Abstract
The overall goal of the present study was to determine the effects of different doses of (+)-methamphetamine (meth) on locomotor activity of Balb/C mice. Four experiments were designed to test a wide range of meth doses in BALB/c female mice. In Experiment 1, we examined locomotor activity induced by an acute administration of low doses of meth (0.01 and 0.03 mg/kg) in a 90-min session. Experiment 2 was conducted to test higher meth doses (0.3 – 10 mg/kg). In Experiment 3, separate sets of mice were pre-treated with various meth doses once or twice (one injection/week) prior to a locomotor challenge with a low meth dose. Finally, in Experiment 4, we tested whether locomotor activation would be affected by pretreatment with a low or moderate dose of meth one month prior to the low meth dose challenge. Results show that low doses of meth induce hypolocomotion whereas moderate to high doses induce hyperlocomotion. Prior exposure to either one moderate or high dose of meth or to two, low doses of meth attenuated the hypolocomotor effect of a low meth dose one week later. This effect was also attenuated in mice tested one month after administration of a moderate meth dose. These results show that low and high doses of meth can have opposing effects on locomotor activity. Further, prior exposure to the drug leads to tolerance, rather than sensitization, of the hypolocomotor response to low meth doses.
Keywords: Methamphetamine, Mice, Hypolocomotion, Hyperlocomotion, Stereotypy
1. Introduction
Methamphetamine (meth) dependence is a serious worldwide public health problem with major medical [1–5], psychiatric [6–9], socioeconomic and legal consequences [2]. The effects of meth use include euphoria and many of the same stimulant effects seen with cocaine, but these effects may last much longer [reviewed in [10]].
Meth exerts multiple effects in the central nervous system and acts as a highly potent releaser of monoamines by increasing cytoplasmic concentrations of dopamine (DA), serotonin (5-HT), and norepinephrine (NE) by: i) blocking the activity of the intracellular vesicular monoamine transporter 2 (VMAT2) [11] [12], ii) inducing reverse transport of monoamines [13], iii) decreasing the expression of the DA transporter (DAT) at the cell surface [14], iv) inhibiting the activity of monoamine oxidase and v) increasing the activity and the expression of tyrosine hydroxylase [15]. Prolonged meth use leads to down-regulation of DA D2-receptors and uptake sites and relative hypodopaminergic activity [16–18].
Currently, there is no FDA approved pharmacotherapy for this addictive disorder. A variety of medications have failed to show efficacy in clinical trials, including a DA partial agonist (aripiprazole), GABAergic agents (gabapentin) and serotonergic agents [reviewed in [19]]. However, immunotherapies, including vaccines, have shown therapeutic potential for various addictions [reviewed in [20]]. Meth vaccines have produced antibodies, have reduced meth self-administration, and have inhibited meth discriminative stimulus effects in rats and pigeons [21–23]. Because meth, like other psychostimulants, induces locomotor activation in rodents [24, 25], potential antagonists like immunotherapies can be tested for their ability to block meth-induced locomotion in mice.
Two distinct locomotor behaviors have been observed in rats and mice following meth administration [24, 25]. While moderate doses of meth induce hyperlocomotion in rats and mice, higher doses induce stereotypy [24, 25]. A third type of locomotor behavior namely hypolocomotion has also been observed in rodents upon treatment with very low doses of the DA agonist apomorphine [26] or, high doses of the NE agonist clonidine [27] [28]. These agents preferentially stimulate DA or NE autoreceptors respectively [26] [27] [28]. Interestingly, low doses of apomorphine causes hypolocomotion in mice by stimulating presynaptic dopamine autoreceptors whereas higher doses induce hyperlocomotion in mice by stimulating postsynaptic dopamine receptors [29, 30]. Additionally, very low doses of cocaine have produced hypolocomotion in rodents [31, 32].
In view of these reports, this study was undertaken to test the effects of various doses of meth on the locomotor activities of female, BALB/c mice. This strain and sex of mouse was chosen because our current vaccine studies employ female mice of this strain. We specifically tested whether very low doses of meth induce hypolocomotion, while moderate and higher doses induce hyperlocomotion and stereotypy respectively. We also tested behavioral responses from repeated meth dosing and after sustained abstinence from meth. The time points chosen for these chronic dosing and abstinence studies were based on those that are commonly used in the field of locomotor sensitization.
2. Materials and Methods
2.1. Drugs and Chemicals
(+)-Methamphetamine hydrochloride (meth) was purchased from Sigma-Aldrich (St Louis, MO, USA). Meth was dissolved in sterile phosphate buffered saline (PBS), which also served as the vehicle control. Injection volumes varied between 75–100 μl based on the procedure and the weight of the animal. Concentrations were expressed as the freebase form. The injection route was intraperitoneal (i.p.). All other reagents used in these studies were obtained from Fisher Scientific (Springfield, NJ), unless otherwise specified.
2.2. Animals
Experimentally-naïve, inbred strains of pathogen-free female BALB/c (AnNHsd) mice (Harlan Laboratories, TX) aged 8–12 weeks were used in the experiments. Mice were housed in plastic cages with isolator tops in a temperature and humidity controlled room on a 12 h light (6:00 a.m. to 6:00 p.m.) and dark cycle (6:00 p.m. to 6:00 a.m.) with access to food and water ad libitum. All experimental procedures were conducted during light cycle (i.e. between 6:00 a.m. to 6:00 p.m.). All procedures were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine and were conducted in accordance with Principle of Laboratory Animal Care (National Institutes of Health Publication).
2.3. Quantification of locomotor activity
In order to evaluate the effect of varying doses of meth on the locomotor activity, BALB/c mice were placed into the acrylic test cage (Opto-M3; 27.5″ × 27.5″ × 8.0″; Columbus Instruments, Ohio) equipped with infrared sensors. All the test cages were housed within a soundproof room with controlled lighting. All animals were habituated to the test cages and dim light by placing them in the cages for 60 minutes at least three times each separated by a day. On the test day, mice were placed in the test cages 60 minutes before the treatment to set the baseline. Their locomotor activity was recorded in terms of numbers of beam-breaks per 10 minutes by using the software supplied by the manufacturer (Columbus Instruments). After a baseline evaluation, mice were treated with varying doses of meth or PBS and their locomotor activity was recorded for another 90 minutes, as indicated in the results and figure legends. At the end of experiment, first 30 minutes of average baseline data (n=5) was deducted from rest of the data to normalize the locomotor activity.
2.4 Experiment procedural detail
2.4.1. Effect of low doses of methamphetamine on the locomotor activity of BALB/c mice
Different groups of mice (n=5/group) were either treated with varying doses of meth that ranged from 0.001 mg/kg to 1.0 mg/kg body weight or with PBS. The locomotor activity was measured as explained above.
2.4.2. Effect of high doses of methamphetamine
Different groups mice (n=5/group) were either treated with varying doses of meth that ranged from 0.01 mg/kg to 10 mg/kg or PBS. The locomotor activity was measured as explained above.
2.4.3. Effect of pre-exposure to methamphetamine on the low dose meth-induced hypolocomotion
This set of experiments was designed to test the effect of pre-exposure to different doses of meth on the low dose meth-induced hypolocomotion. In the first experiment, mice were pre-exposed only once to different doses of meth and were then challenged a week later with 0.01 mg/kg meth on the day of observation. In the second experiment, mice were pre-exposed two times a week apart (i.e. day 0 and day 7) with the same dose range of meth and then were challenged with 0.01 mg/kg meth a week later (day 14).
2.4.4. Effect of abstinence from meth on hypolocomotion in meth pre-exposed mice
Different groups (n=5) of mice were injected with different doses of meth (Fig. 4). One month later, they were challenged with 0.01 mg/kg meth. For comparison, other groups of drug-naïve mice were challenged with PBS, or 2.5 mg/kg or 0.01 mg/kg of meth on the test day. The locomotor activities were measured as explained above.
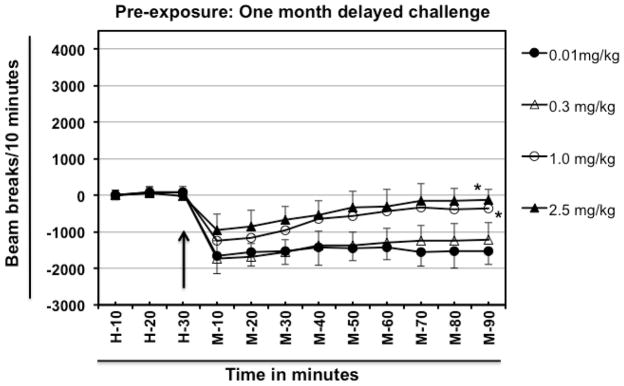
Fig. 4.
Effect of abstinence from meth on hypolocomotion in meth pre-exposed mice. Different groups of experimentally naïve, 8–10-week old female BALB/c mice (n=5/group) were intraperitoneally injected with varying doses of meth (day 0) and were then rested for a month. On day-30 different groups of mice were challenged intraperitoneally with a fixed dose of meth (0.01 mg/kg). Normalized baseline and other data were obtained as explained in Fig. 1. The arrow represents the time of meth injection. In the legend, left hand side values represent the dose of meth which was used for pre-exposure and the right hand side values represent the challenge dose. For example, 2.5/0.01 mg/kg means mice were exposed to 2.5 mg/kg meth, rested for a month and then challenged with 0.01 mg/kg meth. Data shown are mean ± S.E.M. Only some of the S.E.M. bars are shown for the clarity of the graphs. *P<0.001 for comparison vs. 0.01/0.01 mg/kg group.
2.4. Statistical Analysis
All data are shown as mean ± S.E.M. Statistical significance was assessed by two-way repeated measure analysis of variance (ANOVA) followed by Bonferroni post-hoc analysis for multiple comparisons by using Prism software (Prism 5.0; GraphPad Software, San Diego, California, USA. The level of significance was defined as P<0.05.
3. Results
3.1. Effect of low doses of methamphetamine on the locomotor activity of BALB/c mice
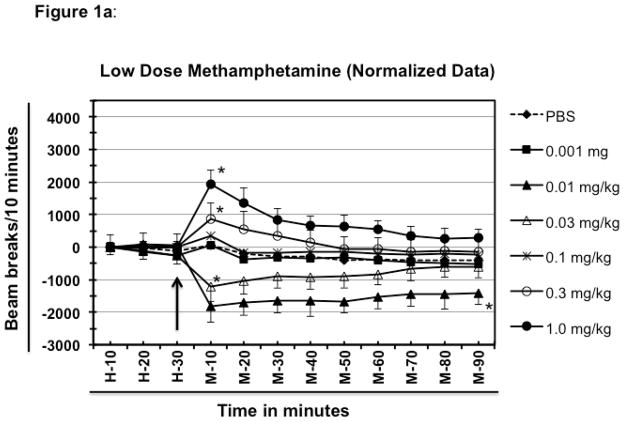
Extremely low dose of meth (0.001 mg/kg) failed to induce any noticeable change in the locomotor activity as compared to the group of mice treated with PBS as shown in figure 1a. One mg/kg meth resulted in significantly higher locomotor activity than PBS in mice (F [6, 252] =348.6; P<0.0001). The maximum hyperactivity was recorded at 10 minutes after the treatment (1922 beam breaks vs. 53 beam breaks) and started declining afterwards, but never came back to the baseline within the 90 minute experiment (+300 beam breaks).
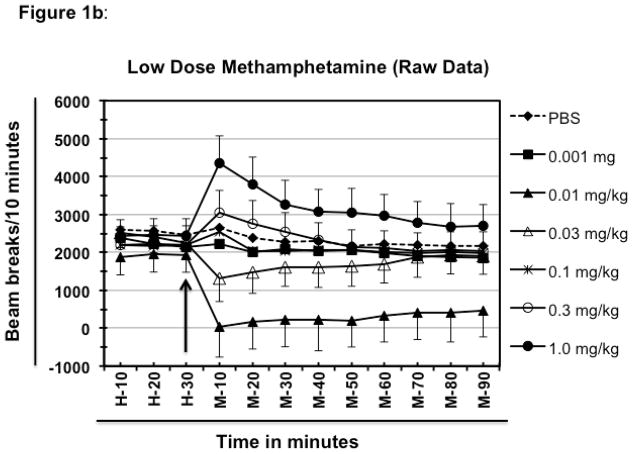
Fig. 1.
Effect of low doses of methamphetamine on the locomotor activity of BALB/c mice. Different groups of experimentally naïve, 8–10-week old female BALB/c mice (n=5/group) were placed in the test cage 60 minutes before drug or PBS injection to establish the baseline. Average value of first 30 minutes of baseline data was deducted from rest of the data for each mouse to normalize the data (figure 1a shows normalized data whereas, figure 1b shows the raw data). The other half of the 60 minutes of baseline is represented here as H-10 to H-30. The arrow represents the time of meth or PBS injection. The time following meth (or PBS) injection is represented as M-10 through M-90. The legends of the graph show the various doses of meth (mg/kg of animal body weight) that was injected into the test animals. All the injections were delivered intraperitoneally. Data shown are mean ± S.E.M. Only some of the S.E.M. bars are shown for the clarity of the graphs. *P<0.001 for comparison vs. PBS group.
After setting the baseline for PBS and highest activity for this set of experiment, other doses of meth were evaluated for their ability to induce or suppress the locomotor activity as compared to the PBS-treated groups. Treatment of mice with 0.01 mg/kg meth resulted in a significantly (P<0.0001) lower locomotor activity (hypolocomotor) compared to the PBS-treated group (−1833 vs. +45 for PBS). The negative breaks indicate less activity than during the 30 minute baseline (data were normalized as explained in material and methods). The maximum hypoactivity was achieved at 10 minutes of the treatment, which remained significantly low even at the end of the experiment (−1405 beam breaks for meth compared to −415 for PBS at 90 min.; P<0.0001 at 90 min.). Doses less than 0.01 mg/kg of meth resulted in locomotor activity similar to that observed after treatment with the PBS or with 0.001 mg/kg meth (data not shown).
Mice treated with a dose 3 fold higher (0.03 mg/kg vs. 0.01mg/kg) also showed hypolocomotion as compared to the PBS-treated group (figure 1a; P <0.0001 at 10 and 20 min. after meth dosing); however, the magnitude of hypolocomotion was less than that caused by 0.01 mg/kg of meth at 10 minutes of treatment (+45 for PBS vs. −1220 for 0.03 mg/kg vs. −1833 for 0.01 mg/kg) indicating that the hypolocomotion is a dose dependent phenomenon. Hypolocomotion produced by 0.03 mg/kg only marginally declined with time and remained fairly stable until 60 minutes of treatment after which activity levels started showing a tendency to move closer to the baseline and at 90 minutes of treatment the values were very similar to the PBS-treated group (−615 for 0.03 mg/kg compared with −415 for PBS at 90 min. of treatment). When compared with hypolocomotion produced by 0.01 mg/kg dose, activity levels of this group showed increased propensity to move closer to the basal level. The difference between the values at 10 minutes (maximum hypoactivity) and at 90 minutes (lowest hypoactivity) of treatment for 0.01 mg/kg group was almost 1.3 times (−1833 at 10 min. and −1405 at 90 min.), whereas the difference between values for the same time point was approximately 2 times for 0.03 mg/kg meth-treated group (−1220 at 10 min. and −615 at 90 min.) indicating that with longer time this group might reach to the basal level faster than the group treated with 0.01 mg/ml of meth (Fig. 1a).
The group of mice treated with 0.1 mg/kg of meth showed only a marginal increase in activity levels over that of the PBS-treated group at 10 minutes (342 beam breaks vs. 53 for PBS). It is noteworthy that this is the time point where a maximum change in the locomotor activity (either hyper or, hypo -locomotion) of other groups is observed (Fig. 1a). After 10 minutes locomotor activity of this group fell close to the baseline and remained fairly close to the values of PBS-treated groups at all time points of observation. A separate group of mice was treated with a dose of 0.3 mg/kg of meth. This dose resulted in a moderate increase in the locomotor activity at 10 minutes as compared to PBS-treated group, but the values were considerably less than that observed with 1 mg/kg dose (at 10 min. of treatment, 53 for PBS; 855 for 0.3 mg/kg and 1922 for 1 mg/kg; P<0.001). After 10 min, the locomotor activity gradually declined, returning near the baseline at 50 min. and remaining close thereafter (Fig. 1a).
3.2. Effect of high doses of methamphetamine
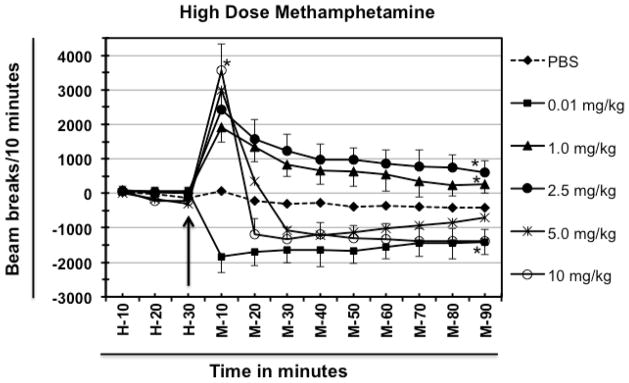
Treatment of mice with 2.5 mg/kg dose resulted in significant hyperlocomotor activity at 10 minutes as compared to the PBS-treated group [2422 beam breaks vs. 84 for PBS; ([F 5, 216] = 278.1; P<0.0001)] which started declining afterwards but always remained higher than that of the PBS group even at 90 minutes after treatment (615 beam breaks vs. −391 beam breaks for PBS at 90 min). As shown in figure 2, when compared to the group of mice treated with 1.0 mg/kg meth, the group treated with 2.5 mg/kg meth showed a dose-dependent increase in the locomotor activity (2422 for 2.5 mg/kg; 1922 for 1.0 mg/kg and 84 for PBS-treated group, respectively at 10 min.). Additionally, the locomotor responses after 10 minutes were always higher for 2.5 mg/kg group than the 1.0 mg/kg group, though both groups showed higher locomotor activity even at 90 minutes compared to the PBS-treated group (Fig. 2).
Fig. 2.
Effect of high doses of methamphetamine. Different groups of experimentally naïve, 8–10-week old female BALB/c mice (n=5/group) were injected with indicated doses of meth. Data for locomotor activity were normalized as explained in Fig. 1. Baseline is represented here as H-10 to H-30. The arrow represents the time of meth or PBS injection. The time following meth (or PBS) injection is represented as M-10 through M-90. All the injections were delivered intraperitoneally. Data shown are mean ± S.E.M. Only some of the S.E.M. bars are shown for the clarity of the graphs. *P<0.001 for comparison vs. PBS group.
The group of mice treated with 5 mg/kg meth showed an increase in their locomotor activity at 10 minutes of the treatment as compared to the group treated with 2.5 mg/kg meth. The group of mice treated with 10 mg/kg meth showed more locomotor activity compared to the 5 mg/kg group (3562 for 10 mg/kg group vs. 2998 beam breaks for 5 mg/kg group vs. 2422 for 2.5 mg/kg group). However, a dramatic difference in the pattern was observed after 10 minutes of meth treatment; as the decline in the locomotor activity in the group treated with 5- or 10 mg/kg meth was very much pronounced after 10 minutes and the values for the 10 mg/kg group dropped well below the baseline of PBS-treated group. Whereas, the values remained above the PBS-baseline for 5 mg/kg meth group at 20 minutes but it was well below the values for the 2.5 mg/kg group (at 20 min. −213 for PBS; +1558 for 2.5 mg/kg; +346; −1199 for 10 mg/kg). At 30 minutes after treatment, the locomotor activities of both 5- and 10 mg/kg meth group dropped below the value of PBS-treated group, and remained below this level throughout the experiment (Fig. 2). The 5 mg/kg meth-treated group showed a gradual increase in locomotor activity and then showed a decrease in activity 90 minutes after treatment whereas, the locomotor activity of 10 mg/kg meth group was still considerably low at 90 minutes as compared to the PBS-treated group (−391 for PBS vs. −712 for 5.0 mg/kg meth vs. −1392 for 10 mg/kg meth).
Since both low doses (such as 0.01 mg and 0.03 mg/kg meth) and high doses (such as 5 mg/kg and 10 mg/kg) induced hypolocomotor activity, both patterns were analyzed together. The first significant difference between low and high doses induced locomotor behavior is that the low dose (0.01 mg/kg meth) induced hypolocomotion at 10 minutes whereas, in sharp contrast, high doses (5.0 and 10.0 mg/kg meth) induced hyperlocomotion at 10 minutes and only after that the values came down below the PBS baseline, indicating stereotypy [25] (which is characterized by focused sniffing, repetitive head movements, chewing, licking and biting and little or no locomotion). Therefore, in a separate experimental group of mice the animal behavior in terms of comfort and stress were visually inspected after the onset of hypolocomotion i.e. after 20 min. of meth injection. The animals treated with low dose of were inactive and almost sleeping whereas animals treated with high doses (5.0 and 10.0 mg/kg meth) showed signs of stereotypy [25] which included focused sniffing, repetitive head movements, chewing, licking and biting and little or no locomotion (data not shown).
3.3. Effect of pre-exposure to methamphetamine on low dose meth-induced hypolocomotion
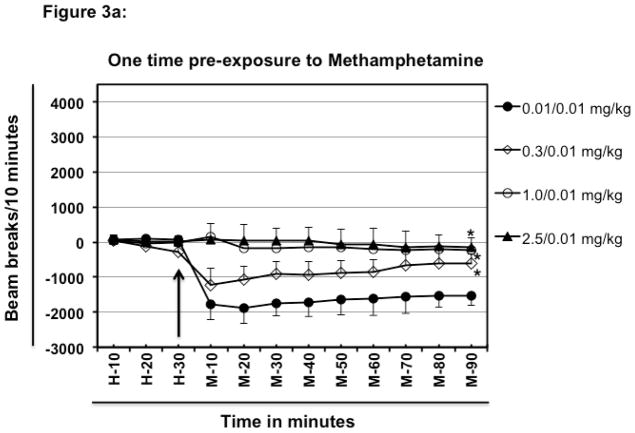
In the first part of the experiment, mice were pre-exposed to meth only once a week before challenge (Fig. 3a; only one injection on day-0 → rested for a week → challenge on day-7 with 0.01 mg/kg meth). Pre-exposure of mice to 0.01 mg/kg meth followed by challenge with 0.01 mg/kg meth after a week resulted in hypolcomotor activity as compared to the basal activity observed during habituation (Fig. 3a; 83 beam breaks at H-30 vs. −1767 beam breaks at M-10 for 0.01/0.01 mg/kg group). By contrast, pre-exposure to a single injection of 0.3 mg/kg meth one week before challenge with 0.01 mg/kg meth, reduced the magnitude of meth-induced hypolocomotion (−1767 beam breaks for 0.01/0.01 mg/kg; vs. −1220 beam breaks for 0.01/0.3 mg/kg at M-10). A single one-week pre-exposure to 1.0 mg/kg or, 2.5 mg/kg meth followed by challenge with 0.01 mg/kg meth completely blocked the hypolocomotion inducing ability of 0.01 mg/kg meth (0.01/0.01 mg/kg compared with 1.0/0.01 mg/kg and 2.5/0.01 mg/kg.; Fig. 3a). These statements are supported by the significant Group effect, F [3, 144] = 160.9; P<0.0001).
Fig. 3.
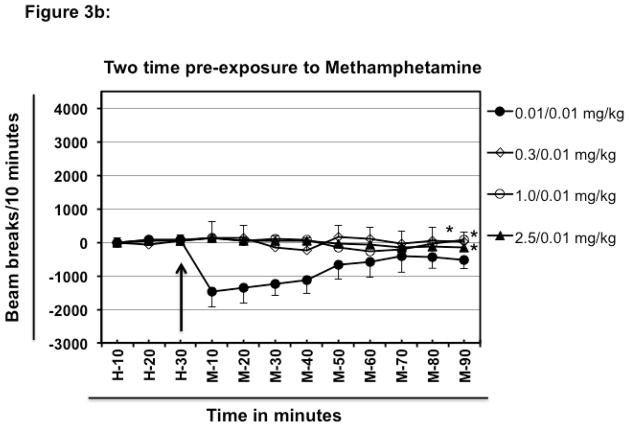
Effect of pre-exposure to methamphetamine on low dose meth-induced hypolocomotion. Different groups of experimentally naïve, 8–10-week old female BALB/c mice (n=5/group) were intraperitoneally injected with varying doses of meth or PBS (day 0) and were then rested for a one week. After that, on day 7 different groups of mice were habituated and challenged intraperitoneally with fixed dose of meth (0.01 mg/kg) (Fig. 3a). Other groups of mice (n=5/group) were intraperitoneally injected with varying doses of meth on day-0 and day-7. Each group received the same dose of meth on both day-0 and day-7. On day-14 each group of mice was habituated and challenged intraperitoneally with fixed dose of meth (0.01 mg/kg; Fig. 3b). Normalized baseline and other data were obtained as explained in Fig. 1. The arrow represents the time of meth injection. In the legend, left hand side values represent the dose of meth which was used for pre-exposure and the right hand side values represent the challenge dose. For example in Fig. 3a, 2.5/0.01 mg/kg means mice were exposed to 2.5 mg/kg meth, rested for one week and then challenged with 0.01 mg/kg meth. In Fig. 3b, mice were exposed twice a week apart before challenge after resting for a week (i.e. only one injection on day-0 → rested for a week → only one injection on day-7 → rested for a week → challenge on day-14). Data shown are mean ± S.E.M. Only some of the S.E.M. bars are shown for the clarity of the graphs. *P<0.001 for comparison vs. 0.01/0.01 group.
Exposure to 0.01 mg/kg meth two times, one- week apart followed a week later by challenge with 0.01 mg/kg meth (Fig. 3b; only one injection on day-0 → rested for a week → only one injection on day-7 → rested for a week → challenge on day-14) resulted in hypolocomotor activity after 10 minutes of meth injection as compared to the basal activity observed during habituation (Fig. 3b; 75 beam breaks at H-30 vs. −1472 beam breaks at M-10 for 0.01/0.01 mg/kg group). However, after 40 minutes of treatment, the locomotor activity of the 0.01/0.01 mg/kg group quickly started coming closer to the basal level (observed during H-10-H-30) and almost reached the basal level by 90 minutes. This is a remarkable departure from the pattern induced by one time pre-exposure to 0.01 mg/kg meth followed by challenge with 0.01 mg/kg meth where this regimen resulted in sustained hypolocomotion and even at 90 mintes of observation hypolocomotive activity was present (0.01/0.01 mg/kg of Fig. 3a and Fig. 3b). Two-time exposure separated by a week to 0.3 or 1.0 or 2.5 mg/kg meth completely blocked the hypolocomotion inducing ability of 0.01 mg/kg meth a week later (Fig. 3b; 0.01/0.01 mg/kg compared with 0.3/0.01; 1.0/0.01 and 2.5/0.01 mg/kg; Group effect, F [3, 144] = 52.2; P <0.0001).
3.4. Effect of abstinence from meth on hypolocomotion in meth pre-exposed mice
As shown in figure 4, mice pre-exposed to 0.01 or 0.3 mg/kg of meth and rested for a month showed a similar pattern and magnitude of locomotor activity upon the test with 0.01 mg/kg meth at 10 and 20 min. after meth dosing (Group effect, F[3, 144] = 79.57; P <0.0001). Mice pre-exposed to 1.0 or 2.5 mg/kg meth and rested for a month followed by challenge with 0.01mg/kg meth also exhibited hypolocomotion compared to the basal locomotor activity observed during habituation prior to meth injection (H-30 compared with M-10 for all four groups), however, at 10 minutes after challenge, the magnitude of the behavioral response was less than that induced in 0.01/0.01 and 0.03/0.01 mg/kg group. Moreover, the duration of hypolocomotion in 1.0/0.01 and 2.5/0.01 mg/kg group was less than that induced by 0.01 mg/kg meth in 0.01 or 0.3 mg/kg meth pre-exposed mice, as the mice pre-exposed to 1.0 or 2.5 mg/kg meth tended to return to the baseline 40 minutes after the challenge.
4. Discussion
The overall goal of these studies was to determine the effects of a range of meth doses in drug-naïve and drug-experienced BALB/c mice. We observed dose dependent changes in their locomotor activity (a measure of CNS effects). Lower doses of meth (0.01 and 0.03 mg/kg) resulted in “hypolocomotor activity” in the drug-naïve mice compared to the PBS-treated group. Kitahama & Valatx [33] have also reported hypolcomtion in meth-treated BALB/c; however, the experimental procedures and the apparatus employed in their study was different than the experimental procedures and the apparatus used in our study. Hypolocomotion has also been reported for cocaine in mice and rats [31, 32]. Moderate doses of meth such as 0.3, 1.0 and 2.5 -mg/kg of meth induced varying degrees of hyperlocomotion and higher doses of meth (5.0 and 10 -mg/kg meth) induced stereotypy, as others have reported [25, 34, 35].
Kitahama & Valatx [33] have reported that 1 mg/kg d-meth induces hypoactivity in albino mice (BALB/c Orl, AKR/Orl, and A/J/Orl strains of mice) whereas the same dose induced hyperactivity in pigmented (C57BL/cd/Orl., C57BL/6 Orl., and SEC strains of mice) [33]. However, our results demonstrate that 1 mg/kg meth induces hyperactivity in BALB/c mice whereas 0.03 or 0.01 mg/kg meth result in hypoactivity. The difference between our results and Kitahama & Valatx’s [33] results could be due to differences in the experimental conditions and procedure. In our case, BALB/c mice (Harlan Laboratories, TX) were first habituated to the test cage for 60 minutes and then they were tested for their locomotor activity immediately after meth injection in a walled test cage. Whereas, Kitahama and Valatx [33] habituated their mice for only 1 minute, then injected meth and after 30 minutes they recorded the locomotor activity for 3 minutes in an open field test [33]. Therefore, this does not seem like a comparable situation.
The brain’s mesolimbic dopaminergic system has a critical role in the development of addictive behavior [36, 37], but norepinephrine (NE) may be particularly important for these very low dose effects of meth [38, 39]. In an elegantly designed in vitro release and uptake inhibition assay using rat synaptosomes, Rothman et al. [40] have determined that meth is most potent at NE release (IC50 = 12.3 nM), followed by DA release (IC50 = 24.5 nM), and 5-HT release (IC50 = 736 nM). In other words, meth releases monoamines in rats with ratios of about NE:DA = 1:2, NE:5HT = 1:60 [40], causing increased stimulation of post and pre-synaptic receptors. Meth also indirectly prevents the reuptake of these neurotransmitters, causing them to remain in the synaptic cleft for a prolonged period inhibiting NE reuptake (Ki = 48.0 nM), followed by DA reuptake (Ki = 114 nM) and 5-HT reuptake (Ki = 2,137 nM). In other words, meth inhibits reuptake of monoamines in rats with ratios of about: NE:DA = 1:2.35, NE:5HT = 1:44.5 [40].
The low dose meth-induced hypolocomotion could be attributed to its effect on presynaptic NE α-2 autoreceptors or its effect on pre-synaptic D2-like DA receptors or its combined effects on both receptor types. It is well known that low doses of apomorphine, a DA receptor agonist, decreases locomotor activity in rodents and this decrease is attributed to the activation of presynaptic DA autoreceptors [29, 30]. In contrast, higher doses of apomorphine increase locomotor activity through postsynaptic DA receptor stimulation [29, 30]. Clonidine, which is an agonist of NE α-2 autoreceptors, also induces hypolocomotion by activating presynaptic NE autoreceptors [27] [28]. In view of these reports, low dose meth-induced hypolocomotion needs further investigation to establish the identity of receptors involved.
Moderate doses of meth (1.0 mg/kg and 2.5 mg/kg) resulted in significant hyperlocomotion at 10 minutes after dosing and then locomotion declined but always remained elevated over baseline up to 90 minutes after dosing. The locomotion was always higher for the 2.5 mg/kg group than the 1.0 mg/kg group. Treatment of drug-naïve mice with higher doses of meth (5 mg/kg and 10 mg/kg) resulted in a brief period of hyperlocomotion which was followed by stereotypy.
Generally accepted theory states that psychostimulant-induced locomotion is driven by the ventral striatum, while stereotypy is regulated by the dorsal striatum [41]. This hypothesis is primarily derived from 6-hydroxydopamine lesion studies demonstrating that local dopamine depletion in the ventral striatum inhibits amphetamine induced locomotion, while dorsal lesions curb stereotypy [42–44]. Higher doses of meth not only release larger amounts of neurotransmitters, but also modulate different brain regions to produce hyperlocomotion or stereotypy. By contrast, lower doses of meth may be releasing only limited amount of neurotransmitters (DA and NE directly), which could modulate pre-synaptic NE receptors in the locus ceruleus decreasing activation and reducing arousal and general levels of motor activity inducing hypolocomotion.
Repeated use of psychostimulants can produce addiction in humans and behavioral sensitization (reverse “behavioral tolerance”) in rodents [45], characterized by a progressively enhanced locomotor activity and stereotypy. Because the neural alterations that underlie behavioral sensitization are thought to contribute to the development of the compulsive patterns of drug craving that characterizes addiction [46], behavioral sensitization in rodents is widely used as a model for the study of drug addiction [47]. A treatment model leading to sustained abstinence may not necessarily return the affected brain regions and behavior to the normal state. To test this hypothesis, we designed another set of experiments to assess the effect of pre-exposure to different doses of meth on the low dose meth-induced hypolocomotion as discussed below.
Pre exposure to higher doses (such as 1.0 mg/kg or 2.5 mg/kg meth) completely attenuated the hypolocomotion inducing ability of 0.01 mg/kg meth (Fig. 1, 3a and 3b) for at least two weeks. These data indicate that even onetime usage of higher doses of meth can lead to significant changes in brain responses. Repeated exposure to even the smaller 0.01 mg/kg doses of meth reduced the hypolocomotor response (Fig. 1 and 3b). Repeated administration of meth decreases dopamine D1 and D2 receptors and probably the NE α-2 autoreceptors that may be involved in this hypolocomotion [48].
We also tested the reverse model of “incubation”. That is, if the drug-experienced mice are allowed to rest for a month, would they show a restored or greater hypolocomotor response to the low meth dose 0.01 mg/kg? In fact, mice pre-exposed to 0.01 or 0.3 mg/kg of meth and rested for a month showed a similar pattern and magnitude of locomotor activity upon challenge with 0.01 mg/kg meth as was observed for drug-naïve mice with that dose (Fig. 1 and 4). Additionally, mice pre-exposed to moderate doses of meth (1.0 mg/kg or 2.5 mg/kg) meth and rested for a month also exhibited hypolocomotion upon challenge with 0.01mg/kg meth. However, the magnitude of behavioral response was less than that induced by 0.01 mg/kg meth in naïve mice. Thus, tolerance to the hypolocomotor effect is induced by prior exposure to higher, but not lower meth doses, after a prolonged abstinence period.
In conclusion, our data demonstrate that lower doses of meth induce hypolocomotion in BALB/c mice. We have also defined the dose-dependence of hyperlocomotion and stereotypy of meth-exposed BALB/c mice. Additionally, we have also shown the effects of repeated exposure and withdrawal of meth on the behavioral responses. These data will help interpret the potency of any future anti-meth antagonists such as vaccines. Very effective partial blockades may result in hypolocomotion below baseline levels, while a full blockade would simply return the locomotion to the baseline. This result is initially counter-intuitive that the most effective full blockade might produce higher levels of locomotion than a partial blockade. Finally, a very sensitive hypolocomotor assay with rapid desensitization from single meth doses and its recovery after a month of abstinence may be helpful in the design and interpretation of future studies involving abstinence and antagonist treatments of meth addiction.
Highlights.
Lower doses of methamphetamine cause hypolocomotion in BALB/c mice.
Moderate and higher doses of methamphetamine induce hyperlocomotion and stereotypy respectively.
Prior exposure to either one moderate or high dose of methamphetamine or to two, low doses of methamphetamine attenuate the hypolocomotor effect of a low meth dose one week later.
Prior exposure to the methamphetamine leads to tolerance, rather than sensitization, of the hypolocomotor response to low meth doses.
Acknowledgments
We thank Bangyi Mao, Yan Wu, and Eric Weathers for excellent technical assistance.
Footnotes
Financial & competing interests disclosure: Funding support was provided by the Department of Veterans Affairs and the National Institute on Drug Abuse grants DA023898, DA023898, DA030338, DP1DA033502 in the production of this manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rawson RA, et al. Methamphetamine dependence and human immunodeficiency virus risk behavior. J Subst Abuse Treat. 2008;35(3):279–84. doi: 10.1016/j.jsat.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shoptaw S, et al. Psychiatric and substance dependence comorbidities, sexually transmitted diseases, and risk behaviors among methamphetamine-dependent gay and bisexual men seeking outpatient drug abuse treatment. J Psychoactive Drugs. 2003;35(Suppl 1):161–8. doi: 10.1080/02791072.2003.10400511. [DOI] [PubMed] [Google Scholar]
- 3.Vigil O, et al. Is hepatitis C infection associated with increased risk of depression in persons with methamphetamine dependence? Am J Addict. 2007;16(5):418–23. doi: 10.1080/10550490701525731. [DOI] [PubMed] [Google Scholar]
- 4.Haning W, Goebert D. Electrocardiographic abnormalities in methamphetamine abusers. Addiction. 2007;102(Suppl 1):70–5. doi: 10.1111/j.1360-0443.2006.01776.x. [DOI] [PubMed] [Google Scholar]
- 5.Maxwell JC. Emerging research on methamphetamine. Curr Opin Psychiatry. 2005;18(3):235–42. doi: 10.1097/01.yco.0000165592.52811.84. [DOI] [PubMed] [Google Scholar]
- 6.Dore G, Sweeting M. Drug-induced psychosis associated with crystalline methamphetamine. Australas Psychiatry. 2006;14(1):86–9. doi: 10.1080/j.1440-1665.2006.02252.x. [DOI] [PubMed] [Google Scholar]
- 7.Harris D, Batki SL. Stimulant psychosis: symptom profile and acute clinical course. Am J Addict. 2000;9(1):28–37. doi: 10.1080/10550490050172209. [DOI] [PubMed] [Google Scholar]
- 8.McKetin R, et al. The prevalence of psychotic symptoms among methamphetamine users. Addiction. 2006;101(10):1473–8. doi: 10.1111/j.1360-0443.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- 9.Lapworth K, et al. Impulsivity and positive psychotic symptoms influence hostility in methamphetamine users. Addict Behav. 2009;34(4):380–5. doi: 10.1016/j.addbeh.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Karila L, et al. Pharmacological approaches to methamphetamine dependence: a focused review. Br J Clin Pharmacol. 2010;69(6):578–92. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vergo S, et al. Vesicular monoamine transporter 2 regulates the sensitivity of rat dopaminergic neurons to disturbed cytosolic dopamine levels. Brain Res. 2007;1185:18–32. doi: 10.1016/j.brainres.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 12.Volz TJ, Fleckenstein AE, Hanson GR. Methamphetamine-induced alterations in monoamine transport: implications for neurotoxicity, neuroprotection and treatment. Addiction. 2007;102(Suppl 1):44–8. doi: 10.1111/j.1360-0443.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- 13.Fleckenstein AE, et al. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–98. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- 14.McCann UD, et al. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62(2):91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- 15.Pereira FC, et al. Single or multiple injections of methamphetamine increased dopamine turnover but did not decrease tyrosine hydroxylase levels or cleave caspase-3 in caudate-putamen. Synapse. 2006;60(3):185–93. doi: 10.1002/syn.20285. [DOI] [PubMed] [Google Scholar]
- 16.Volkow ND, et al. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002;46(2):79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- 17.Volkow ND, Li TK. Drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neurosci. 2004;5(12):963–70. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosten TR, NT, De La Garza R, Haile CN. Cocaine and Methamphetamine Dependence: Advances in Treatment. American Psychiatric Publishing; Washington, DC: 2012. [Google Scholar]
- 20.Orson FM, et al. Substance abuse vaccines. Ann N Y Acad Sci. 2008;1141:257–69. doi: 10.1196/annals.1441.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMillan DE, et al. Effects of murine-derived anti-methamphetamine monoclonal antibodies on (+)-methamphetamine self-administration in the rat. J Pharmacol Exp Ther. 2004;309(3):1248–55. doi: 10.1124/jpet.103.061762. [DOI] [PubMed] [Google Scholar]
- 22.Byrnes-Blake KA, et al. Monoclonal IgG affinity and treatment time alters antagonism of (+)-methamphetamine effects in rats. Eur J Pharmacol. 2005;521(1–3):86–94. doi: 10.1016/j.ejphar.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Daniels JR, et al. Effects of anti-phencyclidine and anti-(+)- methamphetamine monoclonal antibodies alone and in combination on the discrimination of phencyclidine and (+)-methamphetamine by pigeons. Psychopharmacology (Berl) 2006;185(1):36–44. doi: 10.1007/s00213-005-0299-6. [DOI] [PubMed] [Google Scholar]
- 24.Shen X, et al. mu-Opioid receptor knockout mice are insensitive to methamphetamine-induced behavioral sensitization. J Neurosci Res. 2010;88(10):2294–302. doi: 10.1002/jnr.22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gentry WB, et al. (+)-Methamphetamine-induced spontaneous behavior in rats depends on route of (+)METH administration. Pharmacol Biochem Behav. 2004;79(4):751–60. doi: 10.1016/j.pbb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Andersen PH, Jansen JA. Dopamine receptor agonists: selectivity and dopamine D1 receptor efficacy. Eur J Pharmacol. 1990;188(6):335–47. doi: 10.1016/0922-4106(90)90194-3. [DOI] [PubMed] [Google Scholar]
- 27.Heal DJ, Prow MR, Buckett WR. Clonidine-induced hypoactivity and mydriasis in mice are respectively mediated via pre- and postsynaptic alpha 2-adrenoceptors in the brain. Eur J Pharmacol. 1989;170(1–2):19–28. doi: 10.1016/0014-2999(89)90128-3. [DOI] [PubMed] [Google Scholar]
- 28.Archer T, Fredriksson A. Effects of clonidine and alpha-adrenoceptor antagonists on motor activity in DSP4-treated mice I: dose-, time- and parameter-dependency. Neurotox Res. 2000;1(4):235–47. doi: 10.1007/BF03033254. [DOI] [PubMed] [Google Scholar]
- 29.Irifune M, Nomoto M, Fukuda T. Effects of GBR 12909 on locomotor activity and dopamine turnover in mice: comparison with apomorphine. Eur J Pharmacol. 1995;272(1):79–85. doi: 10.1016/0014-2999(94)00620-m. [DOI] [PubMed] [Google Scholar]
- 30.Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277(5692):93–6. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- 31.George FR. Cocaine produces low dose locomotor depressant effects in mice. Psychopharmacology (Berl) 1989;99(2):147–50. doi: 10.1007/BF00442799. [DOI] [PubMed] [Google Scholar]
- 32.George FR. Cocaine produces low dose locomotor depressant effects in NBR and F344 rats. Pharmacol Biochem Behav. 1990;37(4):795–8. doi: 10.1016/0091-3057(90)90565-y. [DOI] [PubMed] [Google Scholar]
- 33.Kitahama K, Valatx JL. Strain differences in amphetamine sensitivity in mice. I. A diallel analysis of open field activity. Psychopharmacology (Berl) 1979;66(2):189–94. doi: 10.1007/BF00427629. [DOI] [PubMed] [Google Scholar]
- 34.Milesi-Halle A, et al. Sex differences in (+)-amphetamine- and (+)- methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharmacol Biochem Behav. 2007;86(1):140–9. doi: 10.1016/j.pbb.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riviere GJ, et al. Spontaneous locomotor activity and pharmacokinetics of intravenous methamphetamine and its metabolite amphetamine in the rat. J Pharmacol Exp Ther. 1999;291(3):1220–6. [PubMed] [Google Scholar]
- 36.Nishikawa T, et al. Behavioral sensitization and relative hyperresponsiveness of striatal and limbic dopaminergic neurons after repeated methamphetamine treatment. Eur J Pharmacol. 1983;88(2–3):195–203. doi: 10.1016/0014-2999(83)90006-7. [DOI] [PubMed] [Google Scholar]
- 37.Yang MH, et al. Proteomic analysis of methamphetamine-induced reinforcement processes within the mesolimbic dopamine system. Addict Biol. 2008;13(3–4):287–94. doi: 10.1111/j.1369-1600.2007.00090.x. [DOI] [PubMed] [Google Scholar]
- 38.Sofuoglu M, Sewell RA. Norepinephrine and stimulant addiction. Addict Biol. 2009;14(2):119–29. doi: 10.1111/j.1369-1600.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinshenker D, Schroeder JP. There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32(7):1433–51. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- 40.Rothman RB, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39(1):32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 41.Iversen SD, Mason ST. Proceedings: Impaired response control in the rat after 6-hydroxydopamine lesions to the dorsal noradrenaline bundles. Br J Pharmacol. 1975;55(2):293P. [PMC free article] [PubMed] [Google Scholar]
- 42.Robbins T, Iversen SD. A dissociation of the effects of d-amphetamine on locomotor activity and exploration in rats. Psychopharmacologia. 1973;28(2):155–64. doi: 10.1007/BF00421400. [DOI] [PubMed] [Google Scholar]
- 43.Creese I, Iversen SD. The role of forebrain dopamine systems in amphetamine induced stereotyped behavior in the rat. Psychopharmacologia. 1974;39(4):345–57. doi: 10.1007/BF00422974. [DOI] [PubMed] [Google Scholar]
- 44.Iversen SD, et al. Proceedings: Amphetamine and apomorphine responses in the rat after lesion of mesolimbic or striatal dopamine neurones. Br J Pharmacol. 1975;54(2):244P. [PMC free article] [PubMed] [Google Scholar]
- 45.Bartlett E, et al. Selective sensitization to the psychosis-inducing effects of cocaine: a possible marker for addiction relapse vulnerability? Neuropsychopharmacology. 1997;16(1):77–82. doi: 10.1016/S0893-133X(96)00164-9. [DOI] [PubMed] [Google Scholar]
- 46.Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3137–46. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Itzhak Y, Ali SF. Behavioral consequences of methamphetamine-induced neurotoxicity in mice: relevance to the psychopathology of methamphetamine addiction. Ann N Y Acad Sci. 2002;965:127–35. doi: 10.1111/j.1749-6632.2002.tb04156.x. [DOI] [PubMed] [Google Scholar]
- 48.McCabe RT, et al. Methamphetamine-induced reduction in D1 and D2 dopamine receptors as evidenced by autoradiography: comparison with tyrosine hydroxylase activity. Neuroscience. 1987;23(1):253–61. doi: 10.1016/0306-4522(87)90287-9. [DOI] [PubMed] [Google Scholar]