Abstract
Background
HIV infections increased 48% among young, Black men who have sex with men (MSM) in the United States between 2006–2009. Incomplete understanding of this trend undermines prevention strategy development. We investigated a sexual network to characterize the risk environment in which young, Black MSM acquire HIV.
Methods
Persons reported to the state following diagnosis of HIV or syphilis were included, along with sexual partners. We used network mapping alongside descriptive and bivariate statistics to characterize network connections. Generalized linear models assessed predictors of having untraceable sex partners.
Results
The network included 398 individuals and 419 sexual relationships. Three-quarters were Black (n=299); 92% were MSM. Median age at first network appearance was 26 years and decreased over time (P<0.001). HIV prevalence was at least 29% (n=117); serostatus was unknown for 47% of the network, either because they were untraceable (n=150) or refused HIV testing (n=39). One in 5 network members diagnosed with HIV had a subsequent incident sexually transmitted infection. In multivariable models, one-time encounters increased the risk of having an untraceable partner (risk ratio 4.51, 95% CI, 2.27, 8.97), while being acutely HIV infected at diagnosis reduced it (RR 0.27, 95% CI, 0.08, 0.89).
Conclusions
HIV prevalence in this sexual network of young, Black MSM rivals that of sub-Saharan Africa, reflecting dramatically increased risk of acquiring HIV from the moment one entered the network. Prevention efforts for this population must consider the effect of sexual networks on HIV risk, and find ways of leveraging network structure to reduce transmission.
Keywords: HIV, African-American, men who have sex with men, sexual networks
INTRODUCTION
Thirty years since its first cases were reported,1 HIV remains a major global health problem. Though public consciousness of HIV has shifted towards the developing world, the epidemic in the United States (U.S.) continues to expand. In 2011, the Centers for Disease Control and Prevention (CDC) released new HIV incidence estimates for 2006–2009. The stable annual rate of approximately 50,000 new infections belies an uncomfortable finding: while the pace of infections amongst most risk groups has remained static or declined, estimated incidence among young, Black men who have sex with men (MSM) rose 48% across the four-year period.2
North Carolina (NC) has experienced this trend first-hand. A 2005 outbreak of HIV among young, Black MSM college students raised awareness and prompted a reappraisal of existing prevention and testing messages.3 Despite successful efforts to engage at-risk Black MSM on local levels,4 large numbers of Black men continue to acquire HIV infection, statewide. In 2009, the rate of new diagnoses among persons aged 13 and older in NC was 22.5 cases per 100,000 population, while the rate among Black men was nearly 5-fold higher, at 106.3 cases per 100,000.5 Among Black men, 72% of cases were attributable to sex between men.6 These stark numbers underscore the need for more effective strategies to prevent HIV from spreading among those at greatest risk.
Here, we present our retrospective investigation of an expansive sexual network in NC, predominantly made up of young, Black MSM. Using public health data collected for sexual partner contact tracing around cases of HIV and syphilis, we sought to characterize the risk environment in which Black MSM are becoming HIV-infected in NC. Our findings provide context missing from the recent CDC report, and permit a reassessment of prevention needs in this population.
METHODS
Initial investigations centered around two Black MSM, aged 23 and 24, diagnosed with acute HIV infection (AHI) in October and November 2009, respectively. We define AHI as a combination of a positive HIV RNA with either a non-reactive enzyme immunoassay or a negative or indeterminate Western blot. NC’s program for identifying AHI using nucleic acid testing on pooled plasma specimens has been previously described.7,8 Both men had multiple contacts with previous AHI cases already engaged in care, and had an HIV-uninfected sex partner in common. These clients were the initial “nodes” of the network. The University of North Carolina at Chapel Hill’s institutional review board approved the study concept and protocol.
Behavioral Risk Information
In NC, a mandatory, confidential, name-based system is used to report diagnoses of syphilis or HIV to the Department of Health and Human Services (NC-DHHS).9 Each case is assigned to an officer of NC-DHHS’s Partner Counseling and Referral Service (PCRS), who conducts voluntary interviews with each client and collects standardized information. Risk behaviors in the prior year are recorded, with special attention paid to the 8-week interval prior to diagnosis for AHI cases. Both traditional (e.g., injection drug use) and non-traditional (e.g., Internet sex-seeking) risks are recorded, along with all identifying information available for partners. PCRS officers then contact the client’s partners, provide risk-reduction counseling, and offer either voluntary testing in the field or referral to a clinic for testing services. All infected partners are handled as additional cases, but contacts to uninfected partners are not investigated. Data are entered into a secured, centralized information management system. AHI cases are reviewed twice monthly, and details are discussed to explore epidemiological links among cases.
Network Linkages and Data Management
One study team member experienced with PCRS records used standardized forms to retrospectively abstract demographic and sexual health data for each PCRS client and up to 20 sexual partners. Characteristics of each partnership or “dyad” were also recorded. For partners that PCRS was unable to locate, the client’s description of the partner’s characteristics was used. No personal identifiers were abstracted. Instead, clients were assigned unique study identification numbers to assist with de-duplication. Individual-level demographic data included year of birth, gender, race/ethnicity, and sexual identity; sexual health data included available testing and diagnosis history for HIV and sexually transmitted infections (STIs).
Dyadic information for each reported sexual relationship included dates the partnership began and ended; the frequency of encounters; types of sexual activity with each partner; condom utilization; and where the client and partner met. To assess whether or not relationships overlapped with periods of heightened infectivity around the time of an AHI diagnosis,10,11 we constructed a window surrounding the date of each AHI client’s first positive test result, beginning 28 days prior and lasting 90 days thereafter.
Clients were appended to the network until a censoring date of August 1, 2010, or until no additional partner data existed in NC-DHHS records, whichever was earlier. Diagrams of the network’s structure were produced using NetDraw version 2.089 and UCINET version 6.232 (Analytic Technologies, Lexington, Kentucky).
Statistical Analyses
Demographic and sexual health data were assessed with descriptive statistics. Bivariate analyses permitted us to characterize differences among HIV-infected, uninfected, and serostatus-unknown clients. Pearson’s χ2 test determined associations between categorical variables, with Fisher’s exact test employed where appropriate. Differences among continuous variables were assessed with Wilcoxon rank-sum testing. We used a case-control analysis to determine which client, partner, or relationship characteristics were associated with having an untraceable sex partner, defined as having insufficient identifying information for the partner to be located by PCRS. Each sexual dyad served as a separate observation for this analysis, with client, partner, and relationship characteristics as covariates. Up to 20 dyads per client were included. Strengths of associations between each factor of interest and the outcome were assessed with log-linked bivariate generalized linear models (GLM) and statistically significant associations were included in multivariate GLM analyses. Robust standard errors were calculated using an adjustment for clustering in all models, since each client could be represented in multiple dyads. Significance was set at α=0.05 for all analyses, and all statistical testing utilized Stata/IC version 11.2 (StataCorp LP, College Station, Texas).
RESULTS
The final network included 365 men and 33 women; three quarters were Black (n=299). Across all network members, the median age at the initial PCRS interview was 26 years (range, 16 to 56); this decreased significantly over time, from 35.5 in 1995 to 24 in 2010 (p<0.001 for trend). A similar trend was noted for the 29% of the network that was HIV-infected (2 women, 115 men); median age at diagnosis fell from 36.5 in 1993 to 27 in 2010 (p<0.001). Overall, 23% of the network was confirmed to be HIV-uninfected (n=92). Serostatus could not be determined for 47% of the network (n=189), either because PCRS was unable to trace them (n=150) or they refused HIV testing (n=39). Fifty-eight percent of the network self-identified as MSM (217 MSM and 13 MSM who also reported sex with women), but when we included untraceable male partners who had sex with a male client (n=135), the proportion of MSM increased to 92%. Among the 209 clients for whom HIV status was known (Table 1), the majority were less than 30 years old (78%); male (96%); Black (97%); and self-identified as MSM (90%). There were no significant differences in any characteristic between HIV-infected and uninfected clients.
Table 1.
Characteristics of Clients with Confirmed HIV Status
| Number (%) of Subjects or Median (IQR)† | ||||
|---|---|---|---|---|
| Characteristics* (No. Responding) | Total (N=209) | HIV-Infected (n=117) | Uninfected (n=92) | P-value‡ |
| Age in years (n=208) | 25 (22–28.5) | 26 (23–31) | 25 (21–28) | 0.13 |
| <20 | 18 (8.7) | 7 (6.0) | 11 (12.1) | 0.41 |
| 20–29 | 144 (69.2) | 75 (64.1) | 69 (75.8) | |
| 30–39 | 36 (17.3) | 30 (25.6) | 6 (6.6) | |
| 40–49 | 9 (4.3) | 5 (4.3) | 4 (4.4) | |
| ≥50 | 1 (0.5) | 0 | 0 | |
| Gender (n=209) | ||||
| Female | 9 (4.3) | 2 (1.7) | 7 (7.6) | 0.05 |
| Male | 200 (95.7) | 115 (98.3) | 85 (92.4) | |
| Race/Ethnicity (n=208) | ||||
| Black | 202 (97.1) | 111 (94.9) | 91 (100) | 0.20 |
| White | 3 (1.4) | 3 (2.6) | 0 | |
| Hispanic | 2 (1.0) | 2 (1.7) | 0 | |
| Asian | 1 (0.5) | 1 (0.9) | 0 | |
| Sexual orientation (n=208) | ||||
| Homosexual | 187 (89.9) | 103 (88.0) | 84 (92.3) | 0.55 |
| Bisexual | 20 (9.6) | 13 (11.1) | 7 (7.7) | |
| Heterosexual | 1 (0.5) | 1 (0.9) | 0 | |
| Employed (n=65) | 52 (80.0) | 38 (79.2) | 14 (82.4) | 1.00 |
| College or university student (n=67) | 13 (19.4) | 8 (16.3) | 5 (27.8) | 0.31 |
A total of 209 subjects with confirmed human immunodeficiency virus (HIV) status were included. The number of respondents with data for each item varied.
IQR indicates interquartile range.
Wilcoxon rank-sum testing was used to compare medians. Pearson’s χ2 test was used for categorical variables, with Fisher’s exact test employed where appropriate. P-values <0.05 were considered statistically significant.
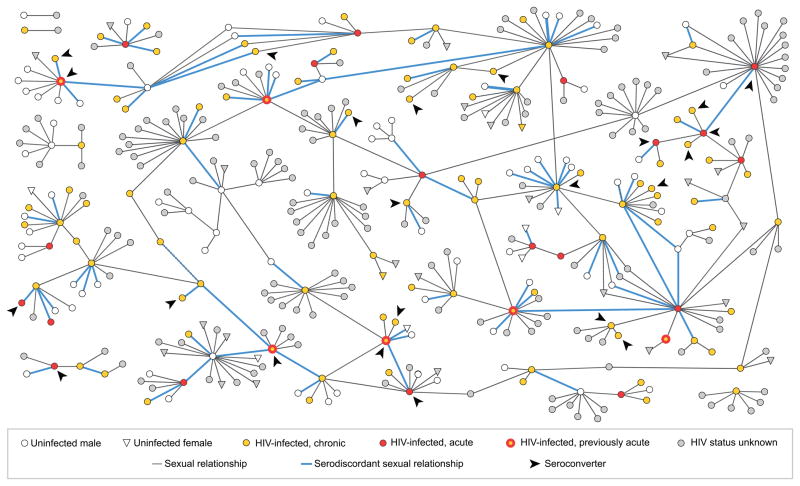
Figure 1 depicts the 398 network members and their sexual relationships. The network’s final structure consisted of seven components, the largest of which contained 363 members (91% of the entire network). Sequential figures depicting sexual relationships, incident STIs, and HIV seroconversions over time can be found in Supplementary Digital Content. Twenty-four individuals acquired HIV after initially appearing as uninfected contacts to known cases of HIV or syphilis, following a median of 1 year in the network (interquartile range [IQR], 0–3 years). Of these seroconverters (arrowheads), 14 were found to have established infection at the time of diagnosis (yellow) and 10 had AHI (red). Overall, 26 clients (22% of known HIV-infected persons) were diagnosed with AHI; all were detected through our statewide pooled nucleic acid screening program.7,8
Figure 1.
A Sexual Network of Black Men Who Have Sex with Men, North Carolina, 1989–2010
The final network consisted of 365 men (circles) and 33 women (inverted triangles). The seven separate components of the network are shown; the largest contains 363 nodes, or 91% of all network members. Of the 117 HIV-infected clients, 24 became HIV-infected after initially appearing in the network as uninfected contacts to known cases of HIV or syphilis (arrowheads); 10 were diagnosed with acute HIV (red symbols) and 14 with established, chronic HIV (yellow symbols). Thick blue lines indicate the 82 confirmed relationships in which one partner was HIV-infected and the other uninfected at the time of sex.
Indicators of sexual risk activity were frequently observed among HIV-infected clients following their diagnosis. Twenty-five of the 117 network members with HIV (21%) had an incident STI reported to the state following the date of their HIV diagnosis, after a median of 1.91 years (interquartile range, IQR, 1.08–2.85); all were MSM. Syphilis accounted for most of these STIs, with 28 cases diagnosed among 22 individuals; the other 3 men had gonorrhea.
Of the 419 dyads in the network, there were only 27 in which the race of the client and partner differed (6%). We noted wide variation in partnership duration, with a median of 92 days (IQR, 2–365). Over half of all relationships had occurred recently, between 2008–2010 (n=226, 54%), with another 42% distributed between 2003–2007 (n=177).
As shown in blue lines in Figure 1 and boldface in Table 2, there were 82 serodiscordant dyads, in which the HIV status of client and sex partner was confirmed to differ; 20% involved clients with AHI. We identified 18 relationships in which clients with AHI named sex partners who were HIV-infected, suggesting possible donor-recipient pairs. Approximately 40% of dyads involved chronically infected clients (n=167), again reflecting ongoing sexual activity among HIV-infected persons following diagnosis. One in five relationships linked HIV-infected clients to partners whose status could not be evaluated (n=96). In 40 dyads, it was not possible to determine clients’ serostatus at the time of sexual activity, since diagnoses of established HIV infection were made after the relationship ended and no prior testing was on record.
Table 2.
Opportunities for Sexual Transmission of HIV across All Dyads
| Partner Serostatus at Time of Sex* | ||||
|---|---|---|---|---|
| Client Serostatus at Time of Sex | Uninfected | Infected | Unknown | Total |
| Uninfected | 66 | 21 | 82 | 169 |
| Infected, acute† | 16 | 18 | 9 | 43 |
| Infected, chronic‡ | 45 | 35 | 87 | 167 |
| Possibly infected§ | 16 | 6 | 18 | 40 |
| Total | 143 | 80 | 196 | 419 |
Underlined numbers indicate relationships where no opportunity for HIV transmission occurred. Boldfaced numbers designate the number of partnerships in which the serostatus was confirmed to be discordant. Partners with unknown serostatus include both those who were untraceable by PCRS and those who were identifiable but refused HIV testing.
A window period for acute HIV infection was defined from 28 days preceding the date of diagnosis to 90 days thereafter. Sexual relationships that occurred during this window were counted as having had exposure to an acutely HIV infected client.
To be counted as a chronic infection at the time of the sexual relationship, a client had to be diagnosed with HIV infection (seronegative or seropositive) more than 90 days prior to the starting date of the relationship.
“Possibly infected” refers to clients with fully reactive enzyme immunoassay and Western blot HIV testing results following the completion of a partnership. In these situations, it was not possible to accurately determine what the client’s serostatus was at the time of the sexual relationship.
We compared characteristics of dyads featuring traceable versus untraceable partners, and found several significant differences (Table 3). Clients with untraceable partners more often reported encounters involving anal intercourse as the only sexual activity (P=0.01), whereas when both oral and anal sex occurred, partners were more often identifiable by PCRS (P=0.03). The number of sexual encounters with a partner influenced traceability, with one-time only partners being more difficult to locate (P<0.001). While the majority of clients reported use of condoms inconsistently or not at all, avoidance of condoms was more often reported by clients with traceable partners (P=0.04). Nearly one in four relationships began by meeting online, with a trend toward such partners being more difficult to trace (P=0.05). Partners met at bars or clubs were readily found by PCRS (P=0.03). In contrast, when clients met their partner at school or work, those individuals were more often untraceable (P=0.002), suggesting that clients may have been reticent to disclose identifying information for classmates or colleagues.
Table 3.
Characteristics of 419 Dyads in Sexual Network
| Number (%) of Dyads or Median | |||
|---|---|---|---|
| Characteristics* | Traceable Partner (n=266) | Untraceable Partner (n=153) | P-value† |
| Age difference between partner and client‡ | |||
| Median | 3 | 4 | 0.13 |
| Range | −28 to 32 | −4 to 17 | |
| Interquartile range | −1 to 7 | 1 to 9 | |
| Type of sexual behavior | |||
| Oral | 21 (10.3) | 5 (5.4) | 0.19 |
| Anal | 55 (27.0) | 39 (41.9) | 0.01 |
| Vaginal | 14 (6.9) | 5 (5.4) | 0.80 |
| Oral and anal | 114 (55.9) | 39 (41.9) | 0.03 |
| Oral and vaginal | 0 | 5 (5.4) | |
| Frequency of sexual encounters | |||
| One time only | 82 (40.1) | 80 (80.8) | <0.001 |
| 2–5 times | 48 (33.8) | 12 (12.1) | <0.001 |
| More than 5 times | 37 (26.1) | 7 (7.1) | <0.001 |
| Condom utilization | |||
| Never | 59 (43.7) | 12 (26.7) | 0.04 |
| Inconsistent | 53 (39.3) | 25 (55.6) | 0.06 |
| Always | 23 (17.8) | 8 (17.8) | 0.91 |
| How partners met | |||
| Online | 69 (40.6) | 31 (52.5) | 0.05 |
| At school or work | 18 (10.6) | 16 (27.1) | 0.002 |
| Through friend or roommate | 22 (12.9) | 3 (5.1) | 0.10 |
| Unknown/couldn’t recall | 24 (14.1) | 2 (3.4) | 0.03 |
| Bar or club | 18 (10.6) | 1 (1.7) | 0.03 |
| Non-sexual physical venue | 8 (4.7) | 4 (6.8) | 0.54 |
| Sex venue | 5 (2.9) | 2 (3.4) | 1.0 |
| Phone chat line | 4 (2.4) | 0 | |
| At church | 2 (1.2) | 0 | |
A total of 419 dyads were examined in this study. The number of respondents with data for each item varied, reflected in column totals for each characteristic.
Wilcoxon rank-sum testing was used to compare medians. Indicator (dummy) variables were used with Pearson’s χ2 test to calculate P-values for individual categories.
Positive numbers indicate that sex partners were older than the client, and negative numbers show that the client was older than the sex partner. For dyads with traceable partners, n=211; for those with untraceable partners, n=43.
Finally, we evaluated whether client, partner, or dyad-specific characteristics were associated with an outcome of having an untraceable partner (Table 4). Bivariate analyses revealed a significant association between having an untraceable partner and having a one-time sexual encounter (risk ratio [RR] 4.44; 95% confidence interval [CI], 2.43, 8.14). Using recreational drugs during sex seemed to independently reduce the risk of having an untraceable partner (RR 0.47; 95% CI, 0.29, 0.77). Being a client with AHI also reduced the risk of a partner being untraceable (RR 0.30; 95% CI, 0.15, 0.56), reflecting PCRS efforts to identify sexual contacts potentially exposed to HIV during the period of heightened infectivity. In a multivariate model, having a one-time sexual encounter (RR 4.51; 95% CI, 2.27, 8.97) and being acutely HIV infected (RR 0.27; 95% CI, 0.08, 0.89) had the strongest influences on the risk of having an untraceable partner.
Table 4.
Client, Sex Partner, and Relationship-Level Predictors of Having an Untraceable Sex Partner
| Characteristics* (No. Responding) | RR† (95% CI) | P-value | Adjusted RR‡ (95% CI) | P-value |
|---|---|---|---|---|
| Client | ||||
| Age (n=419) | 0.98 (0.95, 1.02) | 0.31 | ||
| Black race (n=419) | 1.67 (0.86, 3.24) | 0.13 | ||
| Acutely HIV infected (n=419) | 0.30 (0.15, 0.56) | <0.001 | 0.27 (0.08, 0.89) | 0.031 |
| Recreational drug use around time of sex (n=314) | 0.47 (0.29, 0.77) | 0.003 | 0.61 (0.36, 1.06) | 0.08 |
| Internet user (n=389) | 1.14 (0.65, 1.99) | 0.65 | ||
| Sex Partner | ||||
| Age (n=297) | 1.00 (0.95, 1.04) | 0.85 | ||
| Race differs from client (n=330) | 2.06 (0.98, 4.31) | 0.06 | ||
| Relationship | ||||
| Met offline (n=203) | 0.81 (0.39, 1.72) | 0.59 | ||
| One-time encounter (n=333) | 4.44 (2.43, 8.14) | <0.001 | 4.51 (2.27, 8.97) | <0.001 |
| Index was receptive partner for anal intercourse (n=203) | 1.94 (1.01, 3.75) | 0.05 | ||
| Year sexual relationship ended (n=419) | 0.96 (0.90, 1.02) | 0.17 | ||
A total of 419 dyads were included; the number of dyads for which specific data existed varied and is noted above.
RR indicates risk ratio, here reflecting the risk of being an unreachable sex partner.
Adjusted risk ratios for a multivariate generalized linear model including acute HIV status of the index, frequency of sexual encounters, and use of recreational drugs around the time of sex.
DISCUSSION
We observed an extensive, dynamic sexual network with high HIV prevalence, overwhelmingly made up of Black MSM, whose members exhibited a steady decrease in age over time – both at their first appearance in a contact tracing investigation and at HIV diagnosis. The parallels between our findings and national trends suggest that this sexual network is a representative subunit of the HIV epidemic in the U.S., reflecting features that amplify HIV incidence among young MSM – especially those of color.
The likelihood of acquiring HIV depends on both the presence of HIV in the risk environment and engagement in behaviors placing one at risk for infection. Since multiple studies have shown that the types and frequencies of risk behaviors are similar between White and Black MSM,12–14 it logically follows that the risk environment of Black MSM in the U.S. must account for their disproportionate burden of incident HIV. We noted several key features of the network that support this idea. First, the prevalence of HIV in this network was approximately 30%, although this is almost certainly an underestimate given that some serostatus-unknown network members are likely to be HIV-infected. To put this figure in perspective, in 2006 only 0.4% of the U.S. population was living with HIV, with nearly half of all infections acquired through male-to-male sexual contact.15 Among Blacks, the prevalence was 1.7% and among Black men, it reached 2.4%.15 The likelihood of exposure to HIV within this network is comparable to the generalized epidemics of sub-Saharan Africa, where prevalence approaches 15–20%.16,17. Furthermore, the risk for HIV acquisition in this network is probably much greater than in Africa, since anal intercourse is the most efficient route for HIV sexual transmission.18 Second, the density of connections observed in the network was a likely contributor to the spread of HIV.19 More than 220 sexual relationships occurred from 2008–2010, and we noted a number of possible transmission events from persons with acute and established HIV infection. Though high concentrations of virus in blood and body fluids make individuals with AHI maximally infectious to their partners,11,20 persons with untreated chronic infection also transmit the virus, albeit less efficiently.10 One in five dyads were serodiscordant, but when we included the 189 clients whose HIV status was unknown, then nearly one out of every three relationships involved possible HIV exposures to uninfected persons. The frequency of serodiscordant partnerships is noteworthy, given recent evidence implicating inconsistent ascertainment of partner status as a key risk factor for HIV among Black MSM.14 Third, we observed that men often rejoin the same sexual network from which they acquired HIV, creating opportunities to contract episodic STIs and thus increase the risk of HIV transmission to others.21 Both syphilis and gonorrhea compromise mucosal barriers and increase the viral load of HIV-infected persons, thus potentiating HIV transmission.22 And finally, the preference of clients to seek out same-race sex partners suggested assortative mixing patterns.23 Recent work demonstrated a false perception among MSM of color that such preferential mixing offers protection against acquiring HIV.24 In actuality, mathematical modeling shows assortative mixing is associated with explosive early rates of incident infection, often followed by multi-peak or prolonged epidemic periods.25
We showed that clients with AHI were less likely to have an untraceable partner, reflecting the efforts of PCRS in locating partners potentially exposed during periods of heightened infectiousness. The long-standing success of our state’s PCRS in identifying new cases of HIV9 highlights the pivotal role that partner notification services may have as novel ARV-based prevention and treatment strategies are implemented nationally. Through fieldwork, PCRS officers could readily identify high-risk uninfected individuals and make appropriate referrals to assess candidacy for pre-exposure prophylaxis (PrEP). Established relationships with experienced HIV care providers would enable direct, timely linkage to care – as demonstrated by our statewide acute HIV screening program. “Real-time” reconstruction of sexual networks, using bioinformatics methods and centralized PCRS data, could permit earlier intervention with clients appearing in contact tracing investigations multiple times over many years. Incorporation of network analysis would require adjustments to existing PCRS methods, but the ability to “target” deployment of resources may have greater impact than the generalized prevention efforts currently in place.26 Though CDC has encouraged partner notification services to be included as part of comprehensive HIV prevention and surveillance programs,27 less than half of Americans diagnosed with HIV are offered such services28 – meaning that untold numbers of uninfected and infected sexual contacts miss key opportunities for connection to prevention and treatment resources, nationwide.
This study has limitations. A variety of socioeconomic and societal factors unmeasured by our study also act to concentrate HIV in disadvantaged populations, of which young, Black men make up a disproportionately large segment.29–31 This is only one of many sexual networks in NC, and its structure and characteristics may not be entirely generalizable to others. We were unable to uniformly obtain viral genetic sequence data necessary to confirm HIV transmission events suggested by our epidemiological data, and we lacked sufficient clinical information to estimate the proportion of HIV-infected clients who were virologically suppressed on antiretrovirals. And finally, our calculations are based on the assumption that each partner was unique, but overlap could exist among untraceable partners and with clients in the network. It is also likely that at least some of the serostatus-unknown members are actually HIV-infected. If de-duplication led to a decrease in the total number of network members, and the number of individuals with HIV was greater than we observed, then the prevalence of HIV in the network could in fact be substantially higher than our conservative estimate of 29%.
In summary, our investigation of a large sexual network provides context missing from national incidence statistics, and yields insight into the characteristics of the risk environment in which young, Black MSM are becoming HIV-infected. The confluence of high HIV prevalence, serodiscordant relationships, comorbid STIs, assortative mixing, and involvement in sexual networks at younger ages creates a risk environment in which fewer lapses in safer-sex behaviors are needed before these young, Black MSM become HIV-infected. Although sobering, these findings allow us to reflect on existing challenges and refocus our efforts more strategically. Through the development and application of combined structural, behavioral, and biomedical HIV prevention strategies tailored for this population, we can reduce the spread of HIV among these men of color.
Supplementary Material
Acknowledgments
Funding/Support
CBH is supported by the National Center for Advancing Translational Sciences (8KL2TR000084-05); LHW was supported by HRSA's Special Projects of National Significance Initiative: Systems Linkages and Access to Care Initiative (H97HA22695); LHW and EP are supported by the National Institute of Mental Health (1R01MH093275-01); and the National Institute for Allergy and Infectious Diseases provided support for JJE (5P30AI050410-13), SB, AS, and JK (5U01AI067854-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Health Resources and Services Administration.
We thank the NC-DHHS Communicable Disease Branch: Delbert Williams, PhD, John Barnhart, MPH, Todd Vanhoy, Rhonda Ashby, and the Disease Intervention Specialists that serve the people of NC. We also appreciate the assistance provided by Peter Mucha, PhD and Sonia Napravnik, PhD.
Footnotes
Conflicts of Interest
No conflicts of interest to report for any author.
Presentation of Work
This work was presented at the 19th Conference on Retroviruses and Opportunistic Infections, March 5–8, 2012, in Seattle, WA (Abstract #1105).
Author Contributions
Conceived and designed the study: CBH MSC LBHW JJE PAL SB AS.
Acquired and organized data: SB, AS, EP, EMF, PAL, JK, CBH.
Analyzed and interpreted data: CBH, LBHW, MSC, JJE.
Wrote the first draft of the manuscript: CBH, MSC, LBHW.
Critical review of the manuscript for important intellectual content: MSC, JJE, LBHW, EMF, PAL, SB, AS, EP, JK.
ICMJE criteria for authorship read and met: CBH SB PAL AS EP JK EMF JJE MSC LBHW.
Agree with manuscript results and conclusions: CBH SB PAL AS EP JK EMF JJE MSC LBHW.
Competing Interests
No author has relevant competing interests to report.
References
- 1.CDC. Pneumocystis pneumonia – Los Angeles. Mmwr. 1981;30:250–252. [PubMed] [Google Scholar]
- 2.Prejean J, Song R, Hernandez A, et al. Estimated HIV Incidence in the United States, 2006–2009. PLoS ONE. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hightow LB, MacDonald PD, Pilcher CD, et al. The unexpected movement of the HIV epidemic in the Southeastern United States: transmission among college students. J Acquir Immune Defic Syndr. 2005 Apr 15;38(5):531–537. doi: 10.1097/01.qai.0000155037.10628.cb. [DOI] [PubMed] [Google Scholar]
- 4.Hightow-Weidman LB, Smith JC, Valera E, Matthews DD, Lyons P. Keeping them in “STYLE”: finding, linking, and retaining young HIV-positive black and Latino men who have sex with men in care. AIDS patient care and STDs. 2011 Jan;25(1):37–45. doi: 10.1089/apc.2010.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Communicable Disease Surveillance Unit. Epidemiologic profile for HIV/STD prevention and care planning. Raleigh: Epidemiology and Special Studies Unit, HIV/STD Prevention and Care Branch, North Carolina Department of Health and Human Services; Dec, 2010. [Google Scholar]
- 6.Communicable Disease Surveillance Unit. Epidemiologic profile for HIV/STD prevention and care planning. Raleigh: Communicable Disease Branch, Epidemiology Section, Division of Public Health, North Carolina Department of Health and Human Services; 2011. [Google Scholar]
- 7.Pilcher CD, McPherson JT, Leone PA, et al. Real-time, universal screening for acute HIV infection in a routine HIV counseling and testing population. JAMA. 2002 Jul 10;288(2):216–221. doi: 10.1001/jama.288.2.216. [DOI] [PubMed] [Google Scholar]
- 8.Pilcher CD, Fiscus SA, Nguyen TQ, et al. Detection of acute infections during HIV testing in North Carolina. The New England journal of medicine. 2005 May 5;352(18):1873–1883. doi: 10.1056/NEJMoa042291. [DOI] [PubMed] [Google Scholar]
- 9.CDC. Partner counseling and referral services to identify persons with undiagnosed HIV--North Carolina, 2001. MMWR Morbidity and mortality weekly report. 2003 Dec 5;52(48):1181–1184. [PubMed] [Google Scholar]
- 10.Pilcher CD, Joaki G, Hoffman IF, et al. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS (London, England) 2007 Aug 20;21(13):1723–1730. doi: 10.1097/QAD.0b013e3281532c82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pilcher CD, Tien HC, Eron JJ, Jr, et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. The Journal of infectious diseases. 2004 May 15;189(10):1785–1792. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- 12.Millett GA, Peterson JL, Wolitski RJ, Stall R. Greater risk for HIV infection of black men who have sex with men: a critical literature review. American journal of public health. 2006 Jun;96(6):1007–1019. doi: 10.2105/AJPH.2005.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millett GA, Flores SA, Peterson JL, Bakeman R. Explaining disparities in HIV infection among black and white men who have sex with men: a meta-analysis of HIV risk behaviors. AIDS (London, England) 2007 Oct 1;21(15):2083–2091. doi: 10.1097/QAD.0b013e3282e9a64b. [DOI] [PubMed] [Google Scholar]
- 14.Oster AM, Wiegand RE, Sionean C, et al. Understanding disparities in HIV infection between black and white MSM in the United States. AIDS (London, England) 2011 May 15;25(8):1103–1112. doi: 10.1097/QAD.0b013e3283471efa. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. HIV prevalence estimates--United States, 2006. MMWR Morbidity and mortality weekly report. 2008 Oct 3;57(39):1073–1076. [PubMed] [Google Scholar]
- 16.El-Sadr WM, Mayer KH, Hodder SL. AIDS in America--forgotten but not gone. The New England journal of medicine. 2010 Mar 18;362(11):967–970. doi: 10.1056/NEJMp1000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2010. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2010. [Google Scholar]
- 18.Varghese B, Maher JE, Peterman TA, Branson BM, Steketee RW. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sexually transmitted diseases. 2002 Jan;29(1):38–43. doi: 10.1097/00007435-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Potterat JJ, Rothenberg RB, Muth SQ. Network structural dynamics and infectious disease propagation. International journal of STD & AIDS. 1999 Mar;10(3):182–185. doi: 10.1258/0956462991913853. [DOI] [PubMed] [Google Scholar]
- 20.Pilcher CD, Shugars DC, Fiscus SA, et al. HIV in body fluids during primary HIV infection: implications for pathogenesis, treatment and public health. AIDS (London, England) 2001 May 4;15(7):837–845. doi: 10.1097/00002030-200105040-00004. [DOI] [PubMed] [Google Scholar]
- 21.Cohen MS. Sexually transmitted diseases enhance HIV transmission: no longer a hypothesis. Lancet. 1998;351(Suppl 3):5–7. doi: 10.1016/s0140-6736(98)90002-2. [DOI] [PubMed] [Google Scholar]
- 22.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004 Jan;2(1):33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 23.Raymond HF, McFarland W. Racial mixing and HIV risk among men who have sex with men. AIDS and behavior. 2009 Aug;13(4):630–637. doi: 10.1007/s10461-009-9574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millett GA, Ding H, Marks G, et al. Mistaken assumptions and missed opportunities: correlates of undiagnosed HIV infection among black and Latino men who have sex with men. Journal of acquired immune deficiency syndromes. 2011 Sep 1;58(1):64–71. doi: 10.1097/QAI.0b013e31822542ad. [DOI] [PubMed] [Google Scholar]
- 25.Anderson RM, Gupta S, Ng W. The significance of sexual partner contact networks for the transmission dynamics of HIV. Journal of acquired immune deficiency syndromes. 1990;3(4):417–429. [PubMed] [Google Scholar]
- 26.Rothenberg R, Kimbrough L, Lewis-Hardy R, et al. Social network methods for endemic foci of syphilis: a pilot project. Sexually transmitted diseases. 2000 Jan;27(1):12–18. doi: 10.1097/00007435-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 27.CDC. Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 2008 Nov 7;57(RR-9):1–83. quiz CE81-84. [PubMed] [Google Scholar]
- 28.Katz DA, Hogben M, Dooley SW, Jr, Golden MR. Increasing public health partner services for human immunodeficiency virus: results of a second national survey. Sexually transmitted diseases. 2010 Aug;37(8):469–475. doi: 10.1097/OLQ.0b013e3181e7104d. [DOI] [PubMed] [Google Scholar]
- 29.Aral SO. The social context of syphilis persistence in the southeastern United States. Sexually transmitted diseases. 1996 Jan-Feb;23(1):9–15. doi: 10.1097/00007435-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Laumann EO, Youm Y. Racial/ethnic group differences in the prevalence of sexually transmitted diseases in the United States: a network explanation. Sexually transmitted diseases. 1999 May;26(5):250–261. doi: 10.1097/00007435-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Friedman SR, Cooper HL, Osborne AH. Structural and social contexts of HIV risk Among African Americans. American journal of public health. 2009 Jun;99(6):1002–1008. doi: 10.2105/AJPH.2008.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.