Abstract
Sensory information is often mapped systematically in the brain with neighboring neurons responding to similar stimulus features. The olfactory system represents chemical information as spatial and temporal activity patterns across glomeruli in the olfactory bulb. However, the degree to which chemical features are mapped systematically in the glomerular array has remained controversial. Here, we test the hypothesis that the dual roles of odorant receptors, in axon guidance and odor detection, can serve as a mechanism to map olfactory inputs with respect to their function. We compared the relationship between response specificity and glomerular formation in genetically-defined olfactory sensory neurons expressing variant odorant receptors. We find that sensory neurons with the same odor response profile can be mapped to different regions of the bulb, and that neurons with different response profiles can be mapped to the same glomeruli. Our data demonstrate that the two functions of odorant receptors can be uncoupled, indicating that the mechanisms that map olfactory sensory inputs to glomeruli do so without regard to stimulus specificity.
Keywords: Olfactory, odorant receptors, mapping, glomeruli, olfactory sensory neuron, electrophysiology
INTRODUCTION
In many sensory systems, information is mapped in neural space with neighboring neurons responding to similar stimulus features. Such sensory maps typically form during development in an activity dependent way, such that neuronal circuits wire with respect to their functional properties (Chklovskii and Koulakov, 2004; Knudsen et al., 1987; Luo and Flanagan, 2007). The olfactory system represents information about chemical structure. Volatile chemicals are transduced by olfactory sensory neurons (OSNs) in the nasal cavity. Each OSN expresses one member of a large family of odorant receptor (OR) genes—there are over 1,000 genes in mice. OSNs that express a defined OR send axonal projections to specific glomeruli in the olfactory bulb (Mombaerts, 2006), and each glomerulus is thought to get input exclusively from OSNs expressing the same OR protein (Bozza et al., 2002; Jourdan et al., 1980; Lancet et al., 1982; Treloar et al., 2002; Wachowiak et al., 2004). The spatial organization of glomeruli in the olfactory bulb is thought to play a role in odor coding (Hildebrand and Shepherd, 1997; Mori et al., 2006; Murthy, 2011; Wilson and Mainen, 2006).
There is a long-standing controversy about whether glomeruli are organized “chemotopically” with respect to physicochemical properties of odorants (Bozza et al., 2004; Farahbod et al., 2006; Johnson et al., 2002; Ma et al., 2012; Meister and Bonhoeffer, 2001; Mori et al., 2006; Soucy et al., 2009; Takahashi et al., 2004; Wachowiak and Cohen, 2001). To complicate matters, even defining chemotopy is problematic given the diversity of chemical structures and potential features that could be mapped (Murthy, 2011). Given these difficulties, we have taken an alternate approach to understand olfactory mapping by asking whether a mechanism exists to systematically map glomeruli based on stimulus sensitivity.
In addition to their sensory function, ORs are involved in axon guidance and influence glomerular position (Mombaerts et al., 1996). As new OR genes are formed during evolution, and nascent OR coding sequences diverge by random mutation, OSNs may develop novel odorant response profiles and/or glomerular positions. Thus, the degree to which odorant sensitivity is mapped by this mechanism is dictated by the correlation between the sensory and axon guidance functions of ORs. Moreover, glomerular position is further influenced by which OSNs express a given OR (Bozza et al., 2009). The olfactory epithelium contains intermingled populations of OSNs (OSN-types) that are restricted to project to separate domains in the olfactory bulb. Random mutations in OR gene regulatory elements that shift OR expression to a new OSN-type would be expected to shift glomerular position as is seen with OR transgenes and targeted alleles (Rothman et al., 2005). Thus, it is critical to understand how changes in OR gene function correlate with resulting changes in axon guidance.
We have asked whether mutations in ORs that change response specificity necessarily change axon guidance and vice versa. We used mice in which OSNs expressing the highly homologous ORs M71 or M72 are labeled (Feinstein and Mombaerts, 2004). M71- and M72-expressing OSNs project to distinct glomeruli in the dorsal bulb, while M71/M72 hybrid mutations (in which the M71 OR is mutated to encode amino acids from M72) exhibit a wide variety of axon guidance phenotypes (Feinstein and Mombaerts, 2004). For some of the hybrids, OSNs expressing ORs with different primary amino acid sequences project to the same glomeruli (Feinstein and Mombaerts, 2004; See also Ishii et al., 2001). These observations prompted us to examine the relationship between odorant responsiveness and glomerular formation in these mutants.
Here we define the odorant response profiles of the model ORs M71, M72, and M71/M72 hybrid ORs using patch clamp recordings from genetically defined OSNs in gene targeted mice. We show for the first time that M71 and M72 have distinct odorant response profiles. A mutation in M71 that redirects axons to a new glomerulus also dramatically changes the response profile of the OR. More surprisingly, a mutation in M71 that changes the response profile does not change glomerular targeting, resulting in a glomerulus that receives functionally distinguishable inputs. Finally, we demonstrate that the same OR can be mapped to different positions in the olfactory bulb despite having the same response specificity. Taken together, the data indicate that the olfactory system does not have acute mechanisms for ensuring that glomeruli are organized strictly according to response specificity.
MATERIAL AND METHODS
Electrophysiological recordings
Patch clamp recordings were made from the knobs of OSNs in epithelial explants (Ma et al., 1999). The olfactory epithelium from P10–P14 mice was removed and kept in oxygenated bath solution (95% O2-5%CO2), containing (in mM) NaCl 124, KCl 3, MgSO4 1.3, CaCl2 2, NaHCO3 26, NaHPO4 1.25 and glucose 15 (pH 7.4). The epithelium was transferred to a recording chamber, and kept under continuous flow (2–3 ml/min) of oxygenated bath solution. All experiments were performed at room temperature.
The knobs of OSNs were visualized with an upright DIC microscope (Zeiss Axioskop 2 Plus) equipped with a CCD camera (SensiCam QE; Cooke Corporation) and a 40× water immersion objective. Extra magnification was achieved using a 4× video coupler. Dendritic knobs of GFP-labeled OSNs were identified under fluorescence illumination using a standard GFP filter set.
Patch pipettes were pulled from borosilicate glass with a P-97 horizontal puller (Sutter Instrument, Novato, CA), and fire-polished using a microforge (MF 83; Narishige, Japan). Electrophysiological recordings were controlled by an EPC-10 amplifier combined with Pulse software (HEKA, Germany). Perforated patch clamp was performed by including 260 μM amphotericin B in the recording pipette, which was filled with the following solution (in mM): KCl 70, KOH 53, methanesulfonic acid 82.3, EGTA 5, HEPES 10, and sucrose 70 (pH 7.2). The electrodes had tip resistances ranging from 8–10 MΩ when filled with internal solution. The liquid junction potential of 9 mV was corrected in all experiments within the software. Signals were acquired at 10 kHz and low-pass filtered at 2.9 kHz.
Odorants were applied via a multi-barrel pipette placed 20 μm downstream of the cell. Stimuli were delivered using a pressure ejection system (PDES-02D, NPI Electronics, Germany). Odorants were dissolved in dimethyl sulfoxide (DMSO) and stored at −20°C. Odorant concentrations were further diluted in bath solution as necessary. The final concentration of DMSO was less than 0.1%.
Analysis and curve fitting were performed using Igor Pro 5.01 (WaveMetrics, Inc., OR). Dose-response curves were fitted by the Hill equation, I =Imax/(1+(EC50C)n), where I represents the peak of odorant-induced responses. Statistical tests were performed using Statview and SPSS.
Histology
Olfactory bulbs were removed and imaged directly without fixation using a Zeiss LSM5 confocal microscope. Visualization of endogenous GFP and fast red-violet β-gal staining in wholemounts has been described previously (Feinstein and Mombaerts, 2004). Images of olfactory bulb wholemounts were collected as z-stacks and projected into a single image for display.
Immunohistochemistry
P10–P15 mice that were compound heterozygous for the M71-IRES-tauGFP and RFP→M71-IRES-M71-IRES-tauGFP mutations (Feinstein et al., 2004) were dissected and the olfactory epithelia fixed in PLP for 1 hour on ice. Tissue was rinse in phosphate buffered saline, cryoprotected in 30% sucrose, frozen in OCT, and cut in 20μm sections on a cryostat. Sections were collected on SuperPlus slides, and stored at −80C until immunohistochemical reactions.
Sections were quenched with 0.5% hydrogen peroxide, blocked in TNB buffer (0.1M TRIS-HCl, 0.15M NaCl, 0.5% blocking reagent; PerkinElmer) with 2% normal donkey serum and 0.1% Triton X-100 for 1 hour, and incubated with guinea pig anti M71/M72 (1:5000) in blocking solution overnight. Sections were incubated with biotinylated donkey anti guinea pig (1:500; Jackson ImmunoResearch) followed by streptavidin horseradish peroxidase (1:500; PerkinElmer). The signal was enhanced with biotinyl tyramide (1:200; PerkinElmer) and visualized with streptavidin 647 (1:300; Invitrogen). Sections were imaged using confocal microscopy. M71-OSNs were identified by the presence of GFP and absence of RFP labeling. Fluorescence levels associate with M71 stating were quantified by taking the average pixel value within a fixed region of interest encompassing the dendritic knob and proximal cilia of individual OSNs. The same laser intensity and gain settings were used for all experiments. Images were analyzed using Zeiss AIM software and ImageJ.
RESULTS
Previous studies have identified ligands for the ORs M71 and/or M72 including the aromatic compounds acetophenone and benzaldehyde (Bozza et al., 2002; Feinstein et al., 2004) and tiglates (Soucy et al., 2009). However, few data have been published comparing the response profiles of these two ORs. To investigate this further, we exploited mouse strains in which OSNs expressing M71 or M72 are labeled with the fluorescent marker, tauGFP (Bozza et al., 2002; Potter et al., 2001). This allowed us to visualize and target genetically identified OSNs for patch clamp recordings in a semi-intact preparation of the olfactory epithelium (Fig. 1A). Odor-induced currents were measured from the dendrites of visualized neurons using the perforated patch configuration (Ma et al., 1999). Our previous calcium imaging recordings had shown that acetophenone is a better agonist for M71 than M72 suggesting that M71 and M72 have distinguishable odorant response profiles despite their high sequence similarity (11 amino acid differences). We therefore set out to better define the response profiles of M71 and M72 and to define ligands that could selectively activate these ORs.
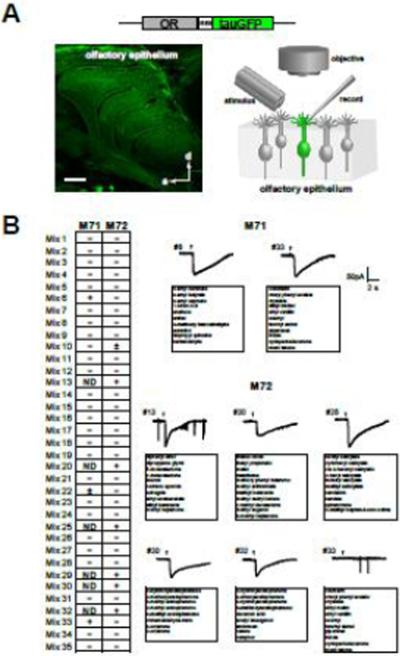
Fig. 1. Screening M71 and M72-OSNs with odorant mixtures.
(A) Diagram of the experimental approach. OSNs that express a defined OR are labeled with GFP in gene-targeted mice. The coding sequence for tauGFP (green) is inserted after the intact OR coding sequence (grey box) and downstream of an internal ribosome entry site (IRES). Confocal image shows green fluorescent OSNs in the nasal cavity. Anterior (a) and dorsal (d) are indicated. Scale bar = 500 μm. Diagram of the recording configuration (right). Perforated patch recordings were made from the dendrites of semi-intact OSNs.
(B) Responses elicited by 35 odorant mixtures tested at 50μM for each odorant in the mixture. + indicates repeatable response, ± small unreliable response, − negative response, ND not tested. (Right) Typical current traces from M71 and M72 OSNs elicited by the odorant mixtures. Identified ligands from the mixes are shown in bold.
Identification of novel M72 selective ligands
To identify new M72-ligands, we screened M72-expressing OSNs (M72-OSNs) with 330 odorant molecules, presented in 35 mixtures, each of which comprised 8–11 odorants at a fixed concentration (Fig. 1B). The mixtures contained chemicals with a variety of functional groups and perceived odor qualities (Table S1). Six out of the 35 mixtures (#13, #20, #25, #29, #30 and #32) elicited robust responses in M72-OSNs (Fig. 1B). To identify the individual effective ligands, we broke down these mixtures and tested the components at a fixed concentration (Fig. 2). This process uncovered 14 previously unknown ligands for M72. As one might predict, some of the new ligands that elicited the best responses were structurally similar to acetophenone (e.g. 4-methoxyacetophenone, 2-methylacetophenone, and 2-hydroxyacetophenone). Others included a monoterpene ketone (menthone) and aromatic esters (ethyl benzoate, methyl benzoate and methyl salicylate). Of the known M72 ligands, methyl salicylate elicited the largest responses. Many of the M72 ligands also activated M71-OSNs to varying degrees (Fig. 2A and 2B). However, three of the M72 ligands (methyl benzoate, menthone, and methyl salicylate) failed to activate M71-OSNs at a fixed concentration (Fig. 2B). Thus, M71 and M72-OSNs have distinguishable response profiles with respect to these novel agonists.
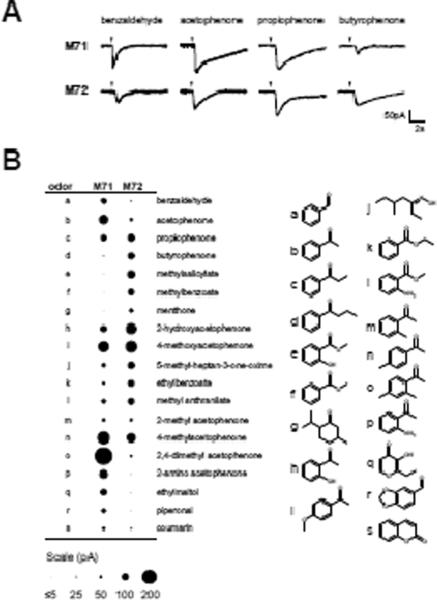
Fig. 2. M71 and M72 have distinct response profiles to individual odorants.
(A) Current recordings in M71 and M72 OSNs in response to acetophenone and its analogues at 10 μM.
(B) Dot plot showing the average response of M71 and M72 OSNs to a set of individual odorants. Size of the dot is proportional to current amplitude. Data are averages from ≥3 OSNs. All odorants were 10 μM except 2-amino acetophenone (1 μM). Odorant structures are shown (right).
Identification of M71-selective ligands
Next, to identify M71-selective ligands, we tested whether any of the 28 odorant mixtures that failed to activate M72-OSNs could activate M71-OSNs. Two mixtures (#6 and #33) elicited robust responses in M71-OSNs (Fig. 1B). To identify the individual effective ligands, we broke down these mixtures and tested individual components at a fixed concentration. This screen identified four novel agonists that activated M71-OSNs—coumarin, piperonal, ethyl maltol, and 2-amino acetophenone. All 4 of these ligands elicited significantly larger responses in M71-OSNs than M72-OSNs (Fig. 2B). We thus defined a set of ligands that can be used to distinguish the response profiles of M71 and M72.
A mutation that changes response specificity shifts glomerular position
Next, we examined the relationship between glomerular formation and odorant specificity in M71/M72 hybrid ORs. We first asked whether a mutation that causes a change in glomerular identity changes odorant specificity. The M71/M72 hybrid J (M71-HybJ) contains a point mutation (D205N) in which an aspartate residue in the predicted transmembrane domain 5 of M71 is mutated to asparagine, the residue at the corresponding position in M72 (Fig. 3A; Feinstein and Mombaerts, 2004). Axons of M71-HybJ expressing OSNs do not project with native M71 axons, but rather, form a new glomerulus that is distinct from both M71 and M72. In fact, the location of convergence is more closely associated with M71 than M72 glomeruli (Fig. 3B). Interestingly, the odorant response profile of M71-HybJ is more similar to M72 than it is to M71. M71-HybJ-OSNs exhibited robust responses to the M72-selective ligands methyl benzoate and methyl salicylate, but not to the M71 selective agonists 2-amino acetophenone, ethyl maltol and piperonal (Fig. 3D). Thus, M71-HybJ has a different response specificity than M71.
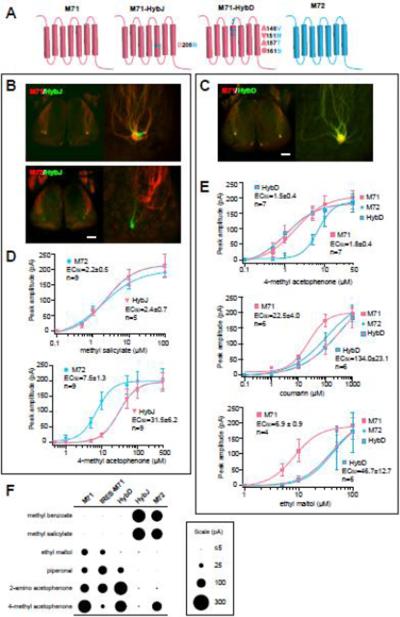
Fig. 3. Analyzing glomerular position and response specificity in M71/M72 hybrids.
(A) Schematic of the ORs M71 and M72 and two Hybrids D and J showing the location of the amino acid substitution. M71-derived sequences are illustrated as red, M72 as blue.
(B) Confocal images of the dorsal bulbs in mice expressing M71-HybJ and M71 (top panels), or M71-HybJ and M72 (bottom panels). Axons expressing M71-HybJ (green) form a glomerulus that is close to, but distinct from, M71 glomeruli (red). The M71-HybJ glomeruli are also separate from M72.
(C) Confocal images of the dorsal bulbs in mice expressing M71-HybD and M71. Axons expressing M71-HybD (green) project with axons expressing M71 (red).
(D) Average dose-response data for M72-OSNs and M71-HybJ-OSNs to the M72-selective ligand, methyl salicylate (top) or the M71/M72 common ligand, 4-methyl acetophenone (bottom). Data are mean ± SEM. EC50 values and number of recorded OSNs are indicated.
(E) Average dose response data for M71, M72, and M71-HybD to three different odorants: the M71/M72 common ligand 4-methyl acetophenone, and the M71-selective ligands ethyl maltol and coumarin (mean ± SEM). EC50 values and number of recorded OSNs are indicated.
(F) Dot plot showing the average response amplitudes elicited by a panel of odorants in OSNs expressing M71, IRES-M71, M72, M71-HybJ and M71-HybD alleles. Each row shows responses for a given odorant. The size of the dot is proportional to the response amplitude. Odorants were presented at 10 μM, except 2-aminoacetophenone (1μM).
While M71-HybJ is similar to M72, the two are not functionally identical. M71-HybJ-OSNs and M72-OSNs shared the same sensitivity to the M72-selective ligand methyl salicylate (Fig. 3D). However, M71-HybJ OSNs were significantly less sensitive to the odorant 4-methyl acetophenone than were M72-OSNs (Fig. 3D). Thus, a single amino acid mutation shifts the response profile of M71 to be very similar, but not identical, to native M72.
Taken together the data show that a single point mutation in TM5 of M71 changes both odorant specificity and axon guidance, mapping a response profile that is similar to M72 to a new glomerulus that is closer in space to M71.
A mutation that changes odorant specificity does not shift glomerular position
Next we asked whether a mutation can change the response profile of an OR without affecting glomerular position. We examined a second M71/M72 hybrid, M71-Hybrid D (M71-HybD) which contains four amino acid changes in predicted transmembrane domain 4 and extracellular loop 2 (Fig. 3A). M71-HybD axons converge with native M71 axons and intermingle within the same glomeruli (Feinstein and Mombaerts, 2004; Fig. 3C).
Despite projecting to the same glomeruli, M71-HybD OSNs have an odorant response profile that is similar to, but distinguishable from, that of M71-OSNs (Fig. 3E). Similar to M71, M71-HybD OSNs responded to 2-aminoacetophenone and piperonal as well as 4-methyl acetophenone, a ligand common to M71 and M72. M71- and M71-HybD-OSNs exhibited similar sensitivity to 4-methyl acetophenone across concentration as well. While, the response profiles of M71 and M71-HybD are similar, they are not identical. First, M71-HybD-OSNs were less sensitive to ethyl maltol than M71-OSNs. In fact, M71-HybD had the same low sensitivity to ethyl maltol as M72-OSNs. Second, M71-HybD-OSNs were less sensitive to coumarin than M71-OSNs. The difference in specificity is unlikely due to the fact that HybD is a hypomorphic allele for two reasons. First, M71 and M71-HybD have the same sensitivity to 4-methyl acetophenone (Fig. 3E). Second, M71-HybD has a different profile than a known hypomorphic version of M71 (see IRES-M71 below and Fig. 3F).
Taken together, the data show that a specific OR mutation can change odorant specificity without changing axon guidance identity, mapping two distinct response profiles to the same glomerulus.
OSNs with similar specificity can be mapped to different regions of the bulb
We next examined whether OSNs that share the same or similar response profiles are necessarily mapped to glomeruli in a unique location in the olfactory bulb. Previous data reveal situations in which OSNs that express the same OR can project to glomeruli in different regions of the olfactory bulb. For example, coding sequence swaps between class I and class II OR genes cause pronounced shifts in glomerular position because class I and class II ORs are expressed by different OSN-types that project to different domains in the bulb (Bozza et al., 2009; Pacifico et al., 2012). Expression of the class II OR M72 from the class I OR locus S50 (M72→S50) remaps M72 to a more anterior position, suggesting that glomerular location is more correlated with OSN-type than odorant response profile. However, the response profiles of OSNs expressing interclass swaps have not been characterized.
To examine this issue, we directly compared the anatomical projections and odorant response profiles of M72- and M72→S50-OSNs. M72→S50 glomeruli are located approximately 1mm from the native M72 glomeruli (Bozza et al., 2009; Fig. 4A). Despite this large shift in position, M72-OSNs and M72→S50-OSNs exhibited indistinguishable response profiles to a set of M71/M72 ligands, acetophenone, methyl salicylate, ethyl tiglate and eugenol (Fig. 4B). For both ORs, the largest responses were observed using methyl salicylate and ethyl tiglate, while intermediate responses were seen with acetophenone. This similarity in response profile was also observed across concentration. The EC50 values for methyl salicylate and acetophenone were the same in both M72 and M72→S50 OSNs (Fig. 4C and 4D). The data demonstrate for the first time that OSN-type has little effect on odorant specificity. Thus, OSNs with the same odorant response specificity can be mapped to two different regions of the bulb, depending on expression in different OSN-types.
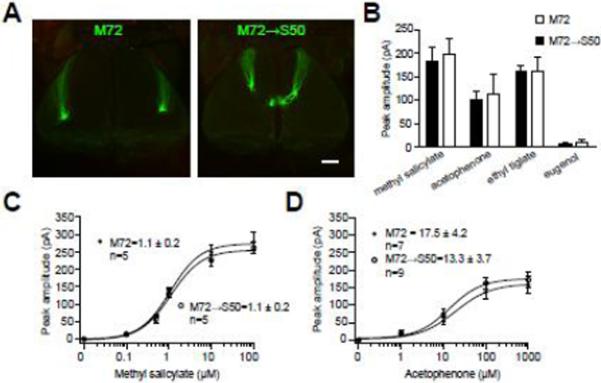
Fig. 4. Axon guidance and odorant specificity in inter-class odorant receptor swaps.
(A) Confocal images of the dorsal bulbs in mice expressing M72 (left) and M72→S50 (right) showing projections to glomeruli in different domains of the dorsal bulb.
(B) Response amplitudes to a set of odorants in M72- (n=5) and M72→S50 (n=5) OSNs. Response profiles are not significantly different.
(C–D) Average dose-response data for M72- and M72→S50 OSNs to two known M72 ligands, methyl salicylate and acetophenone (mean ± SEM). Measured EC50 values and number of OSNs recorded are indicated and are not statistically different between the two populations (t-test, p>0.05).
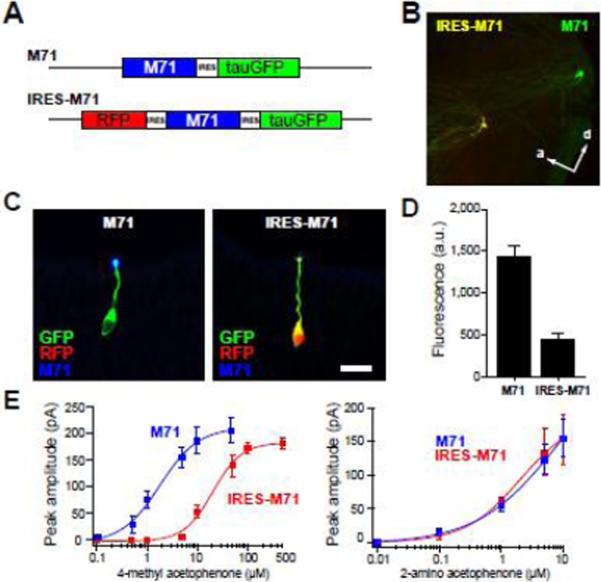
A second factor that shifts glomerular position is OR expression levels. Previous data show that expressing M71 from its native locus, but translated from an internal ribosome entry site (IRES-M71), causes axons to form glomeruli that are shifted anteriorly in comparison to native M71 glomeruli (Feinstein et al., 2004); Fig. 5A and 5B). This shift in position has been attributed to a decrease in M71 protein levels, though this has not been shown directly. We therefore compared M71 protein levels in the dendrites of M71-OSNs and IRES-M71-OSNs using an antibody to M71 (Lomvardas et al., 2006). M71 immunofluorescence in dendritic knobs and cilia was quantified by confocal microscopy in the same mice (Fig. 5C). The average fluorescence intensity was reduced by 68% in IRES-M71 OSNs as compared with M71-OSNs (Fig. 5D).
Fig. 5. Axon guidance and odorant specificity with reduced odorant receptor expression.
(A) Diagram of M71 and IRES-M71 alleles. IRES-M71 expressing cells are labeled with GFP and RFP.
(B) Confocal image of the medial olfactory bulb in a mouse expressing M71 (green) and IRES-M71 (yellow) mutations showing dramatic anterior shift in glomerular position with the IRES mutation. Distance between glomeruli is 1.52mm.
(C) Confocal images of M71 (green) and IRES-M71 (green and red) OSNs stained with an antibody against M71 (blue). M71 expression is high in the dendritic knob of M71 OSNs. Images were taken from the sections derived from the same tissue using identical imaging settings. Scale bar = 20 μm.
(D) Quantification of mean pixel values from M71 fluorescence measured from knobs and cilia in epithelial sections. M71 = 1,436 ± 130 (SEM, n=34 cells); IRES-M72 = 454 ± 75 (n= 22 cells). M71 expression is reduced by 68% (p<0.01, t-test).
(E) Average dose-response data for M71- and IRES-M71 OSNs showing no difference for 2-amino acetophenone and a shift for 4-methyl acetophenone (M71 data same as in Fig. 3E).
Despite the marked decrease in M71 protein, the response profile of IRES-M71-OSNs was strikingly similar to M71-OSNs. M71 and IRES-M71 OSNs responded robustly to the M71 ligands ethyl maltol, piperonal and 2-aminoacetophenone (Fig. 5D). Interestingly, IRES-M71 OSNs had a reduced sensitivity to the M71 ligand 4-methyl acetophenone, but the same sensitivity to 2-aminoacetophenone (Fig. 5E and 5F). Thus, reduced OR expression causes a large shift in glomerular position, but a modest shift in odorant response profile.
The data for both IRES-M71 and M71-HybD provide two examples of distinguishable shifts in response profile with respect to the parent allele, M71—one produces a dramatic shift in glomerular position, while the other produces no change in glomerular position. This further supports the idea that glomerular position does not correlate with response profile. Taken together, the data show that there is no direct relationship between glomerular position and odorant response profile.
DISCUSSION
Odorant receptors play a role in axon guidance and chemosensory reception in OSNs. A correlation between these two functions could serve as a mechanism to map stimulus sensitivity directly in the array of glomeruli in the olfactory bulb. Using the model ORs M71 and M72 and M71/M72 hybrids, we show that changes in the OR protein can alter both odorant specificity and glomerular position. However, we also show that the relationship between glomerular location and odorant specificity breaks down in two ways— ORs with different odorant specificity can map to the same glomeruli, and OSNs expressing the same OR and sharing the same response specificity can be mapped to very different regions of the bulb. The data indicate that the olfactory system does not have a mechanism to acutely map inputs to unique bulbar locations based solely on odorant response profile.
One potential limitation of our study is that we cannot know for certain whether the differences in ligand specificity among our mutant receptors result in differential activity during development. For example, depending on stimuli in the environment, activity in M71 and M71-HybD OSNs may be correlated. We feel this is not an issue for several reasons. First, neuronal activity in OSNs has been shown to affect pruning during development, but not glomerular position or axonal identity (Feinstein and Mombaerts, 2004; Zou et al., 2004). Thus, it is unlikely that the failure of M71 and M71-HybD axons to segregate is due to correlated activity. Second, regardless of whether glomerular position is dictated by activity, the important point of our study is that the final location of glomeruli in adult animals is not directly coupled to ligand specificity. These observations have implications for models of odor mapping (see below).
M71 and M72 as model ORs
M71 and M72 are widely used, model ORs (Bozza et al., 2002; Feinstein and Mombaerts, 2004; Fleischmann et al., 2008; Hague et al., 2004; Otaki et al., 2004; Potter et al., 2001; Rothman et al., 2005; Soucy et al., 2009). The first agonist identified for M71 was acetophenone (Bozza et al., 2002), which was subsequently shown to activate M72 as well (Feinstein et al., 2004). Here, we searched for M71 and M72 ligands using a large panel of odorants while recording from native OSNs using a highly sensitive electrophysiological assay. We have identified several previously unknown M71 and M72 ligands. For M72, the most effective agonists are carbonyl compounds that resemble acetophenone such as butyrophenone, as well as the monoterpene menthone and the esters methyl salicylate and ethyl benzoate. Our screen also revealed an oxime, 5-methyl-3-heptanone oxime, as an M72 ligand. Thus, like other ORs, M72 responds to a broad range of compounds with distinct functional groups. Previous in vivo imaging experiments identified the esters ethyl and isopropyl tiglate as highly effective M72 ligands (Soucy et al., 2009). We find that ethyl tiglate activates M72-OSNs in our assay as well and is as effective as other identified M72 ligands.
We have also identified ligands that preferentially activate M71 or M72. We note that the degree of selectivity is concentration dependent. For example, at low concentrations, we can selectively activate M71, but not M72, with 2-amino acetophenone. However, the odorant activates M71 at high concentrations. We have yet to identify a ligand that activates either M71 or M72 exclusively at all concentrations, though it is possible that such a ligand exists.
M71 and M72 are among the most similar odorant receptors in the mouse repertoire, sharing 98% sequence identity (differing by 11 amino acids). We find that M71 and M72 have similar, but distinguishable odorant response profiles. Other studies in heterologous expression systems (Abaffy et al., 2006) or in native OSNs (Tsuboi et al., 2011) have also shown that homologous ORs share similar response properties. For example, MOR29A and MOR29B are homologous linked ORs that differ by 12 amino acid residues. Both receptors show the same relative specificity to two identified ligands, guaiacol and vanillin. In addition, MOR42-1 and MOR42-3, which differ by 13 amino acid residues and divergent C-termini, share similar but distinguishable response specificity to monocarboxylic and dicarboxylic acids (Abaffy et al., 2006). Our data agree with the idea that structurally similar ORs share similar response profiles. However, our data also show that a single amino acid mutation (D205N) in M71 causes a significant shift in odor specificity. This observation raises the possibility that ORs with one or a few critical amino acid substitutions may show considerable shifts in odorant specificity without a large difference in overall homology.
Previous studies have implicated TM3, 5 and 6 in forming a binding pocket for odorants (Abaffy et al., 2007; Katada et al., 2005). Consistent with this view, we observe that the single residue substitution (D205N) in TM5 of M71 is sufficient to convert the response profile from that of M71 to something that is very similar to M72. The data indicate that D205 in TM5 is an important residues that distinguishes the functional differences between M71 and M72. These data are consistent with models for GPCRs in which residues in TM5 are closely associated with a ligand binding region (Tebben and Schnur, 2011). However, our data also show that mutations in TM4 of an OR can affect ligand specificity.
Odorant response and glomerular formation
Previous data suggest that glomeruli are mapped systematically by response similarity (Mori et al., 2006). Such a mapping scheme would require that ORs with different specificities map to unique glomeruli, and that ORs with similar specificities map to neighboring glomeruli. Indeed, linked, homologous ORs (such as M71/M72 and MOR29A/MOR29B) are mapped to neighboring glomeruli (Feinstein and Mombaerts, 2004; Tsuboi et al., 2011; Tsuboi et al., 1999). However, it is unclear whether this is true for most ORs, or for homologous, non-linked ORs. The data presented here show that this correlation is not due to an inexorable link between the axon guidance and chemosensory functions of ORs, and thus may not hold true in all cases. Moreover, our data provide specific examples in which the relationship between glomerular targeting and odorant specificity break down. Previous data suggest a similar decoupling. Transgenic expression of a specific OR can result in broad expression in the epithelium and the formation of multiple glomeruli in different regions of the bulb (Nakatani et al., 2003; Vassalli et al., 2002). However, no data are available from these studies concerning the odorant response specificity of the OSNs that project to the different glomeruli. Here, we show directly that M72-expressing OSNs that project to glomeruli in different regions of the bulb (due to expression in different OSN-types) share the same odorant response profile.
It is worth noting that mapping of a given odorant specificity to two different bulbar locations likely occurs with endogenous OR genes as well. OSNs expressing a given OR occasionally coalesce into two or more glomeruli per convergence site (Bozza et al., 2002; Zheng et al., 2000; Zou et al., 2004). These multiple glomeruli are almost always separated by one or two glomerular widths and are presumed to be functionally similar. It is not possible to test this idea directly using the approach described here. However, optical imaging from glomeruli in the olfactory bulb supports this notion (our unpublished observations). Therefore, it is likely that functionally similar OSNs can be mapped to non-unique positions in the bulb even under normal conditions.
Mapping by coding sequence changes
Our observations have implications for how the glomerular array is organized over evolutionary time. The OR repertoire is rapidly evolving through gene duplication, mutation and gene conversion (Kambere and Lane, 2007; Nagawa et al., 2002; Nei et al., 2008; Sharon et al., 1999; Young and Trask, 2002). What happens when a new OR gene is created by gene duplication? Transgenic and gene targeting studies indicate that a newly formed OR allele would be readily chosen by a subset of OSNs, as is observed for typical OR alleles in the repertoire (Lewcock and Reed, 2004; Rothman et al., 2005; Serizawa et al., 2003; Vassalli et al., 2002). OSNs expressing the new gene would form glomeruli in a location that is determined by a number of factors including receptor primary sequence, expression level, and the identity of the OSNs choosing the new receptor (Bozza et al., 2009; Feinstein et al., 2004; Mombaerts et al., 1996; Wang et al., 1998). As the coding sequence of the duplicated OR gene diverges by random mutation, the resulting OR may develop a novel odorant response profile (which is determined by the expressed OR) and/or axon guidance identity (which is influenced by the expressed OR). Thus, the relationship between these two OR functions dictates the outcome of this evolutionary mapping.
Here we show that several possible outcomes can occur. Mutations may cause a change in response profile and a concomitant change in glomerular formation, resulting in new glomeruli with novel response profiles. Mutations may also change the response profile of OSNs without changing axonal identity, resulting in a glomerulus that combines functionally distinct inputs. It is difficult to know the degree to which these different scenarios have occurred during olfactory evolution because work is typically done using inbred mouse strains that are homozygous at all loci and retain OR alleles from limited haplotypes. Future work expressing polymorphic ORs in gene targeted mice would help determine the relationship between glomerular formation and response specificity in naturally occurring OR variants.
Our studies make use of genetically designed, hybrid receptors. It is likely that our findings hold for allelic variants of ORs as well. Previous data show that OSNs expressing the MOR28 alleles from 129 and C57BL/6 (that vary by one amino acid residue) project to the same glomerulus but segregate into compartments (Ishii et al., 2001). Similar and more severe phenotypes are observed for allelic variants of the ORs P2, M50, P4 or M72 (Feinstein and Mombaerts, 2004). The data clearly show that allelic variants can encode polymorphic ORs that change axon guidance identity and presumably odorant specificity, similar to what is seen in the M71/M72 hybrids. The strength of our study is that we can directly measure such differences in odorant specificity.
Mapping by shifts in cell type
In addition to coding sequence changes, mutations may occur in enhancers or promoters that bias the probability of choice to different populations of OSNs in the epithelium (Rothman et al., 2005). OSNs in the dorsal epithelium have at least two distinct identities and project to separate glomerular domains (Bozza et al., 2009). Thus, glomerular position would be affected by promoter/enhancer mutations that alter which OSN-type chooses a given OR gene. Our data demonstrate that such a shift would cause a change glomerular position without a concomitant change in odorant response profile. A similar case could be made for mutations that shift expression of an OR gene from the dorsal epithelium, which projects to the dorsal bulb, to the ventral epithelium, which projects to the ventral bulb (Schoenfeld and Cleland, 2005). These data are consistent with our previous findings that that expression of the OR I7 in dorsal or ventral OSNs results in similar response specificity (Bozza et al., 2002). Gene conversion can shuffle coding sequences and promoters during evolution (Kambere and Lane, 2007). Thus, we consider our genetically designed allele (M72→S50) a valid model for mutations that similarly shift OR expression to a different OSN-types during the evolution of native OR genes.
Mapping by shifts in OR expression
Our data also show that knocking down the level or OR protein does not dramatically affect the odorant response specificity despite have a pronounced effect on glomerular position. Previous data indicated that the IRES-M71 mutation reduces OR expression by 10-fold (Feinstein et al., 2004). Using an antibody we estimate a reduction to roughly 1/3 normal OR levels. The discrepancy is likely caused by differences in measurement methods. The previous experiments quantified relative levels of reporter gene co-expression, while we are detecting OR protein by immunohistochemistry and the stability of the proteins being measured could be different. Our data are also relatively conservative as we averaged immunofluorescence in a fixed area of the dendritic knobs and cilia—some fraction of the pixels in each case did not contain cilia/dendrite and thus did not contribute to the difference. In any case, our data show for the first time that the IRES-M71 mutation significantly reduces M71 protein expression. The reduction in M71 protein had only a modest effect on odorant sensitivity, perhaps indicating that OSNs retain a large spare receptor capacity (Cleland and Linster, 1999), or possess homeostatic mechanisms that maintain stimulus sensitivity despite the decrease in OR expression. We favor the former explanation since we see no evidence for increased transduction efficiency in IRES-M71 OSNs (our unpublished observations). It is currently unclear why decreased OR expression affects the sensitivity to one ligand, but not another. This could relate to the affinity of M71 for the two ligands as sensitivity decreased for the ligand with the lower apparent EC50.
Summary
Taken together, our data highlight the idea that glomerular position (and thus odor mapping) comes about by random mutation and selection of OR genes over evolutionary time, rather than by organizing inputs based directly on stimulus specificity (i.e. through activity dependent mechanisms). In this view, the precision of the functional mapping that can result would be limited by the resolution of this selection process. This might explain the lack of a clear, fine-scale functional topography in the organization of glomeruli in the mouse olfactory bulb.
Supplementary Material
ACKNOWLEDGEMENTS
Thanks to Brian Weiland, Dillon Cawley, and Stephanie Leung for technical assistance, Givaudan-Roure for providing odorants, and Gilad Barnea for the M71 antibody. Work was supported by grants from NIH-NIDCD, Whitehall Foundation, and Brain Research Foundation (TB), and the National Center for Research Resources and the National Institute on Minority Health Disparities (PF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abaffy T, Malhotra A, Luetje CW. The molecular basis for ligand specificity in a mouse olfactory receptor: a network of functionally important residues. J Biol Chem. 2007;282:1216–1224. doi: 10.1074/jbc.M609355200. [DOI] [PubMed] [Google Scholar]
- Abaffy T, Matsunami H, Luetje CW. Functional analysis of a mammalian odorant receptor subfamily. J Neurochem. 2006;97:1506–1518. doi: 10.1111/j.1471-4159.2006.03859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza T, Feinstein P, Zheng C, Mombaerts P. Odorant receptor expression defines functional units in the mouse olfactory system. J Neurosci. 2002;22:3033–3043. doi: 10.1523/JNEUROSCI.22-08-03033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza T, McGann JP, Mombaerts P, Wachowiak M. In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron. 2004;42:9–21. doi: 10.1016/s0896-6273(04)00144-8. [DOI] [PubMed] [Google Scholar]
- Bozza T, Vassalli A, Fuss S, Zhang JJ, Weiland B, Pacifico R, Feinstein P, Mombaerts P. Mapping of class I and class II odorant receptors to glomerular domains by two distinct types of olfactory sensory neurons in the mouse. Neuron. 2009;61:220–233. doi: 10.1016/j.neuron.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chklovskii DB, Koulakov AA. Maps in the brain: what can we learn from them? Annu Rev Neurosci. 2004;27:369–392. doi: 10.1146/annurev.neuro.27.070203.144226. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Linster C. Concentration tuning mediated by spare receptor capacity in olfactory sensory neurons: A theoretical study. Neural Comput. 1999;11:1673–1690. doi: 10.1162/089976699300016188. [DOI] [PubMed] [Google Scholar]
- Farahbod H, Johnson BA, Minami SS, Leon M. Chemotopic representations of aromatic odorants in the rat olfactory bulb. J Comp Neurol. 2006;497:350–366. doi: 10.1002/cne.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein P, Bozza T, Rodriguez I, Vassalli A, Mombaerts P. Axon guidance of mouse olfactory sensory neurons by odorant receptors and the beta2 adrenergic receptor. Cell. 2004;117:833–846. doi: 10.1016/j.cell.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Feinstein P, Mombaerts P. A contextual model for axonal sorting into glomeruli in the mouse olfactory system. Cell. 2004;117:817–831. doi: 10.1016/j.cell.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Fleischmann A, Shykind BM, Sosulski DL, Franks KM, Glinka ME, Mei DF, Sun Y, Kirkland J, Mendelsohn M, Albers MW, Axel R. Mice with a “monoclonal nose”: perturbations in an olfactory map impair odor discrimination. Neuron. 2008;60:1068–1081. doi: 10.1016/j.neuron.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hague C, Uberti MA, Chen Z, Bush CF, Jones SV, Ressler KJ, Hall RA, Minneman KP. Olfactory receptor surface expression is driven by association with the beta2-adrenergic receptor. Proc Natl Acad Sci U S A. 2004;101:13672–13676. doi: 10.1073/pnas.0403854101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JG, Shepherd GM. Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci. 1997;20:595–631. doi: 10.1146/annurev.neuro.20.1.595. [DOI] [PubMed] [Google Scholar]
- Ishii T, Serizawa S, Kohda A, Nakatani H, Shiroishi T, Okumura K, Iwakura Y, Nagawa F, Tsuboi A, Sakano H. Monoallelic expression of the odourant receptor gene and axonal projection of olfactory sensory neurones. Genes Cells. 2001;6:71–78. doi: 10.1046/j.1365-2443.2001.00398.x. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ho SL, Xu Z, Yihan JS, Yip S, Hingco EE, Leon M. Functional mapping of the rat olfactory bulb using diverse odorants reveals modular responses to functional groups and hydrocarbon structural features. J Comp Neurol. 2002;449:180–194. doi: 10.1002/cne.10284. [DOI] [PubMed] [Google Scholar]
- Jourdan F, Duveau A, Astic L, Holley A. Spatial distribution of [14C]2-deoxyglucose uptake in the olfactory bulbs of rats stimulated with two different odours. Brain Res. 1980;188:139–154. doi: 10.1016/0006-8993(80)90563-6. [DOI] [PubMed] [Google Scholar]
- Kambere MB, Lane RP. Co-regulation of a large and rapidly evolving repertoire of odorant receptor genes. BMC Neurosci. 2007;8(Suppl 3):S2. doi: 10.1186/1471-2202-8-S3-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katada S, Hirokawa T, Oka Y, Suwa M, Touhara K. Structural basis for a broad but selective ligand spectrum of a mouse olfactory receptor: mapping the odorant-binding site. J Neurosci. 2005;25:1806–1815. doi: 10.1523/JNEUROSCI.4723-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI, du Lac S, Esterly SD. Computational maps in the brain. Annu Rev Neurosci. 1987;10:41–65. doi: 10.1146/annurev.ne.10.030187.000353. [DOI] [PubMed] [Google Scholar]
- Lancet D, Greer CA, Kauer JS, Shepherd GM. Mapping of odor-related neuronal activity in the olfactory bulb by high-resolution 2-deoxyglucose autoradiography. Proc Natl Acad Sci U S A. 1982;79:670–674. doi: 10.1073/pnas.79.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewcock JW, Reed RR. A feedback mechanism regulates monoallelic odorant receptor expression. Proc Natl Acad Sci U S A. 2004;101:1069–1074. doi: 10.1073/pnas.0307986100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomvardas S, Barnea G, Pisapia DJ, Mendelsohn M, Kirkland J, Axel R. Interchromosomal interactions and olfactory receptor choice. Cell. 2006;126:403–413. doi: 10.1016/j.cell.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Luo L, Flanagan JG. Development of continuous and discrete neural maps. Neuron. 2007;56:284–300. doi: 10.1016/j.neuron.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Ma L, Qiu Q, Gradwohl S, Scott A, Yu EQ, Alexander R, Wiegraebe W, Yu CR. Distributed representation of chemical features and tunotopic organization of glomeruli in the mouse olfactory bulb. Proc Natl Acad Sci U S A. 2012;109:5481–5486. doi: 10.1073/pnas.1117491109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Chen WR, Shepherd GM. Electrophysiological characterization of rat and mouse olfactory receptor neurons from an intact epithelial preparation. J Neurosci Meth. 1999;92:31–40. doi: 10.1016/s0165-0270(99)00089-8. [DOI] [PubMed] [Google Scholar]
- Meister M, Bonhoeffer T. Tuning and topography in an odor map on the rat olfactory bulb. J Neurosci. 2001;21:1351–1360. doi: 10.1523/JNEUROSCI.21-04-01351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P. Axonal wiring in the mouse olfactory system. Annu Rev Cell Dev Biol. 2006;22:713–737. doi: 10.1146/annurev.cellbio.21.012804.093915. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. Maps of odorant molecular features in the Mammalian olfactory bulb. Physiol Rev. 2006;86:409–433. doi: 10.1152/physrev.00021.2005. [DOI] [PubMed] [Google Scholar]
- Murthy VN. Olfactory maps in the brain. Annu Rev Neurosci. 2011;34:233–258. doi: 10.1146/annurev-neuro-061010-113738. [DOI] [PubMed] [Google Scholar]
- Nagawa F, Yoshihara S, Tsuboi A, Serizawa S, Itoh K, Sakano H. Genomic analysis of the murine odorant receptor MOR28 cluster: a possible role of gene conversion in maintaining the olfactory map. Gene. 2002;292:73–80. doi: 10.1016/s0378-1119(02)00670-4. [DOI] [PubMed] [Google Scholar]
- Nakatani H, Serizawa S, Nakajima M, Imai T, Sakano H. Developmental elimination of ectopic projection sites for the transgenic OR gene that has lost zone specificity in the olfactory epithelium. Eur J Neurosci. 2003;18:2425–2432. doi: 10.1046/j.1460-9568.2003.02998.x. [DOI] [PubMed] [Google Scholar]
- Nei M, Niimura Y, Nozawa M. The evolution of animal chemosensory receptor gene repertoires: roles of chance and necessity. Nat Rev Genet. 2008;9:951–963. doi: 10.1038/nrg2480. [DOI] [PubMed] [Google Scholar]
- Otaki JM, Yamamoto H, Firestein S. Odorant receptor expression in the mouse cerebral cortex. J Neurobiol. 2004;58:315–327. doi: 10.1002/neu.10272. [DOI] [PubMed] [Google Scholar]
- Pacifico R, Dewan A, Cawley D, Guo C, Bozza T. An Olfactory Subsystem that Mediates High-Sensitivity Detection of Volatile Amines. Cell Reports. 2012 doi: 10.1016/j.celrep.2012.06.006. 10.1016/j.celrep.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter SM, Zheng C, Koos DS, Feinstein P, Fraser SE, Mombaerts P. Structure and emergence of specific olfactory glomeruli in the mouse. J Neurosci. 2001;21:9713–9723. doi: 10.1523/JNEUROSCI.21-24-09713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman A, Feinstein P, Hirota J, Mombaerts P. The promoter of the mouse odorant receptor gene M71. Mol Cell Neurosci. 2005;28:535–546. doi: 10.1016/j.mcn.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, Cleland TA. The anatomical logic of smell. Trends in neurosciences. 2005;28:620–627. doi: 10.1016/j.tins.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Nakatani H, Suzuki M, Saito M, Yoshihara Y, Sakano H. Negative feedback regulation ensures the one receptor-one olfactory neuron rule in mouse. Science. 2003;302:2088–2094. doi: 10.1126/science.1089122. [DOI] [PubMed] [Google Scholar]
- Sharon D, Glusman G, Pilpel Y, Khen M, Gruetzner F, Haaf T, Lancet D. Primate evolution of an olfactory receptor cluster: diversification by gene conversion and recent emergence of pseudogenes. Genomics. 1999;61:24–36. doi: 10.1006/geno.1999.5900. [DOI] [PubMed] [Google Scholar]
- Soucy ER, Albeanu DF, Fantana AL, Murthy VN, Meister M. Precision and diversity in an odor map on the olfactory bulb. Nat Neurosci. 2009;12:210–220. doi: 10.1038/nn.2262. [DOI] [PubMed] [Google Scholar]
- Takahashi YK, Kurosaki M, Hirono S, Mori K. Topographic representation of odorant molecular features in the rat olfactory bulb. J Neurophysiol. 2004;92:2413–2427. doi: 10.1152/jn.00236.2004. [DOI] [PubMed] [Google Scholar]
- Tebben AJ, Schnur DM. Beyond rhodopsin: G protein-coupled receptor structure and modeling incorporating the beta2-adrenergic and adenosine A(2A) crystal structures. Methods Mol Biol. 2011;672:359–386. doi: 10.1007/978-1-60761-839-3_15. [DOI] [PubMed] [Google Scholar]
- Treloar HB, Feinstein P, Mombaerts P, Greer CA. Specificity of glomerular targeting by olfactory sensory axons. J Neurosci. 2002;22:2469–2477. doi: 10.1523/JNEUROSCI.22-07-02469.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi A, Imai T, Kato HK, Matsumoto H, Igarashi KM, Suzuki M, Mori K, Sakano H. Two highly homologous mouse odorant receptors encoded by tandemly-linked MOR29A and MOR29B genes respond differently to phenyl ethers. Eur J Neurosci. 2011;33:205–213. doi: 10.1111/j.1460-9568.2010.07495.x. [DOI] [PubMed] [Google Scholar]
- Tsuboi A, Yoshihara S, Yamazaki N, Kasai H, Asai-Tsuboi H, Komatsu M, Serizawa S, Ishii T, Matsuda Y, Nagawa F, Sakano H. Olfactory neurons expressing closely linked and homologous odorant receptor genes tend to project their axons to neighboring glomeruli on the olfactory bulb. J Neurosci. 1999;19:8409–8418. doi: 10.1523/JNEUROSCI.19-19-08409.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli A, Rothman A, Feinstein P, Zapotocky M, Mombaerts P. Minigenes impart odorant receptor-specific axon guidance in the olfactory bulb. Neuron. 2002;35:681–696. doi: 10.1016/s0896-6273(02)00793-6. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron. 2001;32:723–735. doi: 10.1016/s0896-6273(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Denk W, Friedrich RW. Functional organization of sensory input to the olfactory bulb glomerulus analyzed by two-photon calcium imaging. Proc Natl Acad Sci U S A. 2004;101:9097–9102. doi: 10.1073/pnas.0400438101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Nemes A, Mendelsohn M, Axel R. Odorant receptors govern the formation of a precise topographic map. Cell. 1998;93:47–60. doi: 10.1016/s0092-8674(00)81145-9. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Mainen ZF. Early events in olfactory processing. Annu Rev Neurosci. 2006;29:163–201. doi: 10.1146/annurev.neuro.29.051605.112950. [DOI] [PubMed] [Google Scholar]
- Young JM, Trask BJ. The sense of smell: genomics of vertebrate odorant receptors. Hum Mol Genet. 2002;11:1153–1160. doi: 10.1093/hmg/11.10.1153. [DOI] [PubMed] [Google Scholar]
- Zheng C, Feinstein P, Bozza T, Rodriguez I, Mombaerts P. Peripheral olfactory projections are differentially affected in mice deficient in a cyclic nucleotide-gated channel subunit. Neuron. 2000;26:81–91. doi: 10.1016/s0896-6273(00)81140-x. [DOI] [PubMed] [Google Scholar]
- Zou DJ, Feinstein P, Rivers AL, Mathews GA, Kim A, Greer CA, Mombaerts P, Firestein S. Postnatal refinement of peripheral olfactory projections. Science. 2004;304:1976–1979. doi: 10.1126/science.1093468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.