Summary
Spontaneous activity in the sensory periphery drives infant brain activity and is thought to contribute to the formation of retinotopic and somatotopic maps [1–3]. In infant rats during active (or REM) sleep, brainstem-generated spontaneous activity triggers hundreds of thousands of skeletal muscle twitches each day [4]; sensory feedback arising from the resulting limb movements is a primary activator of forebrain activity, including spindle bursts in somatosensory cortex [1]. The rodent whisker system, with its precise isomorphic mapping of individual whiskers to discrete brain areas, has been a key contributor to our understanding of somatotopic maps and developmental plasticity [5–7]. However, in contrast with other sensory systems—and even though whisker movements are controlled by dedicated skeletal muscles [8, 9]—spontaneous whisker activity has not been entertained as a contributing factor to the development of this system [10]. Here we report in 3- to 6-day-old rats that whiskers twitch rapidly and asynchronously during active sleep. In addition, neurons in whisker thalamus exhibit bursts of activity that are tightly associated with twitches, but occur infrequently during waking. Finally, we observed barrel-specific cortical activity during periods of twitching. This is the first report of self-generated, sleep-related twitches in the developing whisker system, a sensorimotor system that is unique for the precision with which it can be experimentally manipulated. Therefore, the discovery of whisker twitching will allow us to attain a fuller understanding of the contributions of peripheral sensory activity to somatosensory integration and plasticity in the developing nervous system [11–13].
Results and Discussion
Newborn Rats Exhibit Whisker Twitches During Active Sleep
Previous studies of whisker movements in infant rats have focused primarily on the emergence of synchronized movements of the whiskers (i.e., whisking) in awake animals [14–16]. Because whisking does not develop until the end of the second postnatal week, self-generated whisker movements have been overlooked as possible contributors to the perinatal development of whisker system morphology or plasticity. Here we asked whether asynchronous whisker movements (i.e., twitching) occur during active sleep in 3- to 6-day-old rats (n=5), similar to the twitches that occur in other limbs controlled by skeletal muscles [17].
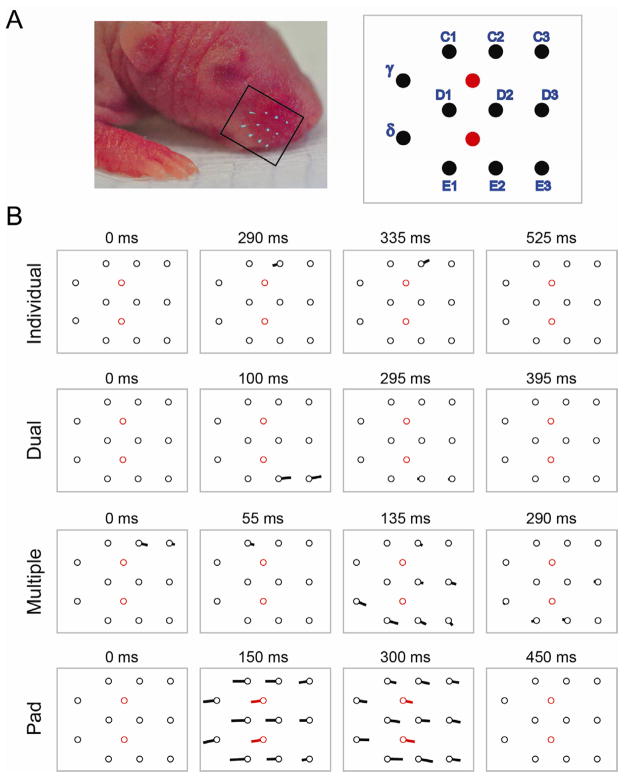
Using high-speed videography (200 frames/s), we assessed movements of the whiskers and mystacial pad as pups cycled between sleep and wakefulness (Figure 1A). During periods of active wakefulness when high-amplitude limb movements (e.g., kicking, stretching) were seen, whisker and mystacial pad movements were noisy and seemingly haphazard. All movements subsided during the transition from wakefulness to quiet sleep. Then, with the onset of active sleep as the distal limbs and tail began to twitch, contemporaneous bouts of whisker twitches were observed. We saw a diversity of whisker movements (Figure 1B; Movie S1), including independent twitches of single whiskers, simultaneous twitches by adjacent or non-adjacent whiskers, and complex movements comprising various subsets of whiskers moving in variable directions. Independent whisker twitches were easily distinguished from events involving pronounced movements of the mystacial pad in which all whiskers moved in unison. This is the first demonstration of whisker twitching in sleeping infant rats, although they have been noted anecdotally in sleeping adults [18].
Figure 1.
Diverse patterns of whisker movements during active sleep in 4–6-day-old rats. (A) Left: One side of the snout, highlighting 11 marked whiskers (boxed area). Right: Labels and relative locations of the 11 marked whiskers (black circles); also shown are the 2 markings on the skin surface of the mystacial pad (red circles). (B) Quiver plots depicting 4 types of whisker and mystacial pad movements observed using high-speed videography. The locations of the whiskers (black circles) and the mystacial pad markings (red circles) correspond to those in (A). The lines emanating from the circles are proportional to the whisker or pad displacement over the previous 25 ms (5 frames); the direction of movement is also indicated. The 4 patterns depicted correspond to Movie S1. See Figure S1 for additional examples of the diversity of whisker trajectories.
Working from a catalog of 51 whisker twitches, we calculated mean maximum whisker displacements from rest of 0.13 ± 0.01 mm (range: 0.02–0.27 mm) and mean angular velocities of 121 ± 7 deg/s (range: 34–255 deg/s). The mean latency from twitch onset to maximum displacement was 65.4 ± 3.4 ms (range: 30–150 ms). Although the majority of these whisker movements were in the protraction and retraction directions, movements in a diversity of other directions were also observed (Figure S1). The patterns of movements observed here are consistent with the known anatomy of the whisker muscle system [8, 9], as well as findings in adults from whisker muscle and facial nucleus stimulation studies [19, 20] and observations of adjacent whisker movements [21].
Extrinsic Whisker Muscles Twitch During Active Sleep
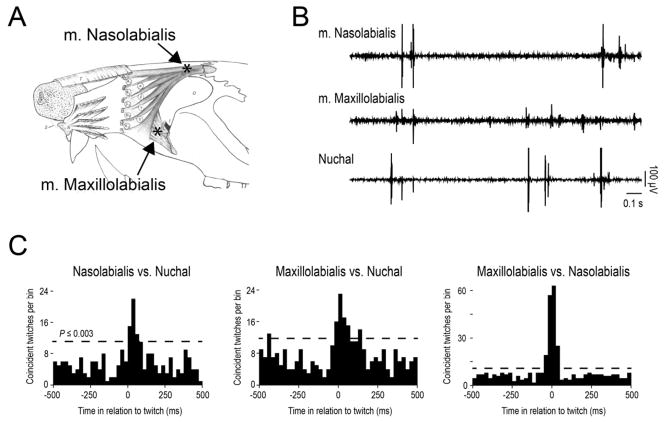
Whiskers are controlled by a complex system of extrinsic and intrinsic muscles [8, 9]. To ensure that the whisker twitches observed using high-speed videography were not due to movement artifact, we recorded electromyographic (EMG) activity from two extrinsic whisker muscles in 4- to 6-day-old rats (n=4), m. maxillolabialis and m. nasiolabialis (Figure 2A). We also recorded EMG activity from the nuchal muscle, the primary elevator of the head, which provides a reliable measure of behavioral state [22]. Both extrinsic whisker muscles twitched during periods of active sleep when twitches occurred in the nuchal muscle (Figure 2B). Twitching in nuchal and extrinsic muscles exhibited strong and significant cross-correlations (Figure 2C). Similar cross-correlations were observed in the three other subjects. In general, these extrinsic whisker muscles exhibited sleep-wake profiles and patterns of twitching similar to those found in other skeletal muscles [23, 24].
Figure 2.
Extrinsic whisker muscles twitch during active sleep. (A) Locations of EMG electrodes (black asterisks) in m. maxillolabialis and m. nasolabialis (also referred to as m. levator labii superioris in [8], from which this drawing is adapted). (B) Raw EMG record depicting twitching in the two extrinsic muscles as well as the nuchal muscle. Sharp spikes in the EMG records indicate myoclonic twitches. (C) Cross-correlograms showing highly correlated bouts of twitch activity for each pair of muscles. The horizontal dashed line indicates statistical significance.
Whisker Thalamus Exhibits Twitch-Dependent Activity
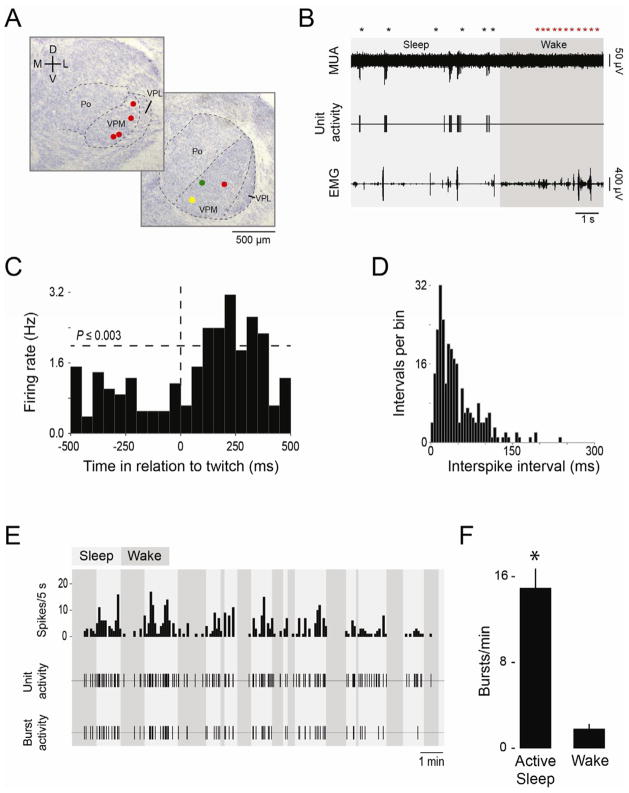
Sensory feedback from twitching limbs increases neural activity in the somatosensory thalamus and cortex of infant rats [1], as well as hippocampus [24]. Moreover, in the whisker system, thalamic and cortical mechanisms are responsive to mechanical whisker stimulation soon after birth [25, 26]. We found that whisker twitches result in sensory feedback to the ventral posteromedial nucleus (VPM), a primary thalamic input of the whisker system. Overall, 7 VPM units were isolated from 3- to 6-day-old rats (n=7) (Figure 3A). For one representative unit (Figure 3B–E), firing rate increased during periods of active sleep (Figure 3B) and exhibited a significant increase in firing rate 100 ms after a twitch, peaking at approximately 250 ms (Figure 3C). Similarly significant relationships between unit activity and twitching were documented in 5 other VPM units (one subject’s EMG record was too noisy for this analysis).
Figure 3.
VPM neurons fire in bursts in close proximity to sleep-related twitching. (A) Coronal sections through the thalamus to show the locations recording sites. VPM, ventral posteromedial thalamus; VPL, ventral posterolateral thalamus; Po, posterior thalamus. (B) Representative data depicting multiunit activity (MUA), single unit activity, and nuchal EMG. Twitch and wake movements (black and red asterisks, respectively) are also shown. Recording site is the yellow circle in (A). (C) Perievent histogram for the VPM unit. Vertical line at 0 ms denotes time of nuchal muscle twitch and horizontal line indicates statistical significance. The twitch-following properties of another VPM unit were further investigated by anesthetizing the whisker pad (see Figure S2). (D) Frequency histogram of interspike intervals (ISI) for the same VPM unit. (E) VPM unit firing rate (spikes/5 s), unit activity, and bursts activity over a 15-min recording session. (F) Mean (+SE) bursts/min during active sleep and wake across all 7 subjects. * significant difference from wake, P<0.001.
All 7 VPM units exhibited bursts of neural activity. We used frequency histograms of interspike interval (ISI; Figure 3D) to define a VPM burst as the occurrence of 2 or more spikes with ISIs ≤150 ms. Across all subjects, the number of spikes per burst varied widely and, for some subjects, more than 10 spikes per burst was not uncommon. Bursts predominated during sleep (Figure 3E). Moreover, their rate of occurrence was significantly higher during active sleep both within each subject individually (Ps<0.05) as well as across all subjects (t6=7.4, P<0.001; Figure 3F). The state-dependent thalamic bursting observed here is consistent with previous observations in neonatal cortex and hippocampus [1, 24] and may reflect the action of corollary discharge mechanisms [27, 28]. (This thalamic bursting should not be conflated with that observed in awake adult rats during so-called “whisker twitching,” defined in that study as rhythmic 7–12 Hz whisker movements [29].)
The temporal relationship between whisker twitching and VPM activity is similar to that reported previously between limb twitching and forebrain activity, for which a causal role for twitching has been established [1, 24]. We were able to assess causality between whisker twitching and thalamic activity in one subject before, during, and after peripheral sensory blockade while recording a twitch-dependent VPM unit (Figure S2). Within 11 min of lidocaine (1%) injection into the mystacial pad, the rate of VPM bursts declined substantially as sleep-related twitching continued, consistent with peripheral sensory blockade [30]. As the effects of the lidocaine dissipated, the rate of VPM bursts returned to pre-injection levels. Therefore, whisker twitches can drive VPM activity during active sleep.
Barrel Cortex Exhibits Spontaneous Activity During Active Sleep
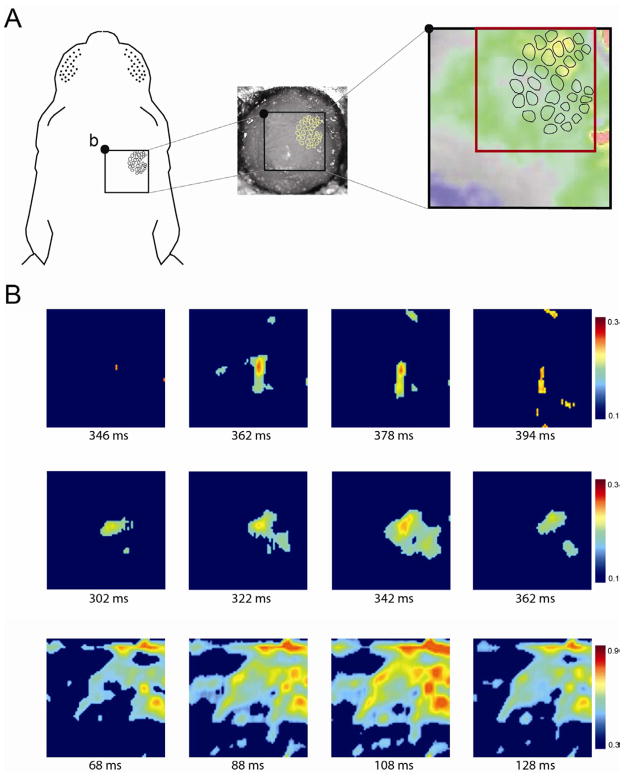
As early as the day of birth, VPM activity is sufficient to activate barrel cortex in a precise topographic manner [26]. In light of the finding above that VPM units increase their firing rate within 100 ms of a twitch, we predicted that periods of twitching would also be accompanied by barrel-specific activation patterns. We used voltage-sensitive dye (VSD) imaging to monitor barrel cortex activity during bouts of active sleep (Figure 4A). We imaged 3 subjects that exhibited significant contralateral VSD responses to whisker stimulation (Figure S3A). In each subject during active sleep, we identified the first 10 events in which there was EMG evidence of a twitch along with behavioral confirmation of twitching. We next examined barrel activity within a 500 ms window of each EMG-defined twitch. In total, 26/30 (86.7%) of these twitches were followed by clear barrel activity. Varying levels of cortical activation were found, from single to multiple barrels (Figure 4B; Movie S2), mirroring the diversity of whisker movements observed using high-speed videography.
Figure 4.
Voltage-sensitive dye (VSD) imaging of barrel cortex activity during active sleep in 4-day-old rats. (A) The experimental set-up for VSD imaging, highlighting the 4 × 4 mm cranial window (black box) to expose the right barrel cortical field. The 2.5 × 2.5 mm region of interest (ROI) is outlined by the red box. b, bregma. See Figure S3 for methods pertaining to the VSDI procedure. (B) Three representative samples of barrel cortex activity, from isolated activity within 1 or 2 barrels (top two rows) to global activity across the barrel field (bottom row). Each image corresponds to the ROI in (A). The number beneath each frame denotes time from the initiation of a nuchal muscle twitch. Color bars at the right of each sequence indicate range of values of dF/F0. For clarity of presentation, obvious instances of noise were removed manually from some VSD images.
Conclusions
The rodent whisker system affords many advantages to investigators interested in understanding the mechanisms that give rise to somatotopic maps, sensorimotor integration, and developmental plasticity [5, 11–13]. After several decades of research, however, important questions remain as to the instructive role played by the sensory periphery in early development. The present findings suggest that past assessments of the importance of the sensory periphery for the developing whisker system were handicapped by incomplete knowledge regarding the sources and timing of sensory input. Thus, contrary to the view that, in infants before the onset of whisking, whisker stimulation only arises from passive interactions with the mother and littermates [10, 12], we have shown here that self-generated, asynchronous whisker twitches drive brain activity during active sleep in a manner that is strikingly similar to the brain activation produced by twitches elsewhere in the body [1, 17, 24].
We focused here on whisker twitching in 3- to 6-day-old rats, an age when thalamocortical projections to layer 4 are established, when the sensitive period for structural cortical plasticity is ending, but when other aspects of cortical plasticity remain [5, 12, 13, 31]. Given that limb twitches occur in utero and exhibit continuity with postnatal twitching [32], it is likely that whisker twitches also commence in utero and therefore may influence subcortical and cortical development in various ways throughout early prenatal and postnatal life.
As with retinal waves [33], the developmental and spatiotemporal features of twitching may provide clues to its functions. For example, a twitch is characterized by discrete motor output, precisely timed sensory feedback, and high signal-to-noise ratio afforded by the background of muscle atonia [17]. These characteristics may make twitches better suited than other forms of motor activity (such as that produced during waking) to produce the isomorphic mapping and multilevel sensorimotor integration that characterizes the whisker system [34–36]. Also, because whisker twitches are diverse in form and direction (see Figure 1B, Figure S1, Movie S1), they provide a range of experiences beyond that provided by whisking, which is largely limited to synchronous whisker movements along the protraction-retraction axis [19]. Accordingly, it is tempting to suggest that this diversity of whisker movements during twitching aids in establishing the “pinwheel” map of whisker directional movement [37, 38]. These and perhaps other features of whisker twitch movements, which predominate during the active-sleep-rich period of early infancy [39], may ultimately help us to better understand the functional value of that sleep state for the developing infant [17].
Experimental Procedures
All surgeries were performed under isoflurane anesthesia and all recording was performed in unanesthetized subjects. For analysis of whisker movements (n=5), whiskers were trimmed to 1 mm and the tips were marked with fluorescent paint for recording under ultraviolet-light illumination. A high-speed digital video camera (IDT, Tallahassee, FL) recorded whisker movements, which were tracked and analyzed offline using ProAnalyst software (Xcitex, Cambridge, MA). EMG activity in two extrinsic whisker muscles and nuchal muscle (n=4) was recorded and analyzed using established methods [19, 24]. For recording of neural activity in the VPM of head-fixed pups (n=7), the apparatus, methods, and analyses have been described previously [4, 24]. Twitch-triggered perievent histograms were constructed and a randomization procedure was used to test the relationship between VPM activity and twitching [24]. State-related differences in mean VPM burst rates were tested within subjects (Wilcoxon matched-pairs signed-ranks test) and between subjects (paired t test). For voltage-sensitive dye (VSD) imaging of barrel cortex (n=3), pups were prepared and recorded similarly as above with the addition of a unilateral craniotomy over the right hemisphere (Figure 4A). A voltage-sensitive dye (RH1838; Optical Imaging, Inc., Rehovot, Israel) was applied topically to the dura and a window was created for imaging (MiCAM Ultima, Scimedia, Costa Mesa, CA). Imaging trials consisted of contralateral and ipsilateral whisker stimulations (Figure S3A) and spontaneous behavior. dF/F0 was calculated and analyzed offline using custom-written scripts in MATLAB (Math Works, Natick, MA) and procedures similar to those described previously [26].
Supplementary Material
Highlights.
Whiskers twitch rapidly and asynchronously in sleeping infant rats.
Whisker twitches result from activation of dedicated whisker skeletal muscles.
Sensory feedback from whisker twitches produces bursts of neural activity in whisker thalamus.
Barrel cortex exhibits discrete activation during periods of twitching.
Acknowledgments
For technical support and guidance we thank Bob McMurray, Cassie Coleman, Ashlynn Gerth, William Todd, Josh Weiner, Jian-Young Wu, and Weifeng Xu. Jenq-Wei Yang and Heiko Luhmann generously provided their custom-written VSDI analysis software. This work was supported by grants from the National Institutes of Health (HD63071, MH66424) to M.S.B.
Footnotes
Supplemental Information includes three figures, two movies, and Supplemental Experimental Procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsáki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- 2.Hanganu IL, Ben-Ari Y, Khazipov R. Retinal waves trigger spindle bursts in the neonatal rat visual cortex. J Neurosci. 2006;26:6728–6736. doi: 10.1523/JNEUROSCI.0752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohns EJ, Blumberg MS. Synchronous bursts of neuronal activity in the developing hippocampus: Modulation by active sleep and association with emerging gamma and theta rhythms. J Neurosci. 2008;28:10134–10144. doi: 10.1523/JNEUROSCI.1967-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcano-Reik AJ, Prasad T, Weiner JA, Blumberg MS. An abrupt developmental shift in callosal modulation of sleep-related spindle bursts coincides with the emergence of excitatory-inhibitory balance and a reduction of somatosensory cortical plasticity. Behav Neurosci. 2010;124:600. doi: 10.1037/a0020774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- 6.Killackey HP, Rhoades RW, Bennett-Clarke CA. The formation of a cortical somatotopic map. Trends Neurosci. 1995;18:402–407. doi: 10.1016/0166-2236(95)93937-s. [DOI] [PubMed] [Google Scholar]
- 7.Petersen CCH. The functional organization of the barrel cortex. Neuron. 2007;56:339–355. doi: 10.1016/j.neuron.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Dörfl J. The musculature of the mystacial vibrissae of the white mouse. J Anat. 1982;135:147–154. [PMC free article] [PubMed] [Google Scholar]
- 9.Wineski LE. Facial morphology and vibrissal movement in the Golden hamster. J Morphol. 1985;183:199–217. doi: 10.1002/jmor.1051830208. [DOI] [PubMed] [Google Scholar]
- 10.Hanganu-Opatz IL. Between molecules and experience: Role of early patterns of coordinated activity for the development of cortical maps and sensory abilities. Brain Res Rev. 2010;64:160–176. doi: 10.1016/j.brainresrev.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Crair MC. How do barrels form in somatosensory cortex? Ann N Y Acad Sci. 2011;1225:119–129. doi: 10.1111/j.1749-6632.2011.06024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erzurumlu RS, Gaspar P. Development and critical period plasticity of the barrel cortex. Eur J Neurosci. 2012;35:1540–1553. doi: 10.1111/j.1460-9568.2012.08075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu CS, Ballester Rosado CJ, Lu HC. What can we get from “barrels”: the rodent barrel cortex as a model for studying the establishment of neural circuits. Eur J Neurosci. 2011;34:1663–1676. doi: 10.1111/j.1460-9568.2011.07892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landers M, Zeigler HP. Development of rodent whisking: trigeminal input and central pattern generation. Somatosens Mot Res. 2006;23:1–10. doi: 10.1080/08990220600700768. [DOI] [PubMed] [Google Scholar]
- 15.Grant RA, Mitchinson B, Prescott TJ. The development of whisker control in rats in relation to locomotion. Dev Psychobiol. 2012;54:151–168. doi: 10.1002/dev.20591. [DOI] [PubMed] [Google Scholar]
- 16.Welker WI. Analysis of sniffing of the albino rat. Behaviour. 1964;22:223–244. [Google Scholar]
- 17.Blumberg MS. Beyond dreams: Do sleep-related movements contribute to brain development? Front Neurol. 2010;1:140. doi: 10.3389/fneur.2010.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- 19.Hill DN, Bermejo R, Zeigler HP, Kleinfeld D. Biomechanics of the vibrissa motor plant in rat: rhythmic whisking consists of triphasic neuromuscular activity. J Neurosci. 2008;28:3438–3455. doi: 10.1523/JNEUROSCI.5008-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herfst LJ, Brecht M. Whisker movements evoked by stimulation of single motor neurons in the facial nucleus of the rat. J Neurophysiol. 2008;99:2821–2832. doi: 10.1152/jn.01014.2007. [DOI] [PubMed] [Google Scholar]
- 21.Sachdev RNS, Sato T, Ebner FF. Divergent movement of adjacent whiskers. J Neurophysiol. 2002;87:1440–1448. doi: 10.1152/jn.00539.2001. [DOI] [PubMed] [Google Scholar]
- 22.Seelke AMH, Blumberg MS. Developmental appearance and disappearance of cortical events and oscillations in infant rats. Brain Res. 2010;1324:34–42. doi: 10.1016/j.brainres.2010.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seelke AMH, Karlsson KÆ, Gall A, Blumberg MS. Extraocular muscle activity, rapid eye movements and the development of active and quiet sleep. Eur J Neurosci. 2005;22:911–920. doi: 10.1111/j.1460-9568.2005.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohns EJ, Blumberg MS. Neocortical activation of the hippocampus during sleep in newborn rats. J Neurosci. 2010;30:3438–3449. doi: 10.1523/JNEUROSCI.4832-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minlebaev M, Ben-Ari Y, Khazipov R. Network mechanisms of spindle-burst oscillations in the neonatal rat barrel cortex in vivo. J Neurophysiol. 2007;97:692–700. doi: 10.1152/jn.00759.2006. [DOI] [PubMed] [Google Scholar]
- 26.Yang JW, An S, Sun JJ, Reyes-Puerta V, Kindler J, Berger T, Kilb W, Luhmann HJ. Thalamic network oscillations synchronize ontogenetic columns in the newborn rat barrel cortex. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs103. [DOI] [PubMed] [Google Scholar]
- 27.Crapse TB, Sommer MA. Corollary discharge across the animal kingdom. Nat Rev Neurosci. 2008;9:587–600. doi: 10.1038/nrn2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fee MS, Mitra PP, Kleinfeld D. Central versus peripheral determinants of patterned spike activity in rat vibrissa cortex during whisking. J Neurophysiol. 1997;78:1144–1149. doi: 10.1152/jn.1997.78.2.1144. [DOI] [PubMed] [Google Scholar]
- 29.Fanselow EE, Sameshima K, Baccala LA, Nicolelis MAL. Thalamic bursting in rats during different awake behavioral states. Proc Natl Acad Sci USA. 2001;98:15330–15335. doi: 10.1073/pnas.261273898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang JW, Hanganu-Opatz IL, Sun JJ, Luhmann HJ. Three patterns of oscillatory activity differentially synchronize developing neocortical networks in vivo. J Neurosci. 2009;29:9011–9025. doi: 10.1523/JNEUROSCI.5646-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox K. Anatomical pathways and molecular mechanisms for plasticity in the barrel cortex. Neurosci. 2002;111:799–814. doi: 10.1016/s0306-4522(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 32.Robinson S, Blumberg MS, Lane MS, Kreber L. Spontaneous motor activity in fetal and infant rats is organized into discrete multilimb bouts. Behav Neurosci. 2000;14:328–336. doi: 10.1037//0735-7044.114.2.328. [DOI] [PubMed] [Google Scholar]
- 33.Wong R. Retinal waves and visual system development. Annu Rev Neurosci. 1999;22:29–47. doi: 10.1146/annurev.neuro.22.1.29. [DOI] [PubMed] [Google Scholar]
- 34.Diamond ME, von Heimendahl M, Knutsen PM, Kleinfeld D, Ahissar E. ‘Where’ and “what” in the whisker sensorimotor system. Nat Rev Neurosci. 2008;9:601–612. doi: 10.1038/nrn2411. [DOI] [PubMed] [Google Scholar]
- 35.Ferezou I, Haiss F, Gentet LJ, Aronoff R, Weber B, Petersen CCH. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron. 2007;56:907–923. doi: 10.1016/j.neuron.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Matyas F, Sreenivasan V, Marbach F, Wacongne C, Barsy B, Mateo C, Aronoff R, Petersen CCH. Motor control by sensory cortex. Science. 2010;330:1240–1243. doi: 10.1126/science.1195797. [DOI] [PubMed] [Google Scholar]
- 37.Andermann ML, Moore CI. A somatotopic map of vibrissa motion direction within a barrel column. Nat Neurosci. 2006;9:543–551. doi: 10.1038/nn1671. [DOI] [PubMed] [Google Scholar]
- 38.Bruno RM, Khatri V, Land PW, Simons DJ. Thalamocortical angular tuning domains within individual barrels of rat somatosensory cortex. J Neurosci. 2003;23:9565–9574. doi: 10.1523/JNEUROSCI.23-29-09565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.