Abstract
Background
Validity of oral contraceptive pill (OCP) clinical trial results depends on participant compliance. Ethinyl estradiol (EE2) induces increases in hepatic binding globulin (BG) levels. Measuring these BG increases may provide an effective and convenient approach to distinguishing non-compliant from compliant OCP users in research settings. This analysis evaluated the usefulness of measuring increases in corticosteroid, sex hormone and thyroxine binding globulins (CBG, SHBG, TBG) as measures of OCP compliance.
Methods
We used frozen serum from a trial that compared ovarian suppression between normal weight and obese women randomized to one of two OCPs containing EE2 and levonorgestrel (LNG). Based on serial LNG measurements during the trial, 17% of participants were non-compliant. We matched non-compliant participants with compliant participants by age, BMI, ethnicity and OCP formulation. We measured CBG, SHBG and TBG levels, and compared change from baseline to 3-month follow-up between the non-compliant and compliant participants. Construction of receiver operator characteristic (ROC) curves allowed comparison of various BG measures.
Results
Changes in CBG and TBG distinguished OCP non-compliant users from compliant users (area under the ROC curve (AUROC), 0.86 and 0.89, p < 0.01). Changes in SHBG were less discriminating (AUROC 0.69)
Conclusions
EE2 induced increases in CBG and TBG provide a sensitive integrated marker of compliance with an LNG-containing OCP.
Keywords: oral contraceptives, compliance, binding globulins
1. Introduction
Non-compliance during a clinical trial often biases estimates of effect, decreases generalizability and validity, reduces statistical power, and increases study costs. Accurate measurement of compliance during a trial, however, can be expensive and difficult to achieve [1, 2]. Clinical trials of contraceptive methods most often use self-report to measure compliance [3, 4]. Self-report is inexpensive, but vulnerable to error. In contrast, biomarker measures of compliance may be more valid, but can be expensive and may require multiple visits, which might not be compatible with a study protocol. In an oral contraceptive pill (OCP) effectiveness trial, mistaken assumptions of good compliance will lead to an inflated estimate of the OCP failure rate; pregnancies may be more common among non-compliant study participants, specifically those who provide inaccurate information about their compliance during the trial. Similarly, physiological studies to evaluate changes during OCP use will also lose validity and power if compliance is misclassified.
We recently studied OCP-mediated ovarian suppression in normal weight and obese women [5-7]. One hundred eighty-one participants completed the trial and submitted paper diaries indicating correct OCP use. Because sonograms and progesterone levels indicated unexpectedly frequent ovulation during the trial (12% of study cycles), we used stored serum specimens to measure levels of levonorgestrel (LNG) and identified 17% of study participants with frequently or consistently undetectable LNG levels indicating substantial or complete OCP non-compliance, contradicting the information recorded in their diaries [5]. Most of the observed ovulations occurred during OCP non-use, and had we relied on self-reported compliance alone, we would have drawn incorrect conclusions about OCP effectiveness.
Measuring compliance with a biomarker was easy to do in that study because we were already seeing the participants at frequent visits and drawing blood to measure estradiol and progesterone levels. Those stored specimens were available to assess compliance, although the additional assays added substantial costs. Large Phase 3 studies to estimate OCP effectiveness use self-report to identify compliance, and often have infrequent visits and few blood draws. In those studies, using frequent blood draws to measure compliance via repeated OCP hormone assays would not be feasible.
Ethinyl estradiol (EE2)- containing OCPs cause numerous metabolic changes, among which are gradual and reversible increases in estrogen sensitive hepatic proteins [8-22]. Changes in hepatic proteins could serve as an integrated marker of taking OCPs similar to use of the hemoglobin A1C test in management of diabetes; that test measures the percentage of hemoglobin that is glycosylated and serves as an integrated measure of glucose levels over several months [23]. By analogy, EE2-induced increases in hepatic proteins could provide a more long-term indicator of OCP compliance. The present analysis sought to evaluate the validity of measuring three hepatic proteins to differentiate non-compliant from compliant women in an OCP clinical trial: corticosteroid binding globulin (CBG), sex hormone binding globulin (SHBG) and thyroxine binding globulin (TBG).
2. Methods
Data for this nested case-control analysis come from an IRB-approved trial comparing normal weight and obese OCP users [5-7]. The Columbia University Institutional Review Board approved the study, and all participants gave informed consent. In brief, eligible women were aged 18-35 years with a recent history of regular, spontaneous menstrual cycles, willing to take an OCP for three to four months and commit to eight bi-weekly study visits during the third or fourth OCP cycle. Participants had no medical contraindications to OCP use [24].
Participants were randomized to receive monophasic OCPs containing either 30 mcg EE2 and 150 mcg LNG or 20 mcg EE2 and 100 mcg LNG (Portia® and Lessina®, respectively, Barr Laboratories, Inc., Bala Cynwyd, PA). Participants reported OCP use on a paper diary. To assess compliance with OCP use, we measured LNG levels in specimens collected between days 2-21 of the study cycle. LNG levels were quantified by sensitive and specific radioimmunoassays (RIAs) as previously described [5-7].
We categorized the 181 participants who completed the study as non-compliant users (either non-users or inconsistent users) or compliant users based on results of 903 LNG assays, details reported previously [5-7]. Thirty-one women were non-compliant (17%) -18 non-users, all with uniformly undetectable LNG levels, and 13 inconsistent users, with undetectable LNG levels in most samples - and 150 women were compliant users (83%), all with LNG levels >1.0 ng/mL. For the present analysis we excluded women with ongoing OCP use at study entry. For each non-compliant user (n=21), we sought two compliant user ‘controls’, matching on age, BMI, ethnicity and OCP formulation. Because there were few Asian participants, and these resembled the white participants with regard to other variables, we present whites and Asians combined into one group; thus, the racial/ethnic categories were white/Asian, black and Hispanic.
Using stored specimens, we measured CBG, SHBG and TBG at enrollment and on day 21 of the study cycle. The Biomarkers Core Laboratory of Irving Institute for Clinical and Translational Research performed the CBG, SHBG and TBG assays. Specimens from matched cases and controls were analyzed in duplicate in the same run. CBG was measured using a RIA (IBL-America, Minneapolis, MN). Our coefficients of variation (CVs) for CBG assays in this study were 8.6% (intra-assay) and 8.7% (inter-assay). SHBG and TBG were measured using chemiluminescence immunoassay on an automated immunochemistry analyzer (Immulite 1000, Siemens Healthcare Diagnostics Inc., Deerfield, IL). Our CVs in this study for the intra- and inter-assay precision of the SHBG assays were 2.4% and 2.5%, and for the TBG assays CVs were 7.0% and 9.5%, respectively.
We first compared baseline participant characteristics to verify that matching was successful. We calculated change and percent change from baseline to follow-up for each of the three BGs. The distribution of BG change among the compliant women was approximately normal; however, BG levels among the noncompliant women were not normally distributed and thus we used non-parametric comparisons throughout (Kruskal-Wallis non-parametric analysis of variance and Wilcoxon signed rank-sum test, as appropriate).
We constructed receiver operator characteristic (ROC) curves for each BG to characterize: (1) All non-compliant users versus compliant users, (2) Non-users versus compliant users, and (3) Inconsistent users versus compliant users. To evaluate which measure of BG change was most predictive, we compared the area under the ROC (AUROC) curves. The maximum possible AUROC value is 1.0, representing perfect discrimination between groups; the optimal ROC curve is the one with the highest AUROC value [25,26]. An AUROC curve with a value > 0.8 is considered to indicate a good test.
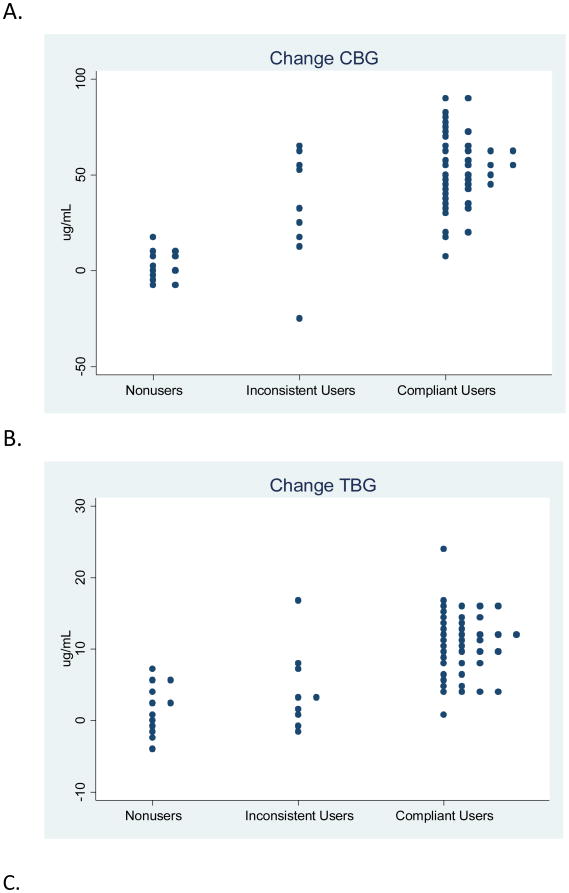
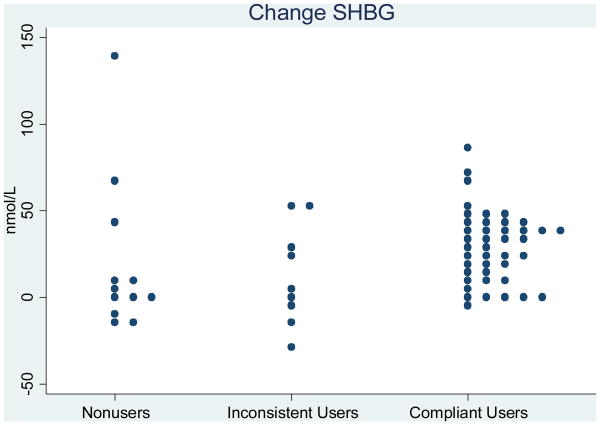
Based on results of prior studies, the expected increase in CBG from baseline to cycle day 21 during use of an LNG-containing OCP was approximately 90% [18-21]. In this analysis, with 21 cases and 42 controls, we had 80% power to detect a CBG change of 20% or greater. Statistical analyses were performed using SAS statistical package 9.2 (SAS Institute, Cary, NC), and Fig. 1 was constructed using STATA release 11 (StataCorp LP, College Station TX).
Fig. 1. Binding globulin changes according to OCP compliance.
3. Results
These results include 21 non-compliant users (further identified as 12 non-users and 9 inconsistent users) and 42 compliant users. We matched the non-compliant users with two compliant controls by age, BMI, ethnicity and OCP formulation. Table 1 summarizes baseline characteristics. By design, non-compliant and compliant users were similar regarding the matching variables. Compliant users tended to have higher levels of education (p=0.05).
Table 1. Baseline Characteristics of Participants.
| Variable | All study Participants | Noncompliant users | Compliant users |
|---|---|---|---|
|
| |||
| N = 181 | N = 21 | N = 42 | |
| Matching variables | |||
|
| |||
| BMI (kg/m2) | 27.2 ± 6.4 | 30.2 ± 6.6 | 30.0 ± 6.4 |
|
| |||
| BMI group | |||
| Normal weight | 106 (59%) | 8 (38%) | 17 (40%) |
| Obese | 75 (41%) | 13 (62%) | 25 (60%) |
|
| |||
| Age | 25.2 ± 4.4 | 26.4 ± 5.4 | 25.7 ± 3.8 |
|
| |||
| OCP formulation | |||
| 20EE2/100LNG | 86 (48%) | 11 (52%) | 21 (50%) |
| 30EE2/150LNG | 95 (52%) | 10 (48%) | 21 (50%) |
|
| |||
| Race/ethnicity | |||
| Hispanic | 51 (28%) | 9 (43%) | 17 (40%) |
| Black | 67 (37%) | 10 (48%) | 20 (48%) |
| White/Asian | 63 (35%) | 2 (9%) | 5 (12%) |
|
| |||
| Non-matching variables | |||
|
| |||
| Height (cm) | 163.8 ± 6.5 | 163.9 ± 6.1 | 163.0 ± 5.9 |
|
| |||
| Weight (kg) | 72.9 ± 17.9 | 81.3 ± 19.1 | 79.7 ± 18.2 |
|
| |||
| Education | |||
| Less than a Bachelor's degree | 99 (55%) | 18 (86%) | 26 (62%) |
| Bachelor's segree or more | 82 (45%) | 3 (14%) | 16 (38%) |
|
| |||
| Previous pregnancy | |||
| Yes | 76 (42%) | 14 (67%) | 23 (55%) |
| No | 105 (58%) | 7 (33%) | 19 (45%) |
|
| |||
| Previous birth | |||
| Yes | 44 (24%) | 10 (48%) | 14 (33%) |
| No | 137 (76%) | 11 (52%) | 28 (67%) |
|
| |||
| Smokes cigarettes | |||
| Yes | 24 (13%) | 1 (5%) | 4 (10%) |
| No | 157 (87%) | 20 (95%) | 38 (90%) |
Data are mean ± standard deviation or n (%).
We evaluated mean levels of the BGs among compliant users according to the variables in Table 1. Mean BG levels did not vary by age. Mean baseline SHBG was lower among obese women than normal weight women (40.9 versus 62.1, p=0.003) and obese women experienced a larger percent change in SHBG after starting the OCP (88.7% versus 50.1%, p = 0.004). Mean CBG and TBG levels did not vary by BMI group. Baseline and follow-up mean CBG and SHBG levels did not vary by ethnicity, but follow-up mean TBG levels were lowest among Asian/white women, higher in black women and highest in Hispanic women (25.7, 32.5, and 35.5, respectively, p = 0.02). Women who received the higher dose OCP formulation had higher levels of CBG at follow-up than women who received the lower dose (115.0 versus 101.3, p=0.01). Follow-up SHBG and TBG levels did not vary by OCP formulation.
Compliant OCP users had substantial increases in the median values of all three BGs during OCP use (p-values all <0.001). In contrast, non-users had minimal increases in each BG (p-values all >0.05). Among inconsistent users, changes in BG were intermediate. Table 2 shows median BG levels. For each BG, the follow-up level, change and percent change showed only slight overlap between non-users and compliant users, but more overlap between inconsistent users and compliant users as illustrated in Fig. 1. Further analyses, exploring the changes in CBG and TBG simultaneously, indicated that this method may provide more separation between the inconsistent users and compliant users.
Table 2. Median (interquartile range) binding globulin levels.
| Group | Non-users | Inconsistent users | Compliant users | P* | P** | P*** |
|---|---|---|---|---|---|---|
|
|
||||||
| N | 12 | 9 | 42 | |||
|
| ||||||
| CBG (mcg/mL) | ||||||
| Baseline | 55.0 (50.0, 58.8) | 52.5 (40.0, 55.0) | 55.0 (47.5, 60.0) | 0.33 | 0.97 | 0.15 |
| Follow-up | 58.8 (53.8, 68.8) | 90.0 (67.5, 102.5) | 107.5 (95.0, 120.0) | <0.001 | <0.001 | 0.004 |
| Change | 1.3 (-3.8, 8.8) | 32.5 (17.5, 55.0) | 52.5 (40.0, 65.0) | <0.001 | <0.001 | 0.077 |
| % change | 2.5 (-4.0, 15.5) | 57.0 (47.0, 137.0) | 92.5 (70.0, 138.0) | <0.001 | <0.001 | 0.29 |
|
| ||||||
| TBG (mcg/mL) | ||||||
| Baseline | 24.4 (21.7, 28.0) | 20.3 (18.3, 21.7) | 22.1 (19.2, 24.5) | 0.035 | 0.072 | 0.17 |
| Follow-up | 25.6 (21.7, 30.5) | 21.5 (20.4, 23.3) | 31.7 (27.3, 38.4) | 0.001 | 0.015 | 0.002 |
| Change | 1.5 (-1.2, 4.6) | 3.0 (0.6, 6.9) | 11.0 (7.7, 13.9) | <0.001 | <0.001 | 0.002 |
| % change | 6.0 (-4.5, 16.3) | 15.1 (2.6, 34.0) | 51.1 (34.0, 64.4) | <0.001 | <0.001 | 0.005 |
|
| ||||||
| SHBG (nmol/L) | ||||||
| Baseline | 44.0 (32.2, 61.6) | 32.0 (27.9, 47.3) | 45.2 (37.4, 69.7) | 0.45 | 0.89 | 0.24 |
| Follow-up | 48.1 (35.2, 79.4) | 60.5 (34.3, 74.1) | 80.1 (63.3, 97.4) | 0.009 | 0.022 | 0.012 |
| Change | 3.7 (-6.3, 26.6) | 5.0 (-7.1, 27.2) | 30.1 (13.0, 40.9) | 0.045 | 0.034 | 0.088 |
| % change | 8.4 (-10.6, 48.2) | 30.9 (-10.5, 104) | 70.1 (19.6, 104.5) | 0.01 | 0.003 | 0.22 |
Kruskal Wallis non-parametric analysis of variance (2 d.f.).
Nonusers versus compliant users.
Inconsistent users versus compliant users.
Table 3 presents the AUROC values and 95% confidence interval for each AUROC. In distinguishing non-compliant (either non-users or inconsistent users) from compliant users, most measures of CBG and TBG performed well, that is, with an AUROC value > 0.8. In distinguishing OCP non-users from compliant users, these two groups were farther apart than all non-compliant versus compliant users (the previous comparison), and thus most AUROC curves of CBG and TBG had even higher values. Finally, in distinguishing inconsistent users from compliant users, the BG changes were more similar, and the AUROC values were somewhat lower than the previous two comparisons; since the study includes only 9 inconsistent users, it is difficult to draw strong conclusions from this portion of the analysis. For all comparisons, measures of SHBG were less informative than measures of CBG and TBG. All of the AUROC results are consistent with the data shown in Fig. 1 and Table 2. These results were unaffected by adjustment for the matching and non-matching factors identified as having an effect on BGs.
Table 3. Area under the curve for ROC analysis.
| Area under the curve | 95% Confidence interval | |
|---|---|---|
| All Non-Compliant Users (n=21) vs. Compliant Users (n=42) | ||
| Follow-up CBG | 0.87 | (0.76-0.98) |
| Change CBG | 0.86 | (0.75-0.97) |
| Percent change CBG | 0.83 | (0.69-0.96) |
| Follow-up TBG | 0.77 | (0.65-0.90) |
| Change TBG | 0.89 | (0.79-0.99) |
| Percent change TBG | 0.89 | (0.79-0.99) |
| Follow-up SHBG | 0.74 | (0.59-0.89) |
| Change SHBG | 0.69 | (0.53-0.86) |
| Percent change SHBG | 0.72 | (0.58-0.87) |
| Non-users (n=12) vs. compliant users (n=42) | ||
| Follow-up CBG | 0.92 | (0.78-1.00) |
| Change CBG | 0.99 | (0.97-1.00) |
| Percent change CBG | 0.99 | (0.96-1.00) |
| Follow-up TBG | 0.73 | (0.56-0.90) |
| Change TBG | 0.94 | (0.88-1.00) |
| Percent change TBG | 0.96 | (0.91-1.00) |
| Follow-up SHBG | 0.72 | (0.48-0.95) |
| Change SHBG | 0.70 | (0.48-0.92) |
| Percent change SHBG | 0.79 | (0.65-0.92) |
| Inconsistent-users (n=9) vs. compliant users (n=42) | ||
| Follow-up CBG | 0.81 | (0.65-0.96) |
| Change CBG | 0.69 | (0.47-0.90) |
| Percent change CBG | 0.61 | (0.37-0.86) |
| Follow-up TBG | 0.83 | (0.65-1.00) |
| Change TBG | 0.83 | (0.63-1.00) |
| Percent change TBG | 0.80 | (0.59-1.00) |
| Follow-up SHBG | 0.77 | (0.60-0.93) |
| Change SHBG | 0.68 | (0.43-0.93) |
| Percent change SHBG | 0.63 | (0.36-0.90) |
4. Discussion
In this clinical trial, all participants had reported using the study medication in their diaries, but the self-report was not substantiated in 17% of participants based on repeated LNG measurement. In this further evaluation of compliance, we found substantial increases in median CBG and TBG levels among compliant OCP users that clearly distinguished them from non-compliant study participants; however, as shown in the figure, there is some overlap between the groups. CBG and TBG changes taken individually did not accurately distinguish the subset of women who were inconsistent OCP users; thus, future larger studies should evaluate the additional benefit of analyzing CBG and TBG increases in tandem.
Measuring compliance with a biomarker was easy to do in this study because we were already seeing the participants at frequent visits and drawing blood to measure estradiol and progesterone. Those stored specimens were available to assess compliance, although the additional assays added substantial costs. In contrast, large Phase 3 studies to estimate OCP effectiveness often have infrequent visits and few blood draws. In those studies, using frequent blood draws to measure compliance via repeated OCP hormone assays would not be feasible, and thus they frequently use self-report to identify compliance.
Inaccurate measures of compliance, such as self-report, bias results and diminish the validity, precision and statistical power of the trial [1-4]. Using BGs as a measure of compliance in Phase 3 contraceptive effectiveness trials would identify women who are not using the assigned hormonal contraceptive; this could lead to a more valid assessment of whether pregnancies are actually treatment failures. Further, in hormonal contraceptive studies with a physiological endpoint, measuring BGs could assist in the identification and exclusion of non-compliant participants, thus improving the validity of the trial. Obtaining just a single on-treatment BG measure late in a study cycle would provide valuable information on compliance and clearly identify non-users. Because non-use of a hormonal method is often a study entry criterion, obtaining a baseline BG measurement will also be useful, since high values (indicating current use) would enable continuing users to be eliminated from study.
Measurement of BGs has benefits compared to measuring the contraceptive steroid itself. Any study participant could take a single dose of study medication immediately prior to a study visit and a single hormone assay would show her to be compliant. BG levels, however, do not change in one day and thus a single value can better indicate weeks of compliance or non-compliance. The BGs studied here reach peak levels by about 14 days after starting daily OCP use and remain relatively constant thereafter [12, 18, 27, 28], dropping substantially as soon as five days after the last active OCP intake, thus giving flexibility as to when to take a blood sample. More frequent visits and blood samples to repeatedly measure contraceptive steroid levels would often not be practical with large or long duration studies. Even if feasible to obtain multiple specimens, in our laboratory contraceptive steroid analyses are more expensive than measuring BGs using standardized immunoassays. Both SHBG and TBG are widely available in any clinical laboratory; at our laboratory SHBG and TBG assays cost about 80% less than contraceptive steroid assays. CBG may not be routinely available in clinical laboratories, but is offered by reference laboratories. Despite their ready availability, we would consider using these assays to evaluate compliance only in the research context, not for clinical use.
Among the many OCPs containing EE2, the progestin component influences changes in BGs. LNG-containing OCPs cause smaller increases in CBG and TBG and notably smaller increases in SHBG than formulations containing other progestins such as desogestrel and drospirenone [11-15]. We expect, therefore, that BG differences between non-compliant and compliant OCP users would be even larger than observed here in studies of women using those other OCPs. In contrast, new OCP formulations containing estradiol (E2) rather than EE2 cause smaller increases in hepatic proteins [29-31]. Thus, sensitivity of BGs as a measure of compliance in trials of E2 OCPs may be smaller than we observed in this study of an EE2 containing OCP. .
In this study, we used serial contraceptive steroid levels as a gold standard to distinguish non-users from inconsistent users within the group of non-compliant women. The BG changes reported here did not distinguish all inconsistent users from consistent OCP users, but were excellent at distinguishing the non-users. In studies of EE2-containing contraceptives, measurement of BGs provide a simple, readily available way to evaluate non-compliance as an alternative or supplement to assessment of compliance as reported by participant diary.
Acknowledgments
This analysis was a sub-study within a clinical trial supported by NIH Grant R01 HD04578.
Supported in part by Columbia University's CTSA grant No. UL1 RR024156 from NCATS-NCRR/NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Duramed Pharmaceuticals (now Teva) donated oral contraceptives and supported the steroid assay analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boudes P. Drug compliance in therapeutic trials: a review. Control Clin Trials. 1998;19:257–68. doi: 10.1016/s0197-2456(98)00005-1. [DOI] [PubMed] [Google Scholar]
- 2.Haynes RB, Dantes R. Patient compliance and the conduct and interpretation of therapeutic trials. Control Clin Trials. 1987;8:12–9. doi: 10.1016/0197-2456(87)90021-3. [DOI] [PubMed] [Google Scholar]
- 3.Stuart GS, et al. Social desirability bias in family planning studies: a neglected problem. Contraception. 2009 Aug;80:108–12. doi: 10.1016/j.contraception.2009.02.009. Epub 2009 Apr 22. [DOI] [PubMed] [Google Scholar]
- 4.Potter L, Oakley D, de Leon-Wong E, Cañamar R. Measuring compliance among oral contraceptive users. Fam Plann Perspect. 1996;28:154–8. [PubMed] [Google Scholar]
- 5.Westhoff CL, Torgal AH, Mayeda ER, et al. Ovarian suppression in normal-weight and obese women during oral contraceptive use: a randomized controlled trial. Obstet Gynecol. 2010;116(2 Pt 1):275–83. doi: 10.1097/AOG.0b013e3181e79440. [DOI] [PubMed] [Google Scholar]
- 6.Westhoff CL, Torgal AT, Mayeda ER, Shimoni N, Stanczyk FZ, Pike MC. Predictors of noncompliance in an oral contraceptive clinical trial. Contraception. 2011 Nov; doi: 10.1016/j.contraception.2011.09.019. epub. [DOI] [PubMed] [Google Scholar]
- 7.Westhoff CL, Torgal AH, Mayeda ER, Pike MC, Stanczyk FZ. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474–80. doi: 10.1016/j.contraception.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sitruk-Ware R, Nath A. Metabolic effects of contraceptive steroids. Rev Endocr Metab Disord. 2011 Jun;12(2):63–75. doi: 10.1007/s11154-011-9182-4. [DOI] [PubMed] [Google Scholar]
- 9.Van der Vange N, Blankenstein MA, Kloosterboer HJ, Haspels AA, Thijssen JH. Effects of seven low-dose combined oral contraceptives on sex hormone binding globulin, corticosteroid binding globulin, total and free testosterone. Contraception. 1990;41:345–52. doi: 10.1016/0010-7824(90)90034-s. [DOI] [PubMed] [Google Scholar]
- 10.Kuhl H, Gahn G, Romberg G, Althoff PH, Taubert HD. A randomize cross-over comparison of two low-dose oral contraceptives upon hormonal and metabolic serum parameters: II. Effects upon thyroid function, gastrin, STH, and glucose tolerance. Contraception. 1985;32:97–107. doi: 10.1016/0010-7824(85)90119-2. [DOI] [PubMed] [Google Scholar]
- 11.Strufaldi R, Pompei LM, Steiner ML, et al. Effects of two combined hormonal contraceptives with the same composition and different doses on female sexual function and plasma androgen levels. Contraception. 2010:82147–54. doi: 10.1016/j.contraception.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Kuhnz W, Staks T, Jutting G. Pharmacokinetics of levonorgestrel and ethinylestradiol in 14 women during three months of treatment with a tri-step combination oral contraceptive: serum protein binding of levonorgestrel and influence of treatment on free and total testosterone levels in the serum. Contraception. 1994;50:563–79. doi: 10.1016/0010-7824(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 13.Sitruk-Ware R, Plu-Bureau G, Menard J, et al. Effects of oral and transvaginal ethinyl estradiol on hemostatic factors and hepatic proteins in a randomized, crossover study. J Clin EndOCPrinol Metab. 2007;92:2074–9. doi: 10.1210/jc.2007-0026. Epub 2007 Mar 20. [DOI] [PubMed] [Google Scholar]
- 14.Song S, Chen JK, Yang PJ, et al. A cross-over study of three oral contraceptives containing ethinyloestradiol and either desogestrel or levonorgestrel. Contraception. 1992;45:523–32. doi: 10.1016/0010-7824(92)90103-z. [DOI] [PubMed] [Google Scholar]
- 15.Thorneycroft IH, Stanczyk FZ, Bradshaw KD, Ballagh SA, Nichols M, Weber ME. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255–62. doi: 10.1016/s0010-7824(99)00093-1. [DOI] [PubMed] [Google Scholar]
- 16.Wiegratz I, Kutschera E, Lee JH, et al. Effect of four different oral contraceptives on various sex hormones and serum-binding globulins. Contraception. 2003;67:25–32. doi: 10.1016/s0010-7824(02)00436-5. [DOI] [PubMed] [Google Scholar]
- 17.Hammond GL, Langley MS, Robinson PA, Nummi S, Lund L. Serum steroid binding protein concentrations, distribution of progestogens, and bioavailability of testosterone during treatment with contraceptives containing desogestrel or levonorgestrel. Fertil Steril. 1984;42:44–51. doi: 10.1016/s0015-0282(16)47956-2. [DOI] [PubMed] [Google Scholar]
- 18.Ågren UM, Anttila M, Mäenpää-Liukko K, Rantala ML, Rautiainen H, Sommer WF, Mommers E. Effects of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β-oestradiol in comparison to one containing levonorgestrel and ethinylestradiol on markers of endocrine function. Eur J Contracept Reprod Health Care. 2011 Dec;16(6):458–67. doi: 10.3109/13625187.2011.614363. Epub 2011 Sep 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaspard UJ, Romus MA, Gillain D, Duvivier J, Demey-Ponsart E, Franchimont P. Plasma hormone levels in women receiving new oral contraceptives containing ethinyl estradiol plus levonorgestrel or desogestrel. Contraception. 1983;27:577–90. doi: 10.1016/0010-7824(83)90023-9. [DOI] [PubMed] [Google Scholar]
- 20.Endrikat J, Blode H, Gerlinger C, Rosenbaum P, Kuhnz W. A pharmacokinetic study with a low-dose oral contraceptive containing 20 microg ethinylestradiol plus 100 microg levonorgestrel. Eur J Contracept Reprod Health Care. 2002;7:79–90. [PubMed] [Google Scholar]
- 21.Goldzieher JW, Dozier TS, de la Pena A. Plasma levels and pharmacokinetics of ethynyl estrogens in various populations. I. Ethynlylestradiol. Contraception. 1980;21:1–16. doi: 10.1016/0010-7824(80)90134-1. [DOI] [PubMed] [Google Scholar]
- 22.Goldzieher JW, Brody SA. Pharmacokinetics of ethinyl estradiol and mestranol. Am J Obstet Gynecol. 1990;163(6 Pt 2):2114–9. doi: 10.1016/0002-9378(90)90550-q. [DOI] [PubMed] [Google Scholar]
- 23.Del Prato S. Tackling hyperglycemia: a more comprehensive approach. Endocr Pract. 2006;12(1):63–6. doi: 10.4158/EP.12.S1.63. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization, Department of Reproductive Health. Medical eligibility criteria for contraceptive use. 3rd. Geneva: World Health Organization; 2004. [Google Scholar]
- 25.Lu Q, Elston R. Using the optimal receiver operating characteristic curve to design a predictive genetic test, exemplified with Type 2 dabetes. Am J Hum Genet. 2008;82:641–51. doi: 10.1016/j.ajhg.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tape T. Interpreting Diagnostic Tests. [Accessed March 14 2012]; http://gim.unmc.edu/dxtests/Default.html.
- 27.Musa B, Seal U, Doe R. Elevation of certain plasma proteins in man following estrogen administration: a dose-response relationship. J Clin Endocrinol Metab. 1965 Sep;25(9):1163–6. doi: 10.1210/jcem-25-9-1163. [DOI] [PubMed] [Google Scholar]
- 28.Brien TG. Human corticosteroid binding globulin. Clin Endocrinol (Oxf) 1981;14:193–212. doi: 10.1111/j.1365-2265.1981.tb00616.x. [DOI] [PubMed] [Google Scholar]
- 29.Junge W, Mellinger U, Parke S, Serrani M. Metabolic and haemostatic effects of estradiol valerate/dienogest, a novel oral contraceptive: a randomized, open-label, single centre study. Clin Drug Investig. 2011;31:573–84. doi: 10.2165/11590220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Zeun S, Lu M, Uddin A, Zeiler B, Morrison D, Blode H. Pharmacokinetics of an oral contraceptive containing oestradiol valerate and dienogest. Eur J Contracept Reprod Health Care. 2009;14:221–32. doi: 10.1080/13625180902850039. [DOI] [PubMed] [Google Scholar]
- 31.Teichmann A. Pharmacology of estradiol valerate/dienogest. Climacteric. 2003;6(2):17–23. [PubMed] [Google Scholar]