrizio KR, Radoi GE, Vlad T, Asnis GM. The non-steroidal anti-inflammatory drug diclofenac sodium attenuates lipopolysaccharide-induced alterations to reward behavior and corticosterone release. Behav Brain Res. 2004;149:77–85. doi: 10.1016/s0166-4328(03)00211-0. [DOI] [PubMed] [Google Scholar]
- de Paiva VN, Lima SN, Fernandes MM, Soncini R, Andrade CA, Giusti-Paiva A. Prostaglandins mediate depressive-like behaviour induced by endotoxin in mice. Behav Brain Res. 2010;215:146–51. doi: 10.1016/j.bbr.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Deak T, Arakawa H, Bekkedal MY, Panksepp J. Validation of a novel social investigation task that may dissociate social motivation from exploratory activity. Behav Brain Res. 2009;199:326–33. doi: 10.1016/j.bbr.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak T, Bellamy C, Bordner KA. Protracted increases in core body temperature and interleukin-1 following acute administration of lipopolysaccharide: implications for the stress response. Physiol Behav. 2005a;85:296–307. doi: 10.1016/j.physbeh.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Deak T, Bellamy C, D'Agostino LG, Rosanoff M, McElderry NK, Bordner KA. Behavioral responses during the forced swim test are not affected by anti-inflammatory agents or acute illness induced by lipopolysaccharide. Behav Brain Res. 2005b;160:125–34. doi: 10.1016/j.bbr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Deak T, Larish Y. Methods and motives for use of the forced swim test as an animal model of behavioral despair/depression. In: Anderson MJ, editor. Tasks and Techniques: A sampling of the methodologies for the investigation of animal learning, behavior and cognition. Nova Science Publishers, Inc.; Hauppauge, NY: 2006. pp. 3–18. [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Dhir A, Naidu PS, Kulkarni SK. Protective effect of cyclooxygenase-2 (COX-2) inhibitors but not non-selective cyclooxygenase (COX)-inhibitors on ethanol withdrawal-induced behavioural changes. Addict Biol. 2005;10:329–35. doi: 10.1080/13556210500352964. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–8. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Developmental differences in acute ethanol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2007;31:1516–27. doi: 10.1111/j.1530-0277.2007.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH. Models of depression. Vitam Horm. 2010;82:1–21. doi: 10.1016/S0083-6729(10)82001-1. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. Effects of interleukin-1 and endotoxin in the forced swim and tail suspension tests in mice. Pharmacol Biochem Behav. 2005;81:688–93. doi: 10.1016/j.pbb.2005.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. Effects of acute and chronic stressors and CRF in rat and mouse tests for depression. Ann N Y Acad Sci. 2008;1148:118–26. doi: 10.1196/annals.1410.022. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- File SE. NKP608, an NK1 receptor antagonist, has an anxiolytic action in the social interaction test in rats. Psychopharmacology (Berl) 2000;152:105–9. doi: 10.1007/s002130000513. [DOI] [PubMed] [Google Scholar]
- Fishkin RJ, Winslow JT. Endotoxin-induced reduction of social investigation by mice: interaction with amphetamine and anti-inflammatory drugs. Psychopharmacology (Berl) 1997;132:335–41. doi: 10.1007/s002130050353. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Briscoe RJ, Baird TJ, Vallett M, Carl KL, Holloway FA. Cross-generalization of an EtOH “hangover” cue to endogenously and exogenously induced stimuli. Pharmacol Biochem Behav. 1997;57:199–206. doi: 10.1016/s0091-3057(96)00310-3. [DOI] [PubMed] [Google Scholar]
- Genn RF, Tucci SA, Thomas A, Edwards JE, File SE. Age-associated sex differences in response to food deprivation in two animal tests of anxiety. Neurosci Biobehav Rev. 2003;27:155–61. doi: 10.1016/s0149-7634(03)00017-4. [DOI] [PubMed] [Google Scholar]
- Getachew B, Hauser SR, Taylor RE, Tizabi Y. Alcohol-induced depressive-like behavior is associated with cortical norepinephrine reduction. Pharmacol Biochem Behav. 2010;96:395–401. doi: 10.1016/j.pbb.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Quintela A, Dominguez-Santalla MJ, Perez LF, Vidal C, Lojo S, Barrio E. Influence of acute ethanol intake and alcohol withdrawal on circulating levels of IL-6, IL-8, IL-10 and IL-12. Cytokine. 2000;12:1437–40. doi: 10.1006/cyto.2000.0715. [DOI] [PubMed] [Google Scholar]
- Hale RL, Randall CL, Becker HC, Turner KP. Aspirin pretreatment reduces ethanol withdrawal severity in a mouse model of binge drinking. Pharmacol Biochem Behav. 1992;43:1169–73. doi: 10.1016/0091-3057(92)90499-6. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–37. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hayley S, Mangano E, Strickland M, Anisman H. Lipopolysaccharide and a social stressor influence behaviour, corticosterone and cytokine levels: divergent actions in cyclooxygenase-2 deficient mice and wild type controls. J Neuroimmunol. 2008;197:29–36. doi: 10.1016/j.jneuroim.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Hirani K, Khisti RT, Chopde CT. Behavioral action of ethanol in Porsolt's forced swim test: modulation by 3 alpha-hydroxy-5 alpha-pregnan-20-one. Neuropharmacology. 2002;43:1339–50. doi: 10.1016/s0028-3908(02)00330-1. [DOI] [PubMed] [Google Scholar]
- Huang QH, Hruby VJ, Tatro JB. Role of central melanocortins in endotoxin-induced anorexia. Am J Physiol. 1999;276:R864–71. doi: 10.1152/ajpregu.1999.276.3.R864. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Research, C. o. L. S., National Research Council . Guide for the Care and Use of Laboratory Animals. Washington, D.C.: 1996. [Google Scholar]
- Jung TW, Lee JY, Shim WS, Kang ES, Kim SK, Ahn CW, et al. Rosiglitazone relieves acute ethanol-induced hangover in Sprague-Dawley rats. Alcohol Alcohol. 2006;41:231–5. doi: 10.1093/alcalc/agl013. [DOI] [PubMed] [Google Scholar]
- Kaivola S, Parantainen J, Osterman T, Timonen H. Hangover headache and prostaglandins: prophylactic treatment with tolfenamic acid. Cephalalgia. 1983;3:31–6. doi: 10.1046/j.1468-2982.1983.0301031.x. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Dantzer R, Hardwick AJ, Kelley KW, Rothwell NJ, et al. Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proc Natl Acad Sci U S A. 1992;89:9117–20. doi: 10.1073/pnas.89.19.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Kim W, Yoon SJ, Choi BM, Kim JS, Go HJ, et al. Effects of alcohol hangover on cytokine production in healthy subjects. Alcohol. 2003;31:167–70. doi: 10.1016/j.alcohol.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Kitiyakara C, Welch WJ, Verbalis JG, Wilcox CS. Role of thromboxane receptors in the dipsogenic response to central angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2002;282:R865–9. doi: 10.1152/ajpregu.00328.2001. [DOI] [PubMed] [Google Scholar]
- Kokare DM, Singru PS, Dandekar MP, Chopde CT, Subhedar NK. Involvement of alpha-melanocyte stimulating hormone (alpha-MSH) in differential ethanol exposure and withdrawal related depression in rat: neuroanatomical-behavioral correlates. Brain Res. 2008;1216:53–67. doi: 10.1016/j.brainres.2008.03.064. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–9. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Veeneman J, Combe C, Poole S, Luheshi GN, Dantzer R. Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur J Neurosci. 2008;28:2499–510. doi: 10.1111/j.1460-9568.2008.06549.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–52. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob GF. Theoretical Frameworks and Mechanistic Aspects of Alcohol Addiction: Alcohol Addiction as a Reward Deficit Disorder. Curr Top Behav Neurosci. 2011 doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthorst AC, Flachskamm C, Muller-Preuss P, Holsboer F, Reul JM. Effect of bacterial endotoxin and interleukin-1 beta on hippocampal serotonergic neurotransmission, behavioral activity, and free corticosterone levels: an in vivo microdialysis study. J Neurosci. 1995;15:2920–34. doi: 10.1523/JNEUROSCI.15-04-02920.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–32. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Intracerebroventricular interleukin-1 receptor antagonist blocks the enhancement of fear conditioning and interference with escape produced by inescapable shock. Brain Res. 1995;695:279–82. doi: 10.1016/0006-8993(95)00930-o. [DOI] [PubMed] [Google Scholar]
- McKinney A. A review of the next day effects of alcohol on subjective mood ratings. Curr Drug Abuse Rev. 2010;3:88–91. doi: 10.2174/1874473711003020088. [DOI] [PubMed] [Google Scholar]
- McKinney A, Coyle K. Alcohol hangover effects on measures of affect the morning after a normal night's drinking. Alcohol Alcohol. 2006;41:54–60. doi: 10.1093/alcalc/agh226. [DOI] [PubMed] [Google Scholar]
- Mohanty P, Aljada A, Ghanim H, Hofmeyer D, Tripathy D, Syed T, et al. Evidence for a potent antiinflammatory effect of rosiglitazone. J Clin Endocrinol Metab. 2004;89:2728–35. doi: 10.1210/jc.2003-032103. [DOI] [PubMed] [Google Scholar]
- Morse AC, Schulteis G, Holloway FA, Koob GF. Conditioned place aversion to the “hangover” phase of acute ethanol administration in the rat. Alcohol. 2000;22:19–24. doi: 10.1016/s0741-8329(00)00099-9. [DOI] [PubMed] [Google Scholar]
- Nadjar A, Sauvant J, Combe C, Parnet P, Konsman JP. Brain cyclooxygenase-2 mediates interleukin-1-induced cellular activation in preoptic and arcuate hypothalamus, but not sickness symptoms. Neurobiol Dis. 2010;39:393–401. doi: 10.1016/j.nbd.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Otterness IG, Golden HW, Seymour PA, Eskra JD, Daumy GO. Role of prostaglandins in the behavioral changes induced by murine interleukin 1α in the rat. Cytokine. 1991;3:333–8. doi: 10.1016/1043-4666(91)90502-5. [DOI] [PubMed] [Google Scholar]
- Ozaki K, Leonard WJ. Cytokine and cytokine receptor pleiotropy and redundancy. J Biol Chem. 2002;277:29355–8. doi: 10.1074/jbc.R200003200. [DOI] [PubMed] [Google Scholar]
- Parantainen J. Prostaglandins in alcohol intolerance and hangover. Drug Alcohol Depend. 1983;11:239–48. doi: 10.1016/0376-8716(83)90016-9. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Robertson BM, Epler AJ. Hangover and risk for alcohol use disorders: existing evidence and potential mechanisms. Curr Drug Abuse Rev. 2010;3:92–102. doi: 10.2174/1874473711003020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plata-Salaman CR, Oomura Y, Kai Y. Tumor necrosis factor and interleukin-1 beta: suppression of food intake by direct action in the central nervous system. Brain Res. 1988;448:106–14. doi: 10.1016/0006-8993(88)91106-7. [DOI] [PubMed] [Google Scholar]
- Pollak Y, Ovadia H, Orion E, Yirmiya R. The EAE-associated behavioral syndrome: II. Modulation by anti-inflammatory treatments. J Neuroimmunol. 2003;137:100–8. doi: 10.1016/s0165-5728(03)00073-0. [DOI] [PubMed] [Google Scholar]
- Porsolt RD. Animal model of depression. Biomedicine. 1979;30:139–40. [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–91. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S, Rivier C. Influence of the paraventricular nucleus of the hypothalamus in the alteration of neuroendocrine functions induced by intermittent footshock or interleukin. Endocrinology. 1991;129:2049–57. doi: 10.1210/endo-129-4-2049. [DOI] [PubMed] [Google Scholar]
- Romeo E, Pompili E, di Michele F, Pace M, Rupprecht R, Bernardi G, et al. Effects of fluoxetine, indomethacine and placebo on 3 alpha, 5 alpha tetrahydroprogesterone (THP) plasma levels in uncomplicated alcohol withdrawal. World J Biol Psychiatry. 2000;1:101–4. doi: 10.3109/15622970009150572. [DOI] [PubMed] [Google Scholar]
- SAMHSA . Results from the 2006 National Survey on Drug Use and Health: National Findings. Office of Applied Studies; Rockville: NSDUH Series H-32 MD2007. [Google Scholar]
- SAMHSA . Results from the 2007 National Survey on Drug Use and Health: National Findings. Office of Applied Studies; Rockville: NSDUH Series H-34 MD2008. [Google Scholar]
- Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238:522–4. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Liu J. Brain reward deficits accompany withdrawal (hangover) from acute ethanol in rats. Alcohol. 2006;39:21–8. doi: 10.1016/j.alcohol.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarnick DJ, Cordasco DM, Rotrosen J. Prostanoid modulation (mediation?) of certain behavioral effects of ethanol. Pharmacol Biochem Behav. 1985;23:71–5. doi: 10.1016/0091-3057(85)90132-7. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Gustafsson K. Behavioral changes in rats on the day after acute ethanol intoxication. Alcohol. 1987;4:503–7. doi: 10.1016/0741-8329(87)90093-0. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Taira T. Hangover hyperthermia in rats: relation to tolerance and external stimuli. Psychopharmacology (Berl) 1988;94:161–6. doi: 10.1007/BF00176838. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Roth J. Comparison of the onset of hypoactivity and anxiety-like behavior during alcohol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2004;28:598–607. doi: 10.1097/01.alc.0000122767.69206.1b. [DOI] [PubMed] [Google Scholar]
- Smith CM, Barnes GM. Signs and symptoms of hangover: prevalence and relationship to alcohol use in a general adult population. Drug Alcohol Depend. 1983;11:249–69. doi: 10.1016/0376-8716(83)90017-0. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Dunn AJ. Effects of interleukin-1beta and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacol Biochem Behav. 2007;86:651–9. doi: 10.1016/j.pbb.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiergiel AH, Smagin GN, Johnson LJ, Dunn AJ. The role of cytokines in the behavioral responses to endotoxin and influenza virus infection in mice: effects of acute and chronic administration of the interleukin-1-receptor antagonist (IL-1ra). Brain Res. 1997;776:96–104. doi: 10.1016/s0006-8993(97)01009-3. [DOI] [PubMed] [Google Scholar]
- Swift R, Davidson D. Alcohol hangover: mechanisms and mediators. Alcohol Health Res World. 1998;22:54–60. [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol Clin Exp Res. 2004;28:40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- Walker BM, Drimmer DA, Walker JL, Liu T, Mathe AA, Ehlers CL. Effects of prolonged ethanol vapor exposure on forced swim behavior, and neuropeptide Y and corticotropin-releasing factor levels in rat brains. Alcohol. 2010;44:487–93. doi: 10.1016/j.alcohol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese JG, Shlipak MG, Browner WS. The alcohol hangover. Ann Intern Med. 2000;132:897–902. doi: 10.7326/0003-4819-132-11-200006060-00008. [DOI] [PubMed] [Google Scholar]
- Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–74. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Barak O, Avitsur R, Gallily R, Weidenfeld J. Intracerebral administration of Mycoplasma fermentans produces sickness behavior: role of prostaglandins. Brain Res. 1997;749:71–81. doi: 10.1016/s0006-8993(96)01295-4. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Rosen H, Donchin O, Ovadia H. Behavioral effects of lipopolysaccharide in rats: involvement of endogenous opioids. Brain Res. 1994;648:80–6. doi: 10.1016/0006-8993(94)91908-9. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Morse AC, Koob GF, Schulteis G. Dose- and time-dependent expression of anxiety-like behavior in the elevated plus-maze during withdrawal from acute and repeated intermittent ethanol intoxication in rats. Alcohol Clin Exp Res. 2007;31:1811–9. doi: 10.1111/j.1530-0277.2007.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]