Abstract
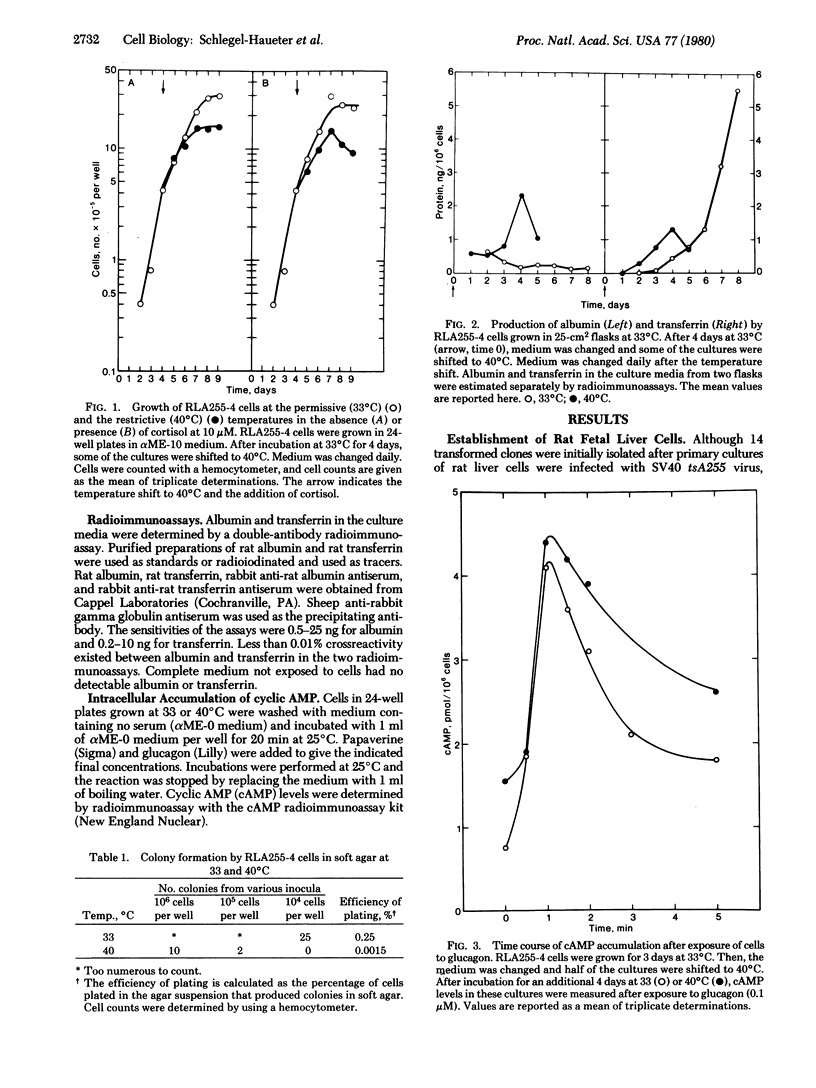
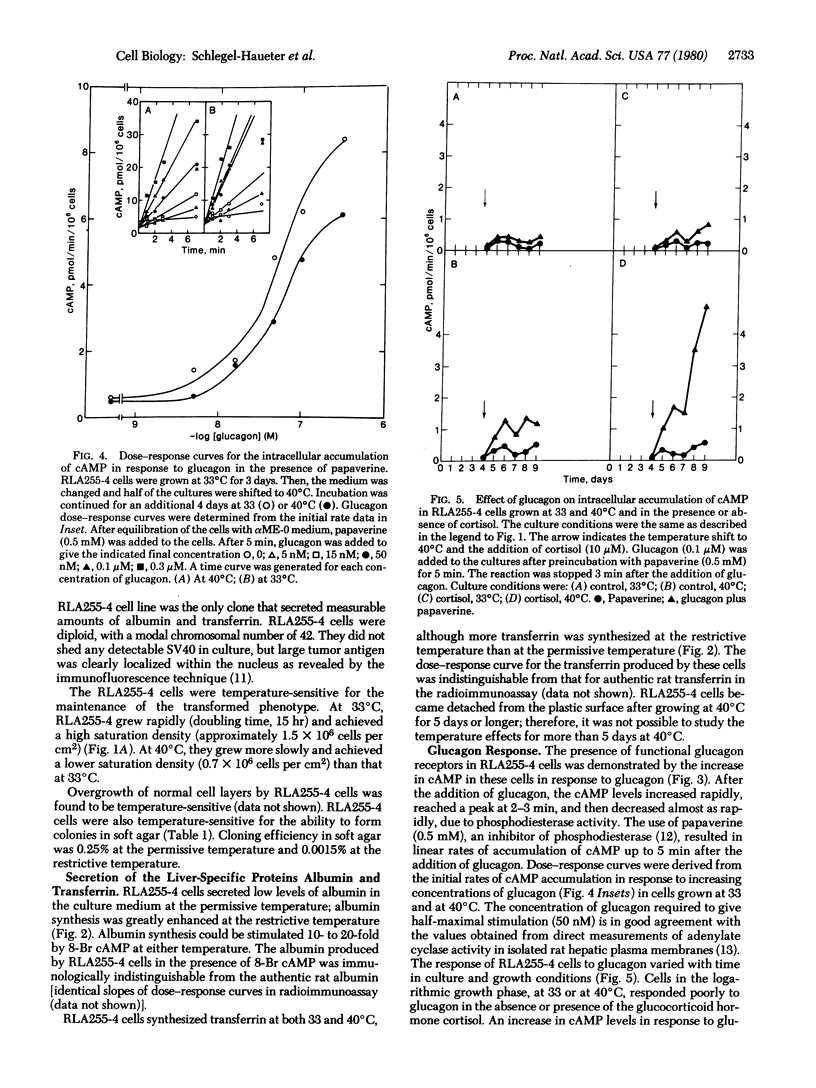
Fetal rat liver cells were transformed with a temperature-sensitive A mutant (tsA255) of simian virus 40. A CLONAL CELL LINE, RLA255-4, which was temperature sensitive in the maintenance of the transformed phenotype, was isolated. This cell line expressed the transformed phenotype (rapid growth, high cell density, overgrowth of normal cells, and cloning in soft agar) at the permissive temperature (33 degrees C) and the nontransformed phenotype (slower growth, lower saturation density, decreased efficiency of overgrowth of normal cells, and lower cloning efficiency in soft agar) at the restrictive temperature (40 degrees C). The tsA255-transformed cells expressed differentiated liver functions under controllable conditions. At the permissive temperature, they produced low levels of albumin and transferrin, whereas at the restrictive temperature the transformed phenotype was lost and the production of these hepatic proteins was greatly enhanced. RLA255-4 cells contained functional receptors for glucagon, as shown by the stimulation of intracellular cyclic AMP accumulation by glucagon. The response to glucagon was dose dependent (Kact = 5 x 10(-8) M) and could be demonstrated in cells grown at both permissive and restrictive temperatures after 7 days in culture (i.e., at a cell density of approximately 4 x 10(5) cells per cm2 or higher). Addition of cortisol to the culture medium enhanced the glucagon response selectively at the restrictive temperature.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. L., Martin R. G. SV40 transformation of mouse brain cells: critical role of gene A in maintenance of the transformed phenotype. J Cell Physiol. 1976 May;88(1):65–76. doi: 10.1002/jcp.1040880109. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Butel J. S. Role of simian virus 40 gene A function in maintenance of transformation. J Virol. 1975 Mar;15(3):619–635. doi: 10.1128/jvi.15.3.619-635.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y. Establishment of clonal human placental cells synthesizing human choriogonadotropin. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1854–1858. doi: 10.1073/pnas.75.4.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y. Human placental cells transformed by tsA mutants of simian virus 40: a model system for the study of placental functions. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1409–1413. doi: 10.1073/pnas.75.3.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffert H. L., Koch K. S., Moran T., Williams M. Liver cells. Methods Enzymol. 1979;58:536–544. doi: 10.1016/s0076-6879(79)58168-3. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y. Simian virus 40 functions required for the establishment and maintenance of malignant transformation. J Virol. 1975 Mar;15(3):599–612. doi: 10.1128/jvi.15.3.599-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Simian virus 40 gene A function and maintenance of transformation. J Virol. 1975 Mar;15(3):636–644. doi: 10.1128/jvi.15.3.636-644.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas C., Chapeville F., Jacquot R. Development of glycogen storage ability under cortisol control in primary cultures of rat fetal hepatocytes. Dev Biol. 1973 May;32(1):82–91. doi: 10.1016/0012-1606(73)90221-2. [DOI] [PubMed] [Google Scholar]
- Plas C., Nunez J. Glycogenolytic response to glucagon of cultured fetal hepatocytes. Refractoriness following prior exposure to glucagon. J Biol Chem. 1975 Jul 25;250(14):5304–5311. [PubMed] [Google Scholar]
- Plas C., Nunez J. Role of cortisol on the glycogenolytic effect of glucagon and on the glycogenic response to insulin in fetal hepatocyte culture. J Biol Chem. 1976 Mar 10;251(5):1431–1437. [PubMed] [Google Scholar]
- Pöch G., Juan H., Kukovetz W. R. Einfluss von herz- und gefässwirksamen Substanzen auf die Aktivität der Phosphodiesterase. Naunyn Schmiedebergs Arch Pharmakol. 1969;264(3):293–294. [PubMed] [Google Scholar]
- Richman R. A., Claus T. H., Pilkis S. J., Friedman D. L. Hormonal stimulation of DNA synthesis in primary cultures of adult rat hepatocytes. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3589–3593. doi: 10.1073/pnas.73.10.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. 3. Binding of glucagon: method of assay and specificity. J Biol Chem. 1971 Mar 25;246(6):1861–1871. [PubMed] [Google Scholar]
- Schaeffer W. I., Polifka M. D. A diploid rat liver cell culture. III. Characterization of the heteroploid morphological variants which develop with time in culture. Exp Cell Res. 1975 Oct 1;95(1):167–175. doi: 10.1016/0014-4827(75)90622-9. [DOI] [PubMed] [Google Scholar]
- Staedel C., Beck J. P. Resurgence of glycogen synthesis and storage capacity in cultured hepatoma cells. Cell Differ. 1978 Apr;7(1-2):61–71. doi: 10.1016/0045-6039(78)90007-6. [DOI] [PubMed] [Google Scholar]
- TJIO J. H., WHANG J. Chromosome preparations of bone marrow cells without prior in vitro culture or in vivo colchicine administration. Stain Technol. 1962 Jan;37:17–20. doi: 10.3109/10520296209114563. [DOI] [PubMed] [Google Scholar]
- Taub M., Chuman L., Saier M. H., Jr, Sato G. Growth of Madin-Darby canine kidney epithelial cell (MDCK) line in hormone-supplemented, serum-free medium. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3338–3342. doi: 10.1073/pnas.76.7.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E. B., Tomkins G. M., Curran J. F. Induction of tyrosine alpha-ketoglutarate transaminase by steroid hormones in a newly established tissue culture cell line. Proc Natl Acad Sci U S A. 1966 Jul;56(1):296–303. doi: 10.1073/pnas.56.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]



