Summary
Background
Soon after birth all mammals must initiate milk suckling to survive. In rodents, this innate behavior is critically dependent on uncharacterized maternally-derived chemosensory ligands. Recently the first pheromone sufficient to initiate suckling was isolated from the rabbit. Identification of the olfactory cues that trigger first suckling in the mouse would provide the means to determine the neural mechanisms that generate innate behavior.
Results
Here we use behavioral analysis, metabolomics, and calcium imaging of primary sensory neurons and find no evidence of ligands with intrinsic bioactivity, such as pheromones, acting to promote first suckling in the mouse. Instead, we find that the initiation of suckling is dependent on variable blends of maternal ‘signature odors’ that are learned and recognized prior to first suckling.
Conclusions
As observed with pheromone-mediated behavior, the response to signature odors releases innate behavior. However, this mechanism tolerates variability in both the signaling ligands and sensory neurons which may maximize the probability that this first essential behavior is successfully initiated. These results suggest that mammalian species have evolved multiple strategies to ensure the onset of this critical behavior.
Introduction
How a mammalian newborn intrinsically displays the first episode of suckling behavior without previous experience is largely unknown. In most species, including humans, suckling involves an uncharacterized, influential, maternally-derived olfactory component [1-8]. Olfaction in the mouse is mediated by at least two distinct olfactory subsystems including the main olfactory epithelium (MOE) and the vomeronasal organ (VNO) [9]. A number of molecularly characterized specialized odor cues promote mammalian behavior via sensory neurons of the VNO, [10-12] and genetic disruption of this organ results in deficits in innate behaviors [11, 13-15]. The MOE is largely tasked with the perception, associative learning of, and discrimination between odors [16-19]. However, behavioral and genetic studies of mice have revealed that unknown subsets of neurons in the MOE are necessary to regulate some innate behaviors such as pup suckling, mating, aggression, and innate avoidance [20-22]. In mice, genetic ablation of MOE function results in neonatal lethality [2]. This has been attributed to dehydration as a result of failure to suckle, as mutant pups do not show the characteristic “white abdomen” of milk in their stomach [1-3, 7, 8, 23]. These observations establish the importance of olfaction to initiate the innate, first episode, of suckling [3, 8], and collectively indicate that salient olfactory cues that initiate suckling behavior are detected by sensory neurons of the MOE.
The identities of the odorants that promote suckling from naïve newly-born pups remain largely unknown [8]. Early behavioral studies in rodents indicate that maternally produced milk, amniotic fluid, or saliva may each contain bioactive odorants [5, 24]. Analysis of the pup olfactory bulb following suckling behavior has found an increase in 2-deoxy-glucose incorporation in a subset of unusually shaped glomeruli, the macro-glomerular complex, suggesting that receptors projecting to these specialized glomeruli are responsive to a specialized suckling odor cue [25-28]. Pheromones are specialized olfactory cues which elicit innate stereotypical behavioral, developmental, or physiological responses when detected by others of the same species [29]. Recently, a classic suckling pheromone has been identified in a non-model system, the European Rabbit. 2-methylbut-2-enal is present in the milk of the rabbit dam, detected by neurons in the MOE, and is sufficient to robustly promote innate suckling [30, 31]. This finding supports the role of specialized olfactory cues, pheromones, to trigger the first episode of naïve suckling behavior. Strikingly, the European rabbit has an unusual, absentee, parenting style; spending all but minutes of each day avoiding the nest to minimize the attraction of predators [3]. This offers a dramatically brief time for the initiation and completion of the first suckling behavior and subsequent suckling experience. Whether other mammals rely on a pheromone to trigger the release of innate suckling behavior is unknown.
Odor-mediated learning has been well established in rodents. Pairing of a neutral odor (a conditioned stimulus, CS) with the suckling of milk (an unconditioned stimulus, US) results in subsequent odor preferences for the CS, which can last until adulthood [32-35]. Furthermore, simply pairing the neutral odor with native olfactory cues from the dam conditions neutral odors to stimulate suckling [36]. The underlying mechanism for this observation has been identified in the rabbit: simply pairing the suckling pheromone (US) with a neutral odor (CS) is sufficient to charge the odor with behavioral significance [36-39]. The olfactory cues that act as the US in rodents remain unknown [40].
Here, we investigate the olfactory ligands emitted from the dam that promote innate first suckling behavior in mice. We find that maternally emitted odors previously thought to contain suckling promoting bioactivity, such as saliva and milk, do not elicit suckling when first experienced, and demonstrate that their bioactivity is conditioned. We find amniotic fluid to promote suckling in newborns, but unlike the single pheromone instructing the rabbit [31], the mouse bioactivity is a blend of at least two different volatile cues. We perform in utero alteration of native amniotic fluid and find that the bioactivity is inconsistent with that expected of a classic pheromone [41]. Instead, our results indicate that the generation of suckling requires experience with maternally emitted olfactory ‘signature mixtures’ of odorants. These are of variable ligand composition and do not contain odors with intrinsic pre-set bioactivity [29]. The biology of gestation and birth reliably ensures odor experience so that the generation of first suckling from this mechanism appears innate. Our study indicates that mammalian species have evolved multiple strategies to ensure the onset of this critical behavior.
Results
Nipple lavage generates a platform to identify bioactive ligands
The newborn mouse pup is too small and weak to access the nipple without maternal positioning or experimenter assistance. To identify the olfactory stimuli underlying innate suckling in mice we developed a robust, behavioral assay to quantify olfactory mediated suckling behavior in the newborn. We modified a strategy from those previously used in rats [5, 24] by hand-stabilizing individual pups a heads-length from the ventrum of an anaesthetized lactating female and assayed the time required to locate the nipple and begin stereotypical orocephalic movements indicative of suckling (Fig. 1A, Movie S1). We quantified the ability of suckling-experienced pups, which were under 12 hrs old but observed to have milk in their stomach, to initiate suckling behavior. All animals rapidly located the nipple and began suckling (Fig. 1B-C). We next confirmed the contribution of olfactory cues in promoting this behavior. Simply washing the nipple and surrounding area by triturating with water prevented the majority of pups tested from suckling within a 15 minute trial (Fig. 1B-C) [5, 24]. Those few animals that did suckle demonstrated a significant behavioral delay (mean latency of 99.8 sec compared to 22.8 sec, P<0.0005). To determine the potential for bias by the experimenter we repeated this analysis by initiating the assay with the pup's nose directly on the nipple. Even in these most favorable circumstances, in which suckling-experienced mice were provided direct tactile and thermal stimulus of a lactating nipple, we found that washing the nipple significantly impaired suckling behavior (Fig. S1A).
Figure 1. Innate pup-suckling behavior serves as a platform to identify the underlying olfactory stimuli.
(A) Mouse pups display stereotyped nipple-search and suckling behavior, including, from top, head scanning, rooting and nuzzling, grasping and attachment. (B) The latency-to-suckle in wild-type mice on an unwashed nipple (mean 22.8 sec + 3.2 SEM) is increased (614.2 sec + 106.8 SEM) when the nipple is washed with water (n = 14). (C) Data from B binned in 10 sec windows, demonstrating the different distribution of suckling latencies. (D) The majority of mice lacking Cnga2 fail to locate the nipple after 2 min, TrpC2-/- mice display behavior similar to wild-type (WT) mice. Removal of sensory cues by washing the dam's nipple results in a deficient suckling response similar to Cnga2y/- mutants, which have a lethal phenotype (n = 16-77). (E) Several biologically relevant, maternal odors, initiate wild-type pup suckling behavior in neonatal mice when painted on a washed nipple (n = 8-27). Statistical differences were tested against WT pups suckling on a unwashed dam (B,C,D) or washed dam (E), with Kruskal-Wallis One Way ANOVA followed by a post hoc Dunn Test: ** Q>Q(0.01); *** Q>Q(0.001). Mice that did not suckle (DNS) at the end of each assay were given a latency value of 900 sec (B,C) or 120 sec (D,E) for statistical testing.
Newborn mammals with a functionally ablated VNO have no reported suckling deficits, but those lacking a functional MOE or olfactory bulb die shortly after birth without the characteristic abdominal milk spot that indicates successful suckling [1-3, 7, 42]. Since suckling requires considerable interactions between the dam and the pup we next quantified the extent to which olfactory mutant pups are able to suckle in our more favorable experimental assay. To enable all newborns to feed expediently, we restricted all subsequent assays to the duration of two minutes (see statistical rationale in supplementary information). As expected, we found mutant pups lacking TrpC2, the primary transduction channel of VNO sensory neurons [13, 15, 43], locate the nipple and suckle as efficiently as wild type pups (Fig. 1D, S1A). Corresponding with their failure to thrive, newborn mice lacking Cnga2, the primary signal transduction channel of MOE sensory neurons [2, 44], show significant defects in initiating suckling from an unwashed nipple in our behavioral assay. Though some of the Cnga2y/-mutant pups (as well as wild-type pups presented with the washed nipple lacking the olfactory cues) are able to initiate suckling under the favorable conditions of our assay, the severity of this suckling defect is such that it results in high levels of neonatal lethality under ethologically relevant maternal-pup interactions [2]. Importantly, the behavior of Cnga2y/- pups on an unwashed nipple quantitatively mimics the defect observed from wild-type pups towards a washed nipple (Fig. 1D). This analysis establishes a quantifiable assay to measure suckling behavior. Washing the nipple provides a robust, investigational platform from which we can experimentally manipulate native odors in order to identify the suckling promoting cue(s) in mice.
Amniotic fluid contains suckling-promoting olfactory ligands
Rodent maternal behavior following birth is highly stereotyped. First, the dam removes the amniotic sac, then licks the pup which cleanses and stimulates respiration, and finally licks clean her own ventrum prior to first nursing ([45] and DWL, unpublished observations). This behavior reliably exposes the naive pup to multiple maternal fluids such as amniotic fluid, saliva, and milk. To determine the maternal source of the suckling promoting bioactivity we swabbed maternal fluids onto water-washed nipples and assayed the ability to initiate pup suckling. Maternal amniotic fluid, saliva, and milk were each sufficient to reinstate robust suckling when swabbed on a washed nipple (Fig. 1E). However, other complex native fluids including saliva from virgin females and maternal urine do not promote suckling, nor does a panel of biologically non relevant odorants (Figs. 1E and S1B). Maternal saliva and amniotic fluid have similarly been shown to promote attachment in newborn rats placed in physical contact with a nipple [5]. Due to both their conserved suckling promoting bioactivities, and biological relevance to the newborn mouse pup, we further evaluated the bioactivity of these fluids to identify the suckling promoting ligands.
The rabbit suckling pheromone can act as an unconditioned stimulus to transfer behavior promoting activity to intrinsically irrelevant odorants [38]. Further, the ability of suckling-experienced rodents to rapidly condition suckling behavior to novel odors is well established [34, 39]. We considered that some of the suckling-promoting bioactivity of the mouse maternal fluids may result from the pairing of an unconditioned stimulus (a pheromone) with odor sources that do not contain inherent bioactivity. To test this, we eliminated odor exposure to maternal exocrine fluids (except amniotic fluid) by performing a Caesarean section (c-section) at e20.5 and then reproduced the essential maternal licking behavior by briefly stroking the pup with a clean brush to gently stimulate respiration (Fig. S2A). We reasoned that the elimination of the pup's perinatal experience with a pheromone-containing fluid would not alter its ability to initiate the first suckling episode on exposure at the nipple. However, when these odor-naïve c-sectioned pups were presented to a water-washed nipple swabbed with maternal odors that previously promoted suckling (Fig. 1E), no significant suckling behavior was initiated (Figs. 2A-E and S2B). In contrast, amniotic fluid rapidly promoted suckling confirming that the c-sectioned mice were healthy and able to suckle (Fig. 2F). This indicates that neither the mouse saliva, milk, nor colostrum, contain a classical suckling pheromone. This is in contrast to the source of the bioactivity in the European rabbit, which is emitted by the mammary gland [31]. To confirm that the response to saliva was indeed conditioned, we experimentally provided odor experience by stroking the neonate with a brush coated with saliva, modeling the natural licking behavior of the mother immediately after birth (Fig. S2C). This was sufficient to reinstate the suckling-promoting activity of saliva odor on the dam's nipple (Fig. 2H-I). This experimental procedure is also sufficient to condition both milk and colostrum with bioactivity (Fig. 2K-L). We likewise experimentally exposed c-sectioned animals to odors which our bioassay previously showed to lack inherent biological significance (Figs. 2C, G and S2B). We found that gently brushing neonates with either simple (vanillin) or complex (virgin saliva) odorous fluids charged them with suckling-promoting activity (Figs. 2J, N and S2C). To ensure that we did not miss an alternative post-natal source of maternal pheromones, such as a temporally-regulated product of glands around the newly parturient mother's nipples, we assayed the ability of pups to locate and attach to the nipples of a mother immediately after delivery, but prior to significant self-grooming or the first suckling episode. Pups delivered by c-section did not respond with robust suckling until amniotic fluid was applied to the nipple (Fig. 2O). In total, we were unable to identify any native fluid, besides amniotic fluid (Fig. 2F), that contains chemosensory cues that intrinsically function to promote suckling behavior in naïve, newborn mice.
Figure 2. Post-natal olfactory cues do not display intrinsic suckling-promoting activity.
(A-G) Mice delivered by c-section, to restrict odor experience with post natal maternal fluids, initiate suckling only when amniotic fluid (F) is painted on a water washed nipple (A), not maternal (B) or virgin saliva (C), milk (D), colostrum (E) or vanillin (G) (n = 8-15). (H-N) Providing odor experience after c-section delivery reinstates the suckling-promoting activity to both biologically relevant (I-L) and non-relevant odors (vanillin, N) painted on a washed nipple (H) (n = 8-15). O, C-section delivered mice do not suckle on a nipple of an post-parturient mother immediately assayed prior to self-grooming, unless amniotic fluid is swabbed on the nipple (n = 10). Statistical comparisons B-G were against A; I-N against H; panel O, left, against O right, all using Kruskal-Wallis One Way ANOVA followed by a post hoc Dunn Test: * Q>Q(0.05); ** Q>Q(0.01); *** Q>Q(0.001).
Suckling promoting bioactivity is a blend of olfactory cues
2-deoxyglucose uptake patterns in the olfactory bulb following suckling behavior of rodent pups have revealed focal activity in the macroglomerular complex [28] and analysis of mouse odorant receptors has found differential expression of subsets of odorant receptors on the day of birth compared to older animals [46]. Therefore, we examined the extent to which perinatal MOE neurons are preferentially tuned to a restricted subset of biologically relevant cues from amniotic fluid. There is conflicting data on the ability of the rodent pup MOE neurons to detect odors pre- or perinatally [36, 47-49]. We developed and validated a calcium-imaging based assay to analyze the relative activity of dissociated MOE sensory neurons from embryonic day 17.5 (e17.5) through birth, compared to the response of adult neurons (Fig. S3A-D). We found an increasing proportion of neurons respond to both relevant sources (amniotic fluid) and simple control odors (vanillin, lyral) during late gestation until birth, but no difference in the response profile between neonates and adults (Figs. 3 and S3E-F). This demonstrates that canonical MOE neurons of newborn pups are sufficiently mature to detect and respond to a wide repertoire of olfactory cues at the time the first suckling episode is initiated. However, in spite of the evidence of temporal differences in the expression of olfactory receptors [46], we observed no detectable functional bias towards cues present in amniotic fluid in newborn MOE.
Figure 3. Newborn olfactory neurons detect the same blend of multiple odor cues as adults.
(A) The number of MOE neurons responsive to odor stimuli increase throughout late gestation (n= 1638 to 2969, ± SEM). (B) The neural response to biologically relevant and non-relevant odors at birth (n = 3650, + SEM) is not statistically different to that of adults (n = 1169, + SEM; ns = P>0.05 by One Way ANOVA followed by Tukey-Kramer HSD post hoc analysis).
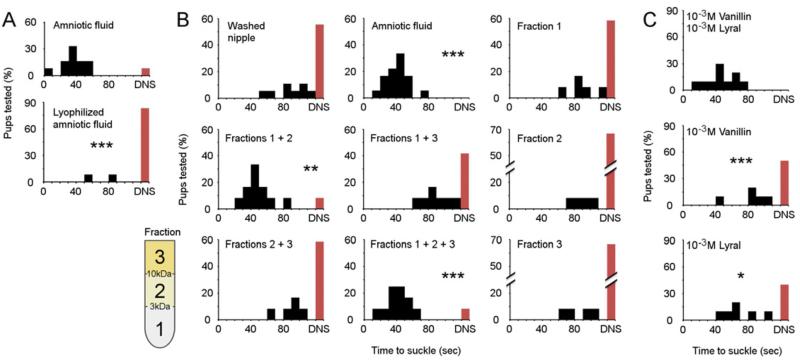
We next investigated the basic physical properties of the bioactive cue(s) present in amniotic fluid itself. Though the MOE is largely tasked with detecting small, volatile odor molecules, some lower volatility peptides have been shown to activate olfactory neurons [50, 51]. We removed volatiles from amniotic fluid by lyophylization and found the residual non-volatile fraction was insufficient to promote suckling when swabbed on washed nipples (Fig. 4A) indicating that the bioactivity is at least partly volatile. We additionally performed a simple three-way molecular weight size fractionation, then assayed suckling behavior in response to a water-washed nipple swabbed with each fraction or combination thereof. We found no fraction alone to be sufficient to initiate suckling (Fig. 4B), indicating that a single chemosensory cue, such as the rabbit mammary pheromone, is insufficient to initiate pup suckling. However, when we blended the two fractions containing molecules of smaller molecular mass (<10kDa) significant suckling activity was reinstated (Fig. 4B). This indicates that the bioactivity is composed of a blend of at least two cues, either a multi-component pheromone or odor blend.
Figure 4. Characteristics of the molecules in amniotic fluid that promote suckling.
(A) Lyophilization of amniotic fluid ablates suckling-promoting activity (n = 12). (B) Physical fractionation of amniotic fluid reveals that pup suckling is not mediated by a single cue. Neither amniotic fluid fraction 1 (<3kDa), 2 (between 3 and 10 kDa) nor 3 (>10kDa) are alone sufficient to promote suckling when painted in a washed nipple. Suckling-promoting activity requires fractions 1 and 2 (<10kDa) (n = 12-18). C, Odor cues that initiate innate suckling behavior are detected as a blend. All c-sectioned pups were brushed with an equal molar lyral/vanillin odor blend and suckling responses were measured either to that blend (top) or each constituent odor alone (beneath). The individual odors are ineffective at promoting suckling (n = 10). Statistical differences were tested against WT pups suckling in response to amniotic fluid (C), a water washed nipple (D), or the odor blend (E) with Kruskal-Wallis One Way ANOVA followed by a post hoc Dunn Test: * Q>Q(0.05); ** Q>Q(0.01); *** Q>Q(0.001).
Finally, we used the conditioning assay (Figure S2C) to determine if suckling behavior is promoted elementally, by individual ligand components which would be consistent with some currently known mouse pheromones [10, 12, 52], or configurally as a classic odor or pheromone blend [19, 53-55]. We investigated the coding strategy of two biologically irrelevant odors to initiate suckling behavior. We first brushed e20.5 c-sectioned delivered pups with a blend of 1mM vanillin and 1mM lyral and then swabbed either the blend or each of the constituent odors alone, on a water-washed dam nipple and assayed suckling behavior. Robust suckling occurred only in response to nipples swabbed with the blend, indicating that the constituent odors of the blend are not sufficiently conditioned elementally by the mouse pups, but as a complex odor (Fig. 4C). We then tested the limits of neonatal discrimination of odor blends by increasing the proportion of either odorant in a blend used to brush the pups. Only when either odorant is 102 times more concentrated than the other, is it effective in promoting suckling as the blend itself (Fig. S4). Therefore, even with this simple combination of two odor cues, the relevant signal that initiates suckling behavior appears to be encoded as a blend.
Mouse suckling bioactivity is not consistent with classic pheromone mechanisms
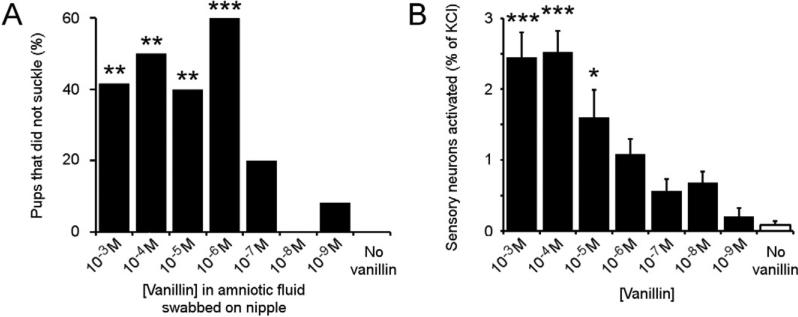
The isolation of behaviorally bioactive volatiles from complex blends is technically challenging. The volatile rabbit suckling-promoting pheromone was identified using gas chromatography-mass-spectrometry (GCMS) analysis coupled directly with an in vivo behavioral assay [31]. However application of this technique to resolve even a simple blend of two bioactive volatiles requires significantly longer assay durations that are beyond the viability limits of our newborn mice. Prior to initiating an effort to create a novel experimental strategy we were motivated to confirm that inherently bioactive ligands, pheromones, were indeed present in amniotic fluid. We considered that a different olfactory mechanism, learning of a signature odor, may alternately underlie the first episode of pup suckling. Unlike pheromones, signature odors are not species wide signaling cues. They are instead proposed to be the variable set of odors that constitute an animal's chemical profile, and therefore cannot be ‘found’ by biochemical fractionation [29]. Though these odors do not possess any intrinsic bioactivity they are ‘learned’ by the receiving animal in a specific context that promotes behavioral interaction, for example signature odors are proposed to underlie maternal recognition of lambs [29, 56, 57]. While we could not eliminate the gestation of the pup in the maternal amniotic fluid, we instead reasoned that the presence of an additional, exogenous odorant would not affect inherent bioactivity of any constitutive pheromone blend but would alter the identity of a signature odor blend [29]. Therefore we altered the odor composition of amniotic fluid by adding a neutral odor, vanillin, to amniotic fluid extracted from pregnant dams. We assayed c-section delivered pups for suckling behavior towards nipples swabbed with either amniotic fluid alone or amniotic fluid supplemented with different concentrations of vanillin. We found that altering the odor constituents of amniotic fluid is indeed sufficient to ablate its suckling-promoting activity (Fig. 5A, S5).
Figure 5. Adding an odor to amniotic fluid alters its suckling promoting activity.
(A) Adding ≥10-6M vanillin to amniotic fluid alters the odor blend resulting in a significant reduction in suckling-promoting activity. Statistical differences were tested against pups suckling in response to untreated amniotic fluid (no vanillin) with Kruskal-Wallis One Way ANOVA followed by a post hoc Dunn Test: ** Q>Q(0.01); *** Q>Q(0.001). (B) Vanillin initiates calcium influx in KCL-responsive newborn olfactory neurons in a concentration dependent manner (n = 2037 to 5141).Statistical differences were tested against the proportion of neurons responding to no vanillin, by One Way ANOVA followed by Tukey-Kramer HSD post hoc analysis: * P<0.05; *** P<0.001).
To ensure that the addition of vanillin alters the odor, but does not inhibit or prevent the detection of a pheromone, we determined the proportion of perinatal MOE neurons that vanillin stimulates by calcium imaging. The addition of 10-6M vanillin significantly interferes with the suckling-promoting activity of amniotic fluid (Fig. 5A), yet when perfused directly onto neurons and assayed by calcium imaging, it activates less than half the maximal sensory neurons observed to respond to vanillin (1.09% neurons activated compared with 2.53%, Fig. 5B). Given the actual concentration of vanillin volatiles at the nasal epithelium of the pup searching for a nipple in our assay is likely to be much lower than the concentration we painted on the nipple; we consider it unlikely that this concentration of vanillin is non-specifically preventing the detection of a functional pheromone blend.
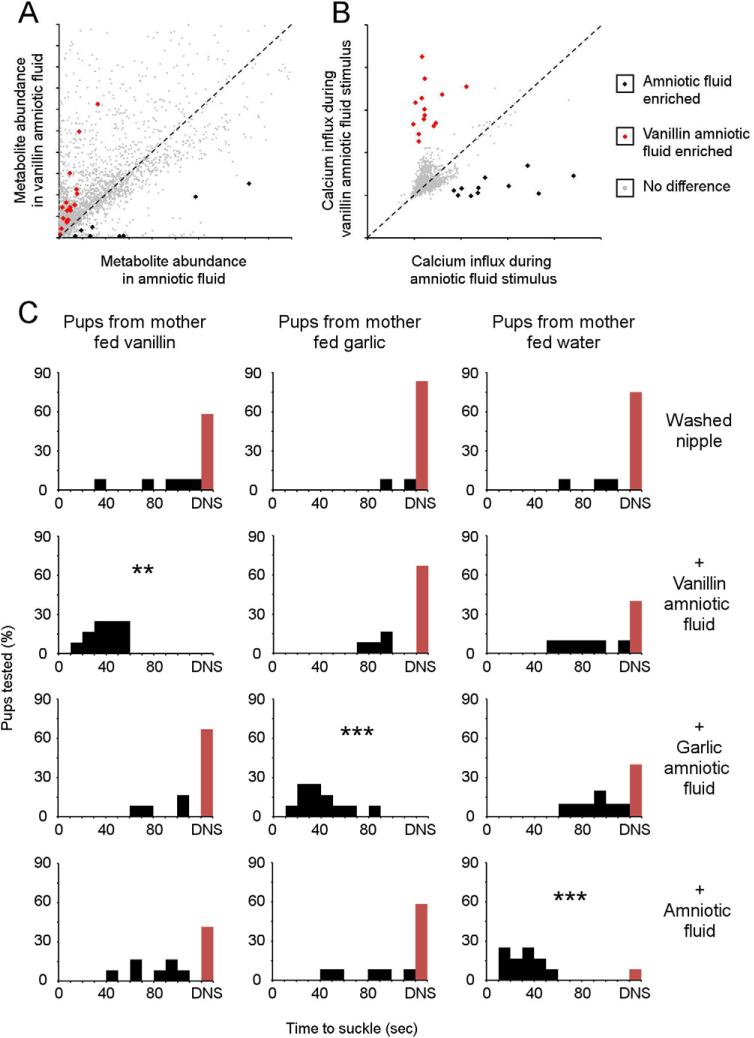
Additionally, we generated a more ethologically relevant manipulation of the amniotic fluid odor profile in utero during gestation. Maternal diet has been shown to alter the odor composition of amniotic fluid in humans and sheep [58, 59] therefore we provided e15.5 pregnant dams ad lib access to one of three types of water: 1) untreated, 2) garlic oil-supplemented, or 3) vanillin-supplemented. We used mass spectrometry based metabolite profiling to compare the molecular composition of garlic oil- or vanillin-supplemented amniotic fluid and found approximately 1% of the metabolites to be highly significantly different (p<0.001) from those present in the amniotic fluid gathered from dams which had consumed unaltered water (Figs. 6A, S6A-E, Table 1) [60]. Based on their masses we can predict that the new metabolites present after maternal consumption of garlic oil were not the same as those produced after the consumption of vanillin. We next determined the extent to which the molecular differences observed by mass spectrometry correlated with altered odor ligand composition. Using calcium imaging, we directly stimulated primary MOE neurons with the amniotic fluids from dams fed each of the three different diets. When we compared the activation of individual neurons following sequential perfusion of each fluid we found between 1.9% and 2.6% of the neurons to be differentially activated (Figs. 6B, S6C-E). Moreover, as expected by the metabolic profiling, the changes in neural activity produced by each fluid targets different subsets of neurons. This analysis confirms that by providing three different types of drinking water to dams during gestation we were able to generate amniotic fluids with a subtle, approximately 2.2%, difference in the total odor profile.
Figure 6. The first suckling episode is promoted by a signature blend of maternal odors.
(A) Analysis of amniotic fluid from control (black) and vanillin-fed (red) pregnant mice shows 29 (black/red dots) of 3091 unique metabolites (grey dots) have highly significant differences in abundance, P<0.001 (n = 5). (B) Calcium imaging of newborn olfactory neurons (n = 2429) perfused with these amniotic fluids indicates diet alters amniotic fluid odor responses. Each neuron is represented by the maximal amount of intracellular calcium (F340/F380) during each amniotic fluid perfusion, after normalization to the starting calcium concentration. Neurons that were differentially activated are indicated by color. (D) Pups suckle only in response to the signature odor of the amniotic fluid corresponding to their gestation (n = 10-12). Statistical differences were tested against pups suckling in response to a washed nipple with Kruskal-Wallis One Way ANOVA followed by a post hoc Dunn Test: ** Q>Q(0.01); *** Q>Q(0.001).
Table 1.
| # significantly different metabolites | % significantly different metabolites | ||||||
|---|---|---|---|---|---|---|---|
| XCMS comparison | Metabolites | (P<0.001) | (P<0.01) | (P<0.05) | (P<0.001) | (P<0.01) | (P<0.05) |
| AF vs AF | 5771 | 3 | 23 | 156 | 0.05 | 0.40 | 2.70 |
| AF vs Vanillin AF | 3091 | 29 | 137 | 374 | 0.94 | 4.43 | 12.10 |
| AF vs Garlic AF | 2796 | 29 | 106 | 335 | 1.04 | 3.79 | 11.98 |
| Garlic AF vs Vanillin AF | 3323 | 54 | 184 | 466 | 1.63 | 5.54 | 14.02 |
| C57BL/6J AF vs BALB/c AF | 3136 | 0 | 19 | 89 | 0.00 | 0.61 | 2.84 |
We next determined if the slight change in neutral odors comprising amniotic fluid altered the bioactivity. We quantified the suckling behavior of c-section delivered pups gestated in each diet-altered amniotic fluid when presented with water-washed nipples swabbed with amniotic fluid collected from each of the three diet groups. Pups gestated in dams that had an altered odor composition of amniotic fluid initiated suckling singularly and specifically towards the corresponding fluid (Fig. 6C). These pups failed to suckle unaltered amniotic fluid from control dams. This effect does not appear to be a consequence of biased olfactory development, as pups gestated in dams fed untreated water singularly suckled in response to amniotic fluid from water fed dams. Conversely, the subtle alteration of amniotic fluid by presumably neutral ligands was sufficient to ablate the bioactivity when detected by pups gestated in dams fed unaltered water. This indicates that the bioactivity of amniotic fluid in its original context is lost in animals that have experienced an altered odor environment. While these responses are inconsistent with a classic pheromone releasing suckling behavior, they fulfill the expectation of signature odor activity.
Previously described signature odors act to enable a ewe to identify and distinguish her individual lambs [57]. Therefore we next investigated the extent to which the signature odor of amniotic fluid serves in maternal recognition. We tested the ability of our c-sectioned delivered pups to innately suckle in response to amniotic fluid of another, genetically distinct, mouse strain (C57BL/6J vs. BALB/c). We found pups from both strains to equally initiate suckling in response to the olfactory cues from each strain (Figure S7). When we performed mass-spectrometry analysis of the metabolites from the amniotic fluid of each strain (C57BL/6J vs. BALB/c) we found that the odor profiles of these distinct laboratory strains were statistically indistinguishable (Table 1).
Discussion
Our data suggest that first suckling behavior in the mouse does not reflect a hardwired response to specific or predetermined cues (i.e. pheromones), but rather involves a learning process through which the neonate must have experience of maternal olfactory stimuli in order to initiate first suckling. The behavior is primed by obligate perinatal learning of a contextually relevant, but variable, blend of maternal odors, and released by re-exposure to the odor blend at the mother's nipple. These findings are consistent with another form of bioactive cue, the learning of signature odors. Signature odors have been proposed to underlie kin recognition between ewe and lamb [29, 57]. Due to their immediate mobility, precocious lambs will attempt to suckle from any available ewe. In order to devote her resources to the fitness of her lamb, the ewe learns the ‘signature odor’ of the lamb immediately at birth and only permits the lamb that displays this learned odor to commence suckling. Our data suggests that in the mouse, the suckling signature odor does not similarly provide information regarding individual identification. Under natural conditions, it is unlikely that a newborn mouse, prior to first suckling, would become separated from the mother and need to distinguish between dams. Indeed, while pre-weanling mice can distinguish lactating from virgin females, they appear unmotivated or unable to distinguish their mother from another dam that can provide milk [61]. Further, our analysis of the behavior reveals that the olfactory cues are not simply a mechanism of attraction to entice the newborn to the proximity of the nipple. We found that artificially providing attraction by starting the assay with the newborn's nose directly on the nipple, yet preventing detection of the salient olfactory cues was not sufficient to initiate suckling. Our data indicate that the mouse amniotic fluid signature odor acts to directly promote the act of suckling.
Previously identified pheromones are olfactory ligands that activate specialized neural circuits to robustly release behavior or neuroendocrine changes [29, 41]. In the mouse, semiochemicals, such as pheromones and kairomones, of known bioactivity are single or small, defined combinations of molecules, which retain their activity in complex native odor sources, and activate specific subsets of olfactory neurons [10-12, 14, 55]. For these molecules to regulate social behavior, the emitting animal must produce the correct ligand(s) of proper abundance and the receiving animal must have an accurately developed neural circuit highly tuned to detect them. In contrast to pheromones, the release of behavior through the signature odor mechanism does not depend on the proper production of a specific ligand from the dam, nor on the development of highly tuned neurons in the pup. This inherent plasticity of the system may favor the likelihood of successful first suckling and ultimately pup survival. The highly tuned detection of pheromones ensures that behavior such as fear and aggression are released in the correct context. Inappropriate display of these behaviors is likely to reduce fitness. In contrast, the odor cues that generate the first suckling behavior of a naive, newborn animal needs to only be effective and meaningful once; immediately following birth. Suckling results in warmth, satiety, and a sweet flavor which are all powerfully rewarding. Indeed, if the litters are dramatically culled and the maternal diet is highly enriched, a fraction of anosmic Cnga2y/- animals are able to locate a nipple within their first few days, perhaps by chance. Following this experience, those pups are subsequently able to nurse as normal and survive into adulthood, presumably by learning other associative sensory cues (LS, unpublished observations)[62].
While our data do not support the existence of a classic pheromone, the US that conditions the signature odor may be a novel form of semiochemical. It is formally possible that a species specific molecule(s) has intrinsic activity which is not sufficient to release suckling on its own, but instead is the US that charges the signature odor with activity. To our knowledge, no such molecule has previously been described in any system. As there is no experimental method to separate the signature odor from the gestating pup or to further identify a specific ligand that is not sufficient to function to release suckling, this possibility cannot currently be resolved. The process of suckling itself is a positive reinforcer of odor-mediated learning. It has been well established that during suckling the locus ceruleus releases norepinephrine into the olfactory bulb [63, 64]. Further, experience dependant changes in AMPA receptor activity in olfactory cortical nuclei has been proposed to underlie early olfactory learning [65]. Such established mechanisms may be important in mediating first suckling. However, it is not clear how they could charge the specific signature odor with significance prior to first experience of suckling behavior.
The success of mammalian suckling required co-evolution of multiple anatomical, physiological, and behavioral systems. In the European rabbit, this included the evolution of a pheromone to release the rabbit pup's behaviour. Because of the rabbit's absentee parenting style, a pheromone may make the brief interactions between the mother and the new-born pup feasible [31]. Our data suggests that the evolution of a specific maternal pheromone and detecting neural circuits is not the only strategy to ensure mammalian suckling is initiated. In the mouse, the process simply requires odor experience and recall, which is a fundamental task of the MOE across vertebrate species. Further, the detection of signature odors appears to require olfactory components that are likely ubiquitous in mammals, unlike the pheromone mediated initiation of suckling in rabbits. This perinatal odor conditioning is inextricably associated with the birthing process itself, maximizing the probability that suckling is successfully initiated. These results suggest that mammalian species have evolved multiple strategies to ensure the onset of this critical behavior. While one mechanism is thought to generate hardwired behavior and the other relies on olfactory learning, the display and stereotypy of the output behavior is essentially indistinguishable; both appear innate. The extent to which other ostensibly innate output behaviors similarly utilize neural mechanisms of experience remains to be investigated.
Methods Summary
Animals
Adult female mice (strains: C57BL/6J, BALB/cByJ, FVB/NJ) were mated overnight with males of the same strain and checked for vaginal plugs the following morning. If present, the embryos were timed at 0.5 days (e0.5). Unless otherwise indicated mice were provided ad lib access to a breeder diet (11% fat) and water. All experimental protocols were approved by a local Institutional Animal Care and Use Committee protocol #06-0298, or a local Ethical Review Committee and performed under Home Office license.
Neonate suckling assay
An anesthetized lactating female was arranged with her ventrum exposed. The nipple was washed by triturating for 30 sec with 50μl distilled water and dabbed dry with cotton swab. Pups were fasted for 3 hours, singly removed from their nest and vertically supported 1 cm from the female's shorn nipple for 120 sec, ensuring movement was unrestricted to sample the area around the nipple. To assay odorants, 3μl of each solution was swabbed on a washed nipple using a Loew-Cornell 2037 round #1 brush. To ensure evaluation of innate behavior, each pup was only assayed once; against one stimulus condition.
Caesarean suckling assays
All behavioral assays except those presented in Figures 1 and S1 were done with c-section delivered pups. Pups were removed from e20.5 pregnant dams, the amniotic sacs opened and the umbilicus was ligated. A #3 Royal fine sable paint brush was used to gently brush each pup with 37°C water. Surviving pups were placed in the warmed nest for 1 hour then used in a suckling assay as described. To test whether prior exposure to an odor promoted suckling, a brush was dipped in the warmed odorous fluid and then used to gently brush each pup. In all suckling experiments each test group contained pups sampled from three or more litters, each litter was randomly distributed between two or more test groups, including one control group. Each pup was tested only once, to ensure no learning occurred. The experimenter was blind to the genotype of the pups and, when possible, the nature of the stimuli.
Supplementary Material
Highlights.
Maternal olfactory signals are required for newborn mice to innately perform the first episode of suckling.
A blend of volatile odors from the mother elicits the behavior.
The bioactivity is not pheromonal, but instead requires experience of a maternal ‘signature odor’.
Signature odors need not rely on species specific neural mechanisms and may be a common way to elicit social behaviors that appear innate.
Acknowledgments
We thank E Wynn for technical support; MA Lawson for statistical advice; TD Wyatt, G Beauchamp, and members of the Stowers and Logan labs for constructive advice and criticism. This work was supported by the NIH-NIDCD and the Skaggs Foundation (LS), and Wellcome Trust grant 098051 (DWL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Complete methods are presented as supplemental information.
References
- 1.Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- 2.Brunet LJ, Gold GH, Ngai J. General Anosmia Caused by a Targeted Disruption of the Mouse Olfactory Cyclic Nucleotide-Gated Cation Channel. Neuron. 1996;17:681–693. doi: 10.1016/s0896-6273(00)80200-7. [DOI] [PubMed] [Google Scholar]
- 3.Hudson R, Distel H. Pheromonal release of suckling in rabbits does not depend on the vomeronasal organ. Physiol Behav. 1986;37:123–128. doi: 10.1016/0031-9384(86)90394-x. [DOI] [PubMed] [Google Scholar]
- 4.Singh PJ, Tucker AM, Hofer MA. Effects of nasal ZnSO4 irrigation and olfactory bulbectomy on rat pups. Physiol Behav. 1976;17:373–382. doi: 10.1016/0031-9384(76)90094-9. [DOI] [PubMed] [Google Scholar]
- 5.Teicher MH, Blass EM. First Suckling Response of the Newborn Albino Rat: The Roles of Olfaction and Amniotic Fluid. Science. 1977;198:635–636. doi: 10.1126/science.918660. [DOI] [PubMed] [Google Scholar]
- 6.Varendi H, Porter RH, Winberg J. Does the newborn baby find the nipple by smell? Lancet. 1994;344:989–990. doi: 10.1016/s0140-6736(94)91645-4. [DOI] [PubMed] [Google Scholar]
- 7.Wong ST, Trinh K, Hacker B, Chan GC, Lowe G, Gaggar A, Xia Z, Gold GH, Storm DR. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 2000;27:487–497. doi: 10.1016/s0896-6273(00)00060-x. [DOI] [PubMed] [Google Scholar]
- 8.Schaal B, Coureaud G, Doucet S, Delaunay-El Allam M, Moncomble AS, Montigny D, Patris B, Holley A. Mammary olfactory signalisation in females and odor processing in neonates: ways evolved by rabbits and humans. Behavioural brain research. 2009;200:346–358. doi: 10.1016/j.bbr.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Munger SD, Leinders-Zufall T, Zufall F. Subsystem organization of the mammalian sense of smell. Annual review of physiology. 2009;71:115–140. doi: 10.1146/annurev.physiol.70.113006.100608. [DOI] [PubMed] [Google Scholar]
- 10.Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- 11.Kimoto H, Haga S, Sato K, Touhara K. Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature. 2005;437:898–901. doi: 10.1038/nature04033. [DOI] [PubMed] [Google Scholar]
- 12.Leinders-Zufall T, Brennan P, Widmayer P, S PC, Maul-Pavicic A, Jager M, Li XH, Breer H, Zufall F, Boehm T. MHC class I peptides as chemosensory signals in the vomeronasal organ. Science. 2004;306:1033–1037. doi: 10.1126/science.1102818. [DOI] [PubMed] [Google Scholar]
- 13.Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papes F, Logan DW, Stowers L. The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell. 2010;141:692–703. doi: 10.1016/j.cell.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of Sex Discrimination and Male-Male Aggression in Mice Deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- 16.Choi GB, Stettler DD, Kallman BR, Bhaskar ST, Fleischmann A, Axel R. Driving opposing behaviors with ensembles of piriform neurons. Cell. 2011;146:1004–1015. doi: 10.1016/j.cell.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleischmann A, Shykind BM, Sosulski DL, Franks KM, Glinka ME, Mei DF, Sun Y, Kirkland J, Mendelsohn M, Albers MW, et al. Mice with a “monoclonal nose”: perturbations in an olfactory map impair odor discrimination. Neuron. 2008;60:1068–1081. doi: 10.1016/j.neuron.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- 19.Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron. 2009;63:854–864. doi: 10.1016/j.neuron.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- 21.Lin DY, Zhang SZ, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434:470–477. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- 22.Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nature neuroscience. 2005;8:1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- 23.Weiss J, Pyrski M, Jacobi E, Bufe B, Willnecker V, Schick B, Zizzari P, Gossage SJ, Greer CA, Leinders-Zufall T, et al. Loss-of-function mutations in sodium channel Nav1.7 cause anosmia. Nature. 2011;472:186–190. doi: 10.1038/nature09975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teicher MH, Blass EM. Suckling in newborn rats: eliminated by nipple lavage, reinstated by pup saliva. Science. 1976;193:422–425. doi: 10.1126/science.935878. [DOI] [PubMed] [Google Scholar]
- 25.Greer CA, Stewart WB, Teicher MH, Shepherd GM. Functional development of the olfactory bulb and a unique glomerular complex in the neonatal rat. J Neurosci. 1982;2:1744–1759. doi: 10.1523/JNEUROSCI.02-12-01744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guthrie KM, Gall C. Anatomic mapping of neuronal odor responses in the developing rat olfactory bulb. The Journal of comparative neurology. 2003;455:56–71. doi: 10.1002/cne.10452. [DOI] [PubMed] [Google Scholar]
- 27.Jastreboff PJ, Pedersen PE, Greer CA, Stewart WB, Kauer JS, Benson TE, Shepherd GM. Specific olfactory receptor populations projecting to identified glomeruli in the rat olfactory bulb. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:5250–5254. doi: 10.1073/pnas.81.16.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teicher MH, Stewart WB, Kauer JS, Shepherd GM. Suckling pheromone stimulation of a modified glomerular region in the developing rat olfactory bulb revealed by the 2-deoxyglucose method. Brain Res. 1980;194:530–535. doi: 10.1016/0006-8993(80)91237-8. [DOI] [PubMed] [Google Scholar]
- 29.Wyatt TD. Pheromones and signature mixtures: defining species-wide signals and variable cues for identity in both invertebrates and vertebrates. Journal of comparative physiology. A, Neuroethology, sensory, neural, and behavioral physiology. 2010;196:685–700. doi: 10.1007/s00359-010-0564-y. [DOI] [PubMed] [Google Scholar]
- 30.Charra R, Datiche F, Casthano A, Gigot V, Schaal B, Coureaud G. Brain processing of the mammary pheromone in newborn rabbits. Behavioural brain research. 2012;226:179–188. doi: 10.1016/j.bbr.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Schaal B, Coureaud G, Langlois D, Ginies C, Semon E, Perrier G. Chemical and behavioural characterization of the rabbit mammary pheromone. Nature. 2003;424:68–72. doi: 10.1038/nature01739. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan RM, Wilson DA. Molecular biology of early olfactory memory. Learn Mem. 2003;10:1–4. doi: 10.1101/lm.58203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan RM, Wilson DA, Leon M. Norepinephrine and learning-induced plasticity in infant rat olfactory system. J Neurosci. 1989;9:3998–4006. doi: 10.1523/JNEUROSCI.09-11-03998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson DA, Sullivan RM. Neurobiology of associative learning in the neonate: early olfactory learning. Behav Neural Biol. 1994;61:1–18. doi: 10.1016/s0163-1047(05)80039-1. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedersen PE, Blass EM. Prenatal and postnatal determinants of the 1st suckling episode in albino rats. Developmental psychobiology. 1982;15:349–355. doi: 10.1002/dev.420150407. [DOI] [PubMed] [Google Scholar]
- 37.Coureaud G, Languille S, Schaal B, Hars B. Pheromone-induced olfactory memory in newborn rabbits: Involvement of consolidation and reconsolidation processes. Learn Mem. 2009;16:470–473. doi: 10.1101/lm.1434009. [DOI] [PubMed] [Google Scholar]
- 38.Coureaud G, Moncomble AS, Montigny D, Dewas M, Perrier G, Schaal B. A pheromone that rapidly promotes learning in the newborn. Curr Biol. 2006;16:1956–1961. doi: 10.1016/j.cub.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen PE, Williams CL, Blass EM. Activation and odor conditioning of suckling behavior in 3-day-old albino rats. J Exp Psychol Anim Behav Process. 1982;8:329–341. [PubMed] [Google Scholar]
- 40.Schaal B. Mammary odor cues and pheromones: mammalian infant-directed communication about maternal state, mammae, and milk. Vitamins and hormones. 2010;83:83–136. doi: 10.1016/S0083-6729(10)83004-3. [DOI] [PubMed] [Google Scholar]
- 41.Karlson P, Luscher M. Pheromones': a new term for a class of biologically active substances. Nature. 1959;183:55–56. doi: 10.1038/183055a0. [DOI] [PubMed] [Google Scholar]
- 42.Risser JM, Slotnick BM. Nipple attachment and survival in neonatal olfactory bulbectomized rats. Physiol Behav. 1987;40:545–549. doi: 10.1016/0031-9384(87)90042-4. [DOI] [PubMed] [Google Scholar]
- 43.Liman ER, Corey DP, Dulac C. TRP2: a candidate transduction channel for mammalian pheromone sensory signaling. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:5791–5796. doi: 10.1073/pnas.96.10.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dhallan RS, Yau KW, Schrader KA, Reed RR. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1990;347:184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- 45.Rosenblatt JSL, D.S. Maternal behavior in mammals. John Wiley and Sons; 1963. [Google Scholar]
- 46.Zhang X, Rogers M, Tian H, Zou DJ, Liu J, Ma M, Shepherd GM, Firestein SJ. High-throughput microarray detection of olfactory receptor gene expression in the mouse. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:14168–14173. doi: 10.1073/pnas.0405350101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coppola DM. The Role of the Main and Accessory Olfactory Systems in Prenatal Olfaction. In: Marchlewska-Koj A, Lepri JJ, Müller-Schwarze D, editors. Chemical Signals in Vertebrates 9. Kluwer Academic/Plenum Publishers; New York: 2001. pp. 189–196. [Google Scholar]
- 48.Pedersen PE, Stewart WB, Greer CA, Shepherd GM. Evidence for olfactory function in utero. Science. 1983;221:478–480. doi: 10.1126/science.6867725. [DOI] [PubMed] [Google Scholar]
- 49.Todrank J, Heth G, Restrepo D. Effects of in utero odorant exposure on neuroanatomical development of the olfactory bulb and odour preferences. Proceedings. Biological sciences / The Royal Society. 2011;278:1949–1955. doi: 10.1098/rspb.2010.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leinders-Zufall T, Cockerham RE, Michalakis S, Biel M, Garbers DL, Reed RR, Zufall F, Munger SD. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14507–14512. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spehr M, Kelliher KR, Li XH, Boehm T, Leinders-Zufall T, Zufall F. Essential role of the main olfactory system in social recognition of major histocompatibility complex peptide ligands. J Neurosci. 2006;26:1961–1970. doi: 10.1523/JNEUROSCI.4939-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haga S, Hattori T, Sato T, Sato K, Matsuda S, Kobayakawa R, Sakano H, Yoshihara Y, Kikusui T, Touhara K. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature. 2010;466:118–122. doi: 10.1038/nature09142. [DOI] [PubMed] [Google Scholar]
- 53.Coureaud G, Thomas-Danguin T, Le Berre E, Schaal B. Perception of odor blending mixtures in the newborn rabbit. Physiol Behav. 2008;95:194–199. doi: 10.1016/j.physbeh.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 54.Harris JA. Elemental representations of stimuli in associative learning. Psychological review. 2006;113:584–605. doi: 10.1037/0033-295X.113.3.584. [DOI] [PubMed] [Google Scholar]
- 55.Novotny MV. Pheromones, binding proteins and receptor responses in rodents. Biochemical Society transactions. 2003;31:117–122. doi: 10.1042/bst0310117. [DOI] [PubMed] [Google Scholar]
- 56.Kendrick KM, Levy F, Keverne EB. Changes in the sensory processing of olfactory signals induced by birth in sleep. Science. 1992;256:833–836. doi: 10.1126/science.1589766. [DOI] [PubMed] [Google Scholar]
- 57.Porter RH, Levy F, Poindron P, Litterio M, Schaal B, Beyer C. Individual olfactory signatures as major determinants of early maternal discrimination in sheep. Developmental psychobiology. 1991;24:151–158. doi: 10.1002/dev.420240302. [DOI] [PubMed] [Google Scholar]
- 58.Mennella JA, Johnson A, Beauchamp GK. Garlic ingestion by pregnant women alters the odor of amniotic fluid. Chem Senses. 1995;20:207–209. doi: 10.1093/chemse/20.2.207. [DOI] [PubMed] [Google Scholar]
- 59.Nolte DL, Provenza FD, Callan R, Panter KE. Garlic in the ovine fetal environment. Physiol Behav. 1992;52:1091–1093. doi: 10.1016/0031-9384(92)90464-d. [DOI] [PubMed] [Google Scholar]
- 60.Smith CA, Want EJ, O'Maille G, Abagyan R, Siuzdak G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal Chem. 2006;78:779–787. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
- 61.Breen MF, Leshner AI. Maternal pheromone: A demonstration of its existence in the mouse (Mus Musculus). Physiology & Behavior. 1977;18:527–529. [Google Scholar]
- 62.Al Ain S, Chraiti A, Schaal B, Patris B. Orientation of newborn mice to lactating females: Identifying biological substrates of semiochemical interest. Developmental psychobiology. 2011 doi: 10.1002/dev.21003. [DOI] [PubMed] [Google Scholar]
- 63.Moriceau S, Sullivan RM. Unique neural circuitry for neonatal olfactory learning. J Neurosci. 2004;24:1182–1189. doi: 10.1523/JNEUROSCI.4578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sullivan RM, Stackenwalt G, Nasr F, Lemon C, Wilson DA. Association of an odor with activation of olfactory bulb noradrenergic beta-receptors or locus coeruleus stimulation is sufficient to produce learned approach responses to that odor in neonatal rats. Behavioral neuroscience. 2000;114:957–962. doi: 10.1037/0735-7044.114.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Franks KM, Isaacson JS. Synapse-specific downregulation of NMDA receptors by early experience: a critical period for plasticity of sensory input to olfactory cortex. Neuron. 2005;47:101–114. doi: 10.1016/j.neuron.2005.05.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.