Abstract
Aurora Kinase-A (Aurora-A) promotes timely entry into mitosis, centrosome maturation, and formation of bipolar spindles. To address the role of Aurora-A in skin development and homeostasis, we interbred a floxed Aurora-A (Aurora-Afl) mouse with the Cre-deleter strain, K14.Cre. Aurora-Afl/fl;Krt14.Cre (Aurora-A−/−) mice died shortly after birth. These mice had translucent skin, and histological evaluation showed that the dorsal skin was very thin and fragile with frank erosions. Although the expression of the basal layer marker Krt14 and the differentiation marker Krt1 was evident in Aurora-A−/− epidermis, there was a marked reduction in the number of suprabasal layers and basal keratinocytes. Dye exclusion assays also showed defects in barrier function. Unlike WT cells, Aurora-A−/− basal progenitors were delayed in forming two layers at E13.5 when embryonic skin begins to stratify. Increased numbers of mitotic cells, apoptotic bodies, and polyploid keratinocytes were evident in Aurora-A−/− epidermis, indicating that a deficiency in Aurora-A promotes aberrant mitosis, mitotic slippage and cell death. Lastly, Aurora-A−/− keratinocytes displayed centrosomal abnormalities that included centrosomes located at non-apical sites in basal cells. Thus, the deletion of Aurora-A in the developing epidermis alters centrosome function of basal keratinocytes and markedly impairs their ability to divide and stratify.
Keywords: skin development, Aurora Kinase A, centrosomes
Introduction
Aurora Kinase A (Aurora-A) belongs to a family of conserved mitotic Serine/Threonine kinases that have fundamental roles in the regulation of cellular division. It is found predominantly at the poles of dividing cells, localized to the centrosomes and proximal spindles. Aurora-A is required for the timely entry into mitosis, centrosome maturation, formation of bipolar spindles, and has been implicated in the breakdown of the nuclear envelope and the completion of cytokinesis (Marumoto et al., 2003; Vader and Lens, 2008). Moreover, a role for Aurora-A was recently demonstrated in the control of mitochondrial fission during mitosis (Kashatus et al., 2011). Its protein stability and activity are tightly coupled to the stages of the cell cycle, peaking at the G2/M transition and suppressed at anaphase through protein degradation that is mediated by the Anaphase promoting complex (Honda et al., 2000; Littlepage and Ruderman, 2002). Numerous interacting factors such as AJUBA, BORA, NEDD9, PAK, PLK1 and TPX2 have been identified as important for Aurora-A’s activation and cellular localization (Karthigeyan et al., 2010). Downstream targets of Aurora-A are diverse and growing (Kettenbach et al., 2011; Koch et al., 2011). Of these, Aurora-A can phosphorylate the cell cycle regulator CDC25B and thereby affect the centrosomal redistribution of CDK1–Cyclin B1 complexes to promote mitotic entry (Dutertre et al., 2004). Aurora-A can also activate the tubulin deacetylase, HDAC6, to regulate cilia disassembly (Pugacheva et al., 2007), and phosphorylate NDEL1 to promote neurite extension (Mori et al., 2009). Thus, Aurora-A has cellular functions that go beyond its ability to regulate cell division.
Aurora-A is also a proto-oncogene that is frequently amplified or overexpressed in epithelial cancers including squamous cell carcinomas (SCCs) (Clausen et al., 2006; Torchia et al., 2009). Aurora-A was further identified as a low penetrance cancer susceptibility gene in both humans and mice (Ewart-Toland et al., 2003; Ewart-Toland et al., 2005). Ectopic Aurora-A expression can transform immortalized rodent cell lines in vitro and promote their growth in immunocompromised mice (Bischoff et al., 1998; Zhou et al., 1998). We have previously shown in genetically engineered cancer mouse models that Aurora-A can co-operate with a classical skin chemical carcinogenesis protocol or with the expression of a gain-of-function p53R172H mutant allele to promote the formation of metastasis-prone skin SCCs (Torchia et al., 2012; Torchia et al., 2009). Due to its role in cell division, Aurora-A may be an attractive target for therapeutic intervention in skin cancers. Currently, there are several small molecule inhibitors to Aurora Kinase A and B in clinical trials (Green et al., 2011). However, the long-term off-tumor effects of these inhibitors remain undefined, especially in skin. Inhibition of Aurora-A activity in cultured cells results in mitotic abnormalities, misalignment of chromosomes, and growth suppression (Barr and Gergely, 2007). Recently, three different laboratories have described the global deletion of Aurora-A in mice (Cowley et al., 2009; Lu et al., 2008; Sasai et al., 2008). In these reports, deletion of Aurora-A resulted in embryonic death prior to implantation (Cowley et al., 2009; Lu et al., 2008; Sasai et al., 2008). From these studies, it is clear that Aurora-A is essential for life, but the early lethality of these mice precluded the study of Aurora-A’s function in tissue specific development or homeostasis. In the present study, we examined the consequences of keratinocyte-specific deletion of Aurora-A to determine its role in the formation and maintenance of skin epithelia.
RESULTS
Skin-specific deletion of Aurora-A
To delete Aurora-A in skin, we interbred floxed Aurora-A mice (Sasai et al., 2008) with a constitutively active Cre recombinase expressed from a truncated Keratin 14 promoter (K14.Cre) (Dassule et al., 2000). This Cre deleter strain begins to express Cre in epithelial tissues at E10.5 and displays Cre activity more uniformly by E12.5 as shown by the activation of the Rosa26 β-galactosidase Cre-reporter (Supplementary Figure 1a). Aurora-Afl/+; K14.Cre mice appeared phenotypically normal. In contrast, Aurora-Afl/fl; K14.Cre mice died shortly after birth, and had obvious skin related abnormalities including fragile, translucent and stretched skin, the failure of eyelids and ears to form normally, fused fingers, and the absence of whisker follicles (Figure 1a). Moreover, toluidine penetration assays in E18.5 embryos revealed defects in skin barrier function, which were more pronounced on the ventral side of the embryos (Figure 1b). Detection of the deleted floxed allele was evident in the skin of both Aurora-Afl/+; K14.Cre and Aurora-Afl/fl; K14.Cre newborn mice (Fig 1c and Supplemental Figure 1b). Deletion of the floxed allele was associated with reduced Aurora-A mRNA levels in whole skin (Supplemental Figure 2a) and the absence of detectable Aurora-A protein in actively dividing keratinocytes of Aurora-Afl/fl; K14.Cre embryos (Supplemental Figure 2b). Collectively, these observations indicate that a defective skin barrier, cause by epidermal Aurora-A deficiency, most likely led to desiccation and perinatal lethality of Aurora-Afl/fl; K14.Cre newborn mice.
Figure 1. Skin abnormalities in mice deficient for epithelial Aurora-A.
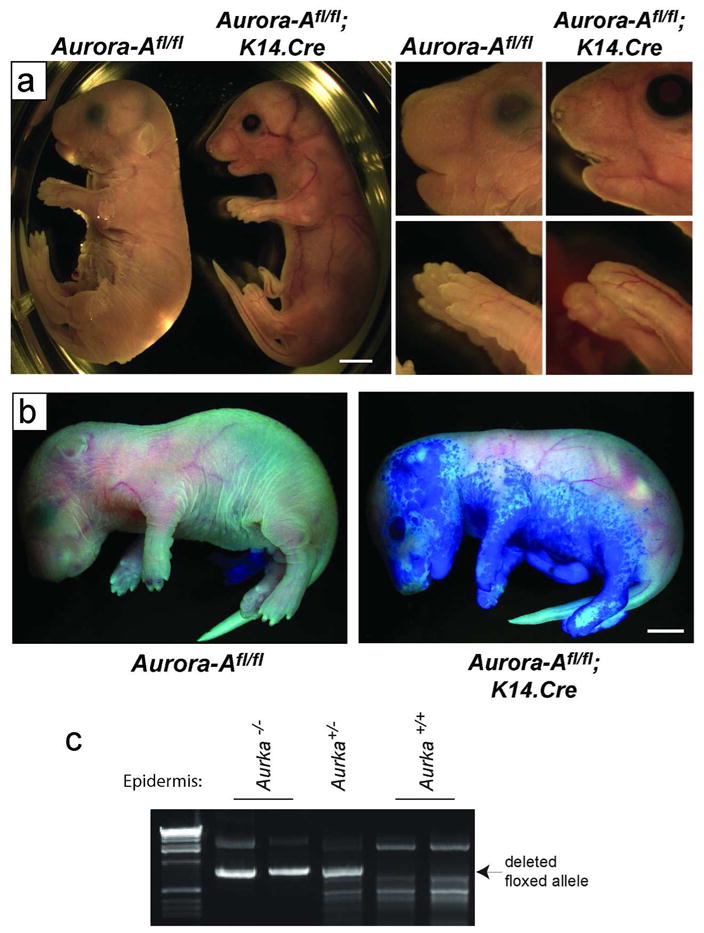
(a) Left panels show gross phenotypic presentation of Aurora-Afl/fl; K14.Cre mice at E18.5. These mice show translucent skin compared to WT controls. Right panels show a close up of the face and paws. Note the improperly formed whiskers, nares, eyelids and nails. (b) Toluidine blue dye penetration assays revealed a defect in barrier function in Aurora-Afl/fl; K14.Cre E18.5 mice. (c) Tails from newborn mice were digested and PCR performed to detect Cre-mediated recombination of the floxed Aurora-A allele. Bars= 2.5 mm.
Histological evaluation of the skin of Aurora-Afl/fl; K14.Cre mice at E16.5 and older showed a thin epidermis, and the absence of mature hair follicles (Figure 2). We focused on the interfollicular keratinocyte compartment for the remainder of this study. The nuclei of the Aurora-A−/− keratinocytes appeared enlarged with multiple nucleoli. Marker analysis revealed the expression of Krt1 and 10 in the suprabasal compartment, whereas Krt14 and Krt5, and the stress Keratin, Krt6 were evident in the basal layer of Aurora-Afl/fl; K14.Cre dorsal skin (Figure 2). The late differentiation markers, Loricrin and Filaggrin, were also detectable in the outermost layer of Aurora-A−/− epidermis. However, the expression of Filaggrin was markedly reduced or sporadic (Figure 2). A similar pattern of expression was observed for Krt1 on the ventral side of E17.5 Aurora-Afl/fl; K14.Cre embryos, whereas Loricrin and Filaggrin expression was markedly reduced or absent (Supplementary Figure 3). The lack of Loricrin and Filaggrin expression on the ventral side likely contributed to a more severe impairment of barrier function in Aurora-Afl/fl; K14.Cre embryos (see Figure 1b). Both Collagen IV and integrinα6 were found at the epidermal/dermal junction of Aurora-A deficient epidermis, and we observed an expression pattern similar to wildtype (WT) for the adherent junction proteins Zo-1, Desmoglein 1/2 and Desmocolin3 (data not shown). Thus, Aurora-A deficiency did not disrupt the integrity of the basement membrane, or cellular junctions of the epidermis.
Figure 2. Aurora-A−/− epidermis exhibits a disrupted epidermal architecture.
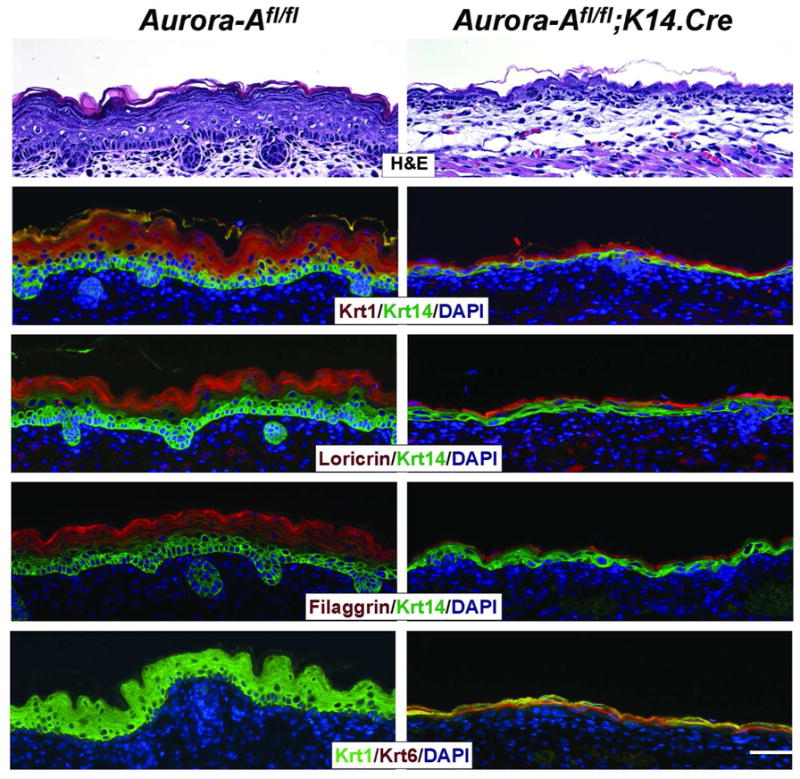
Histological analysis (H&E) of Aurora-A null dorsal epidermis at E16.5 revealed a thin epidermis and absence of mature hair follicle formation. However, marker analysis showed the presence of a basal layer by the detection of Krt14 expression, whereas expression of Krt1 marked the presence of the suprabasal compartment. A similar pattern was observed for Krt10 vs Krt 5 expression (not shown). The late differentiation markers, Loricrin and Filaggrin were visible in the outermost part of Aurora-A−/− epidermis. Bar = 25 μm
Delayed stratification of Aurora-A−/− skin epithelia
The phenotypic presentation and abnormal epidermal architecture of Aurora-Afl/fl; K14.Cre prenatal mice suggested to us that Aurora-A deficiency may impair the formation of the stratified layers of the skin. We therefore examined the embryonic stages between E12–14, when the epithelium stratifies to create the suprabasal compartment (Koster and Roop, 2007). Unlike WT cells, Aurora-A−/− epidermal progenitors were delayed in forming a suprabasal layer in both the ventral and dorsal skin, as evident by histological evaluation and the late appearance of Krt1 expressing cells (Figure 3a). Because suppression of proliferation could account for a delay in the emergence of suprabasal keratinocytes, we examined the S and M phases of the cell cycle in developing skin. At E13.5 similar numbers of keratinocytes in both WT and Aurora-A−/− epidermis were positive for BromodeoxyUridine (BrdU) incorporation indicating that Aurora-A deficiency did not alter the ability of keratinocytes to enter S-phase (Figure 3c). Next, we stained for phospho-Histone H3 to determine the relative number of cells in mitosis (Tapia et al., 2006). As shown in Figure 3b-c for WT epidermis, only a few basal and suprabasal layer cells were positive for phospho-Histone H3, consistent with a short duration spent tranversing M-phase (Dover and Potten, 1988). In contrast, a much higher number of positive cells were found in Aurora-A−/− versus WT skin (a 3 and 6-fold increase in the dorsal and ventral epidermis, respectively). Furthermore, mitotic cells in Aurora-A−/− epidermis lacked a fully formed nuclear envelope as shown by a diffused staining pattern for Lamin A/C, a nuclear membrane structural component (Margalit et al., 2005) (Supplementary Figure 4). As the nuclear envelope is disassembled prior to metaphase in mitosis (Margalit et al., 2005), Aurora-A deficient keratinocytes appear to stall at pro-metaphase of the cell cycle.
Figure 3. Delayed stratification in Aurora-A−/− epidermis.
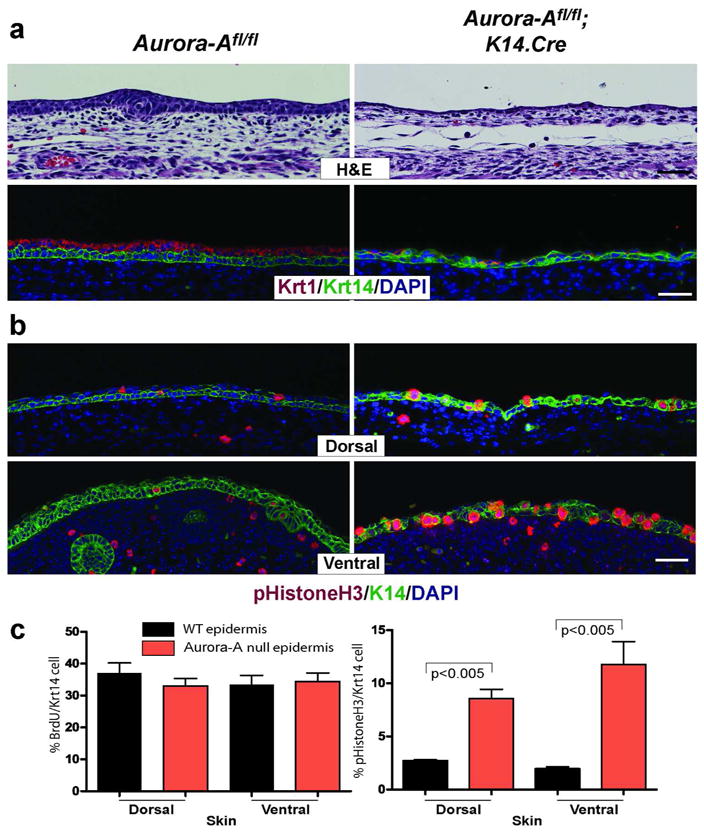
(a) Epidermis between E13–14 was examined by histology and marker analysis. Unlike WT mice that forms two layers at E13.5, Aurora-A−/− epidermis remains primarily a single layer. Consistently, few cells expressed the subrabrasal marker Krt1 in the uppermost layer of the skin. A similar pattern was observed on ventral skin (not shown). (b) Immunofluorescent detection of phopho Histone H3 (Ser10) in E13.5 skin. Note the increased number of positive cells found in both dorsal and ventral skins of Aurora-Afl/fl; K14.Cre mice. (c) Quantitation of BrdU incorporation and detection of mitotic cells per Krt14 positive keratinocytes. Data shown is the average counts per three embryos ± standard deviation. P values are from a t-test. Bar = 25 μm
The outcome of a stalled mitosis in Aurora-A−/− keratinocytes
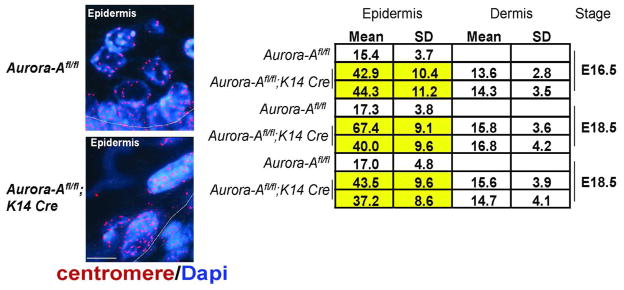
Cells that are stalled in mitosis can undergo cell death or escape without completion of cytokinesis by a process that is called mitotic slippage (Rieder and Maiato, 2004). To determine if apoptosis was a consequence of deleting Aurora-A in keratinocytes, we stained Aurora-A−/− epidermis for the apoptotic marker, active Caspase 3. Unlike WT skin, active Caspase 3 was readily detectable in Aurora-A−/− epidermis. Its detection peaked at E13.5, which coincided with the commencement of stratification (Figure 4). Another outcome of a delayed mitosis is the failure to generate two daughter cells, leaving the cell with twice the normal complement of genetic material. To test this possibility, we enumerated centromeres by staining paraffin sections of embryonic skin with a pan-centromere FISH probe. Increased numbers of centromeres were found in Aurora-A−/− epidermis relative to the nuclei found in WT epidermis or in the dermis of Aurora-Aflfl; K14.Cre mice that remained WT for Aurora-A (Figure 5). Moreover, cells in metaphase or anaphase were rarely observed by histology or by staining with Aurora-B, which localizes to the midzone or anaphase bridges during mitosis (Ruchaud et al., 2007) (data not shown). From these analyses, we conclude that Aurora-A−/− keratinocytes gave rise to polyploid cells.
Figure 4. Increased apoptosis in Aurora-A deficient epidermis.
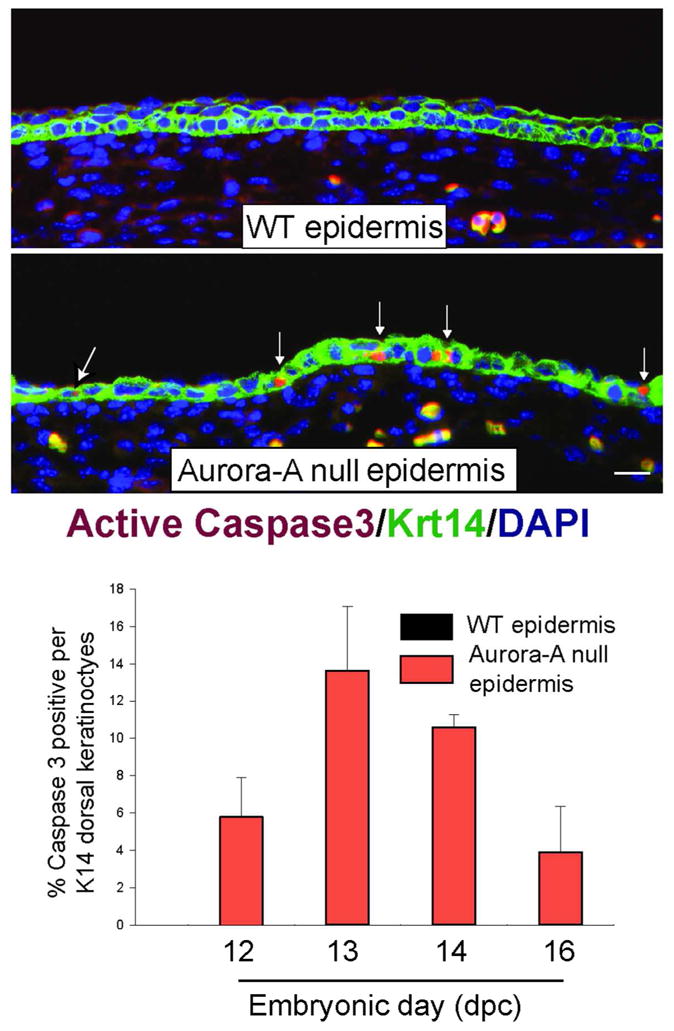
Top panel shows the immunodetection of active Caspase 3 in WT and Aurora-A−/− epidermis at E13.5. Bar= 25 μm. Bottom panel shows the quantitation of apoptosis found in developing skin of Aurora-Afl/fl; K14.Cre or WT embryos (n=2 embryos per genotype and timepoint). Columns represent average ± standard deviation. Note the absence of apoptotic cells in WT skin at any embryonic stage.
Figure 5. Aurora-A deficiency induces polyploidy in keratinocytes.
Left panels show the representative FISH detection of a pan mouse centromere probe on 5-micron thick paraffin sections from E16.5 skin of Aurorafl/fl and Aurora-Afl/fl; K14.Cre embryos. Bar=10 μm. Right, quantification of centromeres. Mean count ± standard deviation is shown. Note the consistent number of centromeres in cells found in WT Aurora-A epidermis or the dermis of Aurora-Afl/fl; K14.Cre mice that do not undergo Cre-recombination. However, much higher numbers are detected in Aurora-A−/− epidermis, indicative of polyploidy.
Centrosome abnormalities in Aurora-A−/− keratinocytes
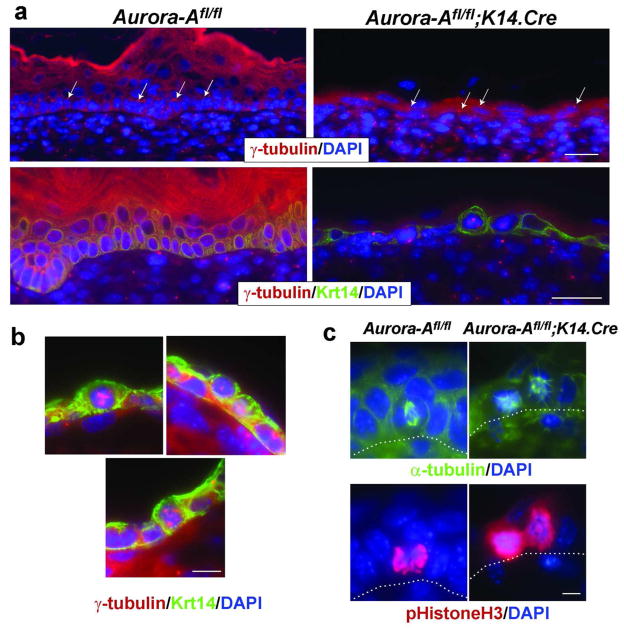
Aurora-A promotes the maturation of centrosomes and formation of bi-polar spindles in preparation for cell division (Vader and Lens, 2008). Therefore, we examined how Aurora-A deficiency affected centrosome function in the developing epidermis. In WT keratinocytes at E16.5 and older, the centrosomes were predominately found apically (Figure 6A, arrows). This orientation was altered in Aurora-A−/− epidermis, with the position of the centrosomes becoming random and located either apically, basolaterally or centrally (Figure 6a, arrows, and see Supplementary Figure 5). As shown in Figure 6b, there were multipolar structures or centrosome clusters in Aurora-A−/− epidermis, reminiscent of spindles anomalies observed in cells with extra centrosomes (Ganem et al., 2009) or those treated with Aurora-A inhibitors, and in Drosophila mutants of Aurora-A (Glover et al., 1995; Manfredi et al., 2007). Since the proper orientation of centrosomes is required for the formation of bi-polar spindles in preparation for metaphase, we next examined the microtubule network in Aurora-A−/− epidermis. In actively dividing WT cells, microtubules are organized between two opposing nucleating poles (i.e., the centrosomes) whose orientation to the basement membrane determines the plane of cell division (Glotzer, 2009). A basal cell that forms a bi-polar spindle perpendicular to the basement membrane will divide asymmetrically and give rise to two cells, a suprabasal and a basal keratinocyte (Figure 6c) (Lechler and Fuchs, 2005; Smart, 1970). In Aurora-A−/− keratinocytes, basal cells with bi-polar spindles were rarely observed at E13.5 or at later embryonic stages. In dividing cells identified by phospho-Histone H3 expression, the microtubule network appeared disorganized (Figure 6c). Taken together, these data indicate that abnormal centrosome function induced by the deletion of Aurora-A leads to a defective cellular division and the mislocation of centrosomes following mitosis of basal cells.
Figure 6. Centrosomal abnormalities in Aurora-A−/− epidermis.
(a) the location of centrosomes was examined in Aurora-A−/− E16.5 epidermal skin by immunostaining with γ-tubulin. Left panels show the apical location of centrosomes (arrows) in basal keratinocytes, relative to the basement membrane. Right panels show non-apical locations of centrosomes in Aurora-A−/− epidermis (arrows) Bar= 25 μm (b) Higher magnification of Aurora-A−/− cells. Bar= 10 μm (c) Detection of the microtubule network by α-tubulin immunostaining. Left panel shows formation of a bipolar spindle in WT keratinocytes undergoing mitosis. Right panels show atypical microtubule formation in Aurora-A deficient basal cells, reminiscent of monopolar spindles. Bar=5 μm.
To determine if the effects of Aurora-A deficiency were similar in post-natal skin, Aurora-A floxed mice were crossed with the Tamoxifen-inducible Cre deleter strain, K14.CreER (Vasioukhin et al., 1999). This Cre-fusion shows robust deletion of floxed alleles in keratinocytes both in vitro and in vivo after treatment with Tamoxifen (Tam) (Supplemental Figure 6a). Similar to Aurora-Afl/fl; K14.Cre embryos, Aurora-A was not detectable in dividing keratinocytes present in the skin of Tamoxifen treated Aurora-Afl/fl; K14.CreER mice (Supplemental Figure 6b). Surprisingly, the deletion of Aurora-A in postnatal epidermis resulted in no obvious gross skin abnormalities (e.g., hair loss or skin erosions) 3 months following the induction of Cre activity by Tamoxifen treatment (n=6 and data not shown). We were able to detect cells that were positive for phospho-Histone H3 or active Caspase 3 in Aurorafl/fl; K14.CreER epidermis 10 days after the induction of Cre (Supplementary Figure 7); however, adult epidermis appears to be reasonably tolerant to the deletion of Aurora-A.
DISCUSSION
In the present study, we utilized a conditional deletion strategy to demonstrate that the expression of Aurora-A is required for normal skin development. The deletion of Aurora-A in embryonic basal keratinocytes resulted in defective centrosome maturation, which markedly impaired the ability of these cells to divide and stratify. Consequently, the initiation of stratification and differentiation was delayed in the developing Aurora-A−/− epidermis, resulting in impaired barrier function and perinatal death. Previous studies have shown that barrier formation begins on the dorsal epidermis and progresses laterally starting at E16 (Hardman et al., 1998). The ventral epidermis is last to acquire a competent barrier. The incorporation and crosslinking of epidermal differentiation proteins such as Loricrin into the cornified envelope coincides with the formation of barrier function (Simpson et al., 2011). In Aurora-A deficient epidermis, barrier formation is defective, leading to a phenotype that is especially prevalent on ventral skin. Nevertheless, dorsal skin in Aurora-Aflfl; K14.Cre embryos was able to express terminal differentiation proteins and form a partial barrier, whereas the ventral epidermis was not. How dorsal skin is able to form two layers and undergo differentiation in light of an obvious cell cycle defect in basal keratinocytes is unclear. It is possible that incomplete Cre-recombination of both floxed Aurora-A alleles between E12.5–13.5 or residual Aurora-A mRNA allowed enough basal progenitors to undergo at least one round of cellular division. Alternatively, basal delamination of non-mitotic cells may contribute to the formation of the first suprabasal layer in Aurora-Afl/fl; K14.Cre skin (Adams and Watt, 1990; Connelly et al., 2010; Liebig et al., 2009; Magee et al., 1987; Poumay et al., 1994).
The phenotypic consequence of deleting Aurora-A in mouse keratinocytes is reminiscent of the effects of expression of Aurora-A mutants in Drosophila (Berdnik and Knoblich, 2002; Glover et al., 1995). Drosophila mutations in Aurora-A result in lower expression of the mutant protein compared to WT flies. The most penetrant of these mutants induced monopolar spindles in dividing cells, delays in mitosis, and polyploidy (Berdnik and Knoblich, 2002; Glover et al., 1995). Invariably, the failure of centrosomes to separate prior to metaphase can explain the effects of suppressing Aurora-A levels in flies. The deletion of Aurora-A in the developing mouse skin mirrored the most severe of the Drosophila mutants, resulting in centrosomal related defects. Additionally, the centrosomes in Aurora-A−/− basal cells appeared to lose the predominant apical placement seen in WT keratinocytes (Lechler and Fuchs, 2007) and were found distributed more randomly. The position of centrosomes, which determines the axis of cell division during mitosis, is set during metaphase in basal keratinocytes (Adams, 1996; Poulson and Lechler, 2010). In the stages that precede metaphase, centrosomes can be found at angles between parallel to perpendicular (0–90°) relative to the basement membrane (Poulson and Lechler, 2010). The observed locations of the centrosomes in Aurora-A−/− keratinocytes may be a consequence of these cells stalling in pro-metaphase, undergoing mitotic slippage, and possibly re-entering the cell cycle.
Surprisingly, the post-natal deletion of Aurora-A resulted in no gross skin defects, suggesting that inhibition of Aurora-A activity may be well tolerated by adult skin, and represents a clear distinction to the effects observed on the prenatal epidermis as we show in this study, or seen in early stage embryos (Cowley et al., 2009; Lu et al., 2008; Sasai et al., 2008). Our observations are also consistent with recent clinical trials of Aurora-A small molecule inhibitors that have shown increased phosho-Histone H3 staining in the skin, but the absence of reported skin-related toxicities (Chakravarty et al., 2011). The mechanism that allows adult skin to tolerate Aurora-A deletion remains unclear but may depend on the relative higher rate of proliferation observed in embryonic versus adult skin. In this regard, Aurora-A inhibition would be expected to have a more profound effect on skin cancer cells, which have a higher rate of proliferation (Torchia et al., 2009), and be more similar to what we observed in embryonic keratinocytes. More importantly, our data suggest that the use of small molecule inhibitors of Aurora-A may be a safe and viable strategy to treat recurrent or metastasis-prone skin SCCs (Ganem et al., 2009) if topical delivery of inhibitors can be achieved.
In summary, we have characterized the cellular and tissue-specific changes associated with Aurora-A deficiency in the developing epidermis. Aurora-A deficient epidermis may be an ideal model to explore the relationship between the regulation of cell division, migration and differentiation in epidermal homeostasis, and a tool to develop novel approaches to maximize the killing of squamous cell carcinoma cells by targeting mitotic regulators.
MATERIALS AND METHODS
Generation of Aurora-A deficient epidermis
The previously described floxed Aurora-A(fl) mice (Sasai et al., 2008), K14.Cre mice (Dassule et al., 2000), or K14.CreER mice (Vasioukhin et al., 1999) were interbred to generate Cre positive and heterozygous or homozygous Aurora-Afl mice. Timed matings were set up at night and the presence of vaginal plugs on the following morning noted as day 0. Embryos were then collected at days corresponding to different mouse developmental stages. The University of Colorado Institutional Animal Care and Use Committee approved animal experiments. Detection of the Cre-recombined floxed Aurora-A allele was performed as previously described (Sasai et al., 2008). For experiments using the K14.CreER strain, mice were topically treated with 125 μg of 4 HydroxyTamoxifen (4HOTam) (Sigma) dissolved in ethanol or injected intraperitoneally for 6 days with Tamoxifen (Sigma) (6 mg/40 g body weight) dissolved in sesame oil.
Dye Penetration and qPCR Assay
Dye penetration was tested on E18.5 embryos as previously described (Hardman et al., 1998). Briefly, embryos were dehydrated and re-hydrated through a series of methanol washes and subsequently incubated in Phosphate Buffered Saline solution saturated with Toluidine blue (Sigma). RNA isolation, reversed transcription, and QPCR analysis was performed as previously described (Torchia et al., 2009). Aurora-A mRNA was detected by Taqman assay# Mn01248177_m1 (Life technologies) and normalized to Gapdh (Life technologies) levels.
Histology, Imuno- and β-galactosidase- staining
Mouse tissues were fixed in 10% neutral buffered formalin (Thermo Fisher Scientific, Pittsburgh, PA), and embedded in Optimal Cutting Temperature compound (Sakura Finetek) for frozen sectioning or processed for paraffin sectioning. The cut planes were either sagittal at the midline or transverse at the midbody. For histological evaluation, dewaxed 5 μM sections were stained with hematoxylin and eosin (H&E). Immunostaining was performed as previously described with modifications (Torchia et al., 2009). Briefly, paraffin embedded tissue was dewaxed and antigen retrieval performed using a Tris EDTA ph10 solution containing 0.05% Tween20. Sections were then blocked and incubated with the following primary antibodies: Keratin (Krt) 1, 5, 10, 14, Loricrin and Filaggrin (Roop et al., 1984; Yuspa et al., 1989), Col IV (Abcam), Itα6 (Chemicon), γ-tubulin (Abcam), α-tubulin (Sigma), phospho-Histone H3 (Ser 10) (Cell Signaling), Active Capase 3 (Cell Signaling). Appropriate secondary antibodies labeled with Alexa488 or 594 were applied and sections mounted on Vectashield Mounting Medium containing DAPI (Vector labs). Staining detection was performed on a Nikon 90i microscope (Nikon Corporation) using standard techniques. For BromodeoxyUridine (BrdU) in vivo labeling experiments, mice were injected with BrdU (Sigma) as previously described (Torchia et al., 2007). Tissue was then harvested after 2hr, fixed, embedded in paraffin, and stained as described above using rat anti-BrdU antibody (Abcam). Staining for β-galactosidase (X-gal) activity was performed on 10 μm OCT sections as previously described (Torchia et al., 2007).
Fluorescence In Situ Hybridization (FISH)
FISH Detection and enumeration of mouse centromeres was conducted at the University of Colorado Cancer Center Cytogenetic Core. Briefly, dewaxed sections were hybridized with SpectrumRed labeled Poseidon All Mouse Centromere Probe (KI-30500, Kreatech Diagnostics, Durham, NC). Chromatin was counterstained with DAPI (Vectashield Mounting Medium, Vector Laboratories). Analysis was performed on epifluorescence microscope using single interference filters sets for red (Texas red), blue (DAPI), and triple (blue, red, green) band pass filters. Number of signals was scored in at least 30 nuclei per selected area in each specimen. Images were acquired and merged using CytoVision application (Applied Imaging).
Cell counting and Statistical Analyses
To enumerate the number of % keratinocytes that were positive for BrdU or phospho-Histone H3, three separate embryos of each genotype were analyzed. Tissue sections from dorsal and ventral skins at E13.5 were stained for either BrdU incorporation/Krt14 or phospho-Histone H3/Krt14. The number of BrdU or phospho-Histone H3 positive cells were scored at 200x magnification after counting on average 294 and 575 Krt14 positive keratinocytes/embryo, respectively. Similar analysis was performed for active Caspase 3/Krt 14 stained tissue sections with an average of 587 Krt 14 positive cells/embryo counted. Dorsal skin was analyzed in two separate embryos for each embryonic timepoint. Statistical tests shown in Figure 5c (phosho-Histone H3) were performed using GraphPad Prism v5.
Supplementary Material
Acknowledgments
This work was supported by grants from NIH: R01CA52607 (DRR) and RO1CA89716 (SS). We thank Dr. Leila Garcia and the University of Colorado Cancer Center Cytogenetics Core for performing the FISH experiments (P30 CA046934), David Sheneman for technical assistance, and Miss Irene Choi for her assistant in the preparation of this manuscript. We also acknowledge support from the University of Colorado Skin Disease Research Center Morphology and Phenotyping Core facility (P30 AR057212).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- Adams JC, Watt FM. Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes alpha 5 beta 1 integrin loss from the cell surface. Cell. 1990;63:425–35. doi: 10.1016/0092-8674(90)90175-e. [DOI] [PubMed] [Google Scholar]
- Adams RJ. Metaphase spindles rotate in the neuroepithelium of rat cerebral cortex. J Neurosci. 1996;16:7610–8. doi: 10.1523/JNEUROSCI.16-23-07610.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AR, Gergely F. Aurora-A: the maker and breaker of spindle poles. J Cell Sci. 2007;120:2987–96. doi: 10.1242/jcs.013136. [DOI] [PubMed] [Google Scholar]
- Berdnik D, Knoblich JA. Drosophila Aurora-A is required for centrosome maturation and actin-dependent asymmetric protein localization during mitosis. Current biology: CB. 2002;12:640–7. doi: 10.1016/s0960-9822(02)00766-2. [DOI] [PubMed] [Google Scholar]
- Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 1998;17:3052–65. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty A, Shinde V, Tabernero J, Cervantes A, Cohen RB, Dees EC, et al. Phase I assessment of new mechanism-based pharmacodynamic biomarkers for MLN8054, a small-molecule inhibitor of Aurora A kinase. Cancer Res. 2011;71:675–85. doi: 10.1158/0008-5472.CAN-10-1030. [DOI] [PubMed] [Google Scholar]
- Clausen OP, Aass HC, Beigi M, Purdie KJ, Proby CM, Brown VL, et al. Are keratoacanthomas variants of squamous cell carcinomas? A comparison of chromosomal aberrations by comparative genomic hybridization. J Invest Dermatol. 2006;126:2308–15. doi: 10.1038/sj.jid.5700375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly JT, Gautrot JE, Trappmann B, Tan DW, Donati G, Huck WT, et al. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat Cell Biol. 2010;12:711–8. doi: 10.1038/ncb2074. [DOI] [PubMed] [Google Scholar]
- Cowley DO, Rivera-Perez JA, Schliekelman M, He YJ, Oliver TG, Lu L, et al. Aurora-A kinase is essential for bipolar spindle formation and early development. Mol Cell Biol. 2009;29:1059–71. doi: 10.1128/MCB.01062-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–85. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- Dover R, Potten CS. Heterogeneity and cell cycle analyses from time-lapse studies of human keratinocytes in vitro. J Cell Sci. 1988;89 ( Pt 3):359–64. doi: 10.1242/jcs.89.3.359. [DOI] [PubMed] [Google Scholar]
- Dutertre S, Cazales M, Quaranta M, Froment C, Trabut V, Dozier C, et al. Phosphorylation of CDC25B by Aurora-A at the centrosome contributes to the G2-M transition. J Cell Sci. 2004;117:2523–31. doi: 10.1242/jcs.01108. [DOI] [PubMed] [Google Scholar]
- Ewart-Toland A, Briassouli P, de Koning JP, Mao JH, Yuan J, Chan F, et al. Identification of Stk6/STK15 as a candidate low-penetrance tumor-susceptibility gene in mouse and human. Nat Genet. 2003;34:403–12. doi: 10.1038/ng1220. [DOI] [PubMed] [Google Scholar]
- Ewart-Toland A, Dai Q, Gao YT, Nagase H, Dunlop MG, Farrington SM, et al. Aurora-A/STK15 T+91A is a general low penetrance cancer susceptibility gene: a meta-analysis of multiple cancer types. Carcinogenesis. 2005;26:1368–73. doi: 10.1093/carcin/bgi085. [DOI] [PubMed] [Google Scholar]
- Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460:278–82. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M. The 3Ms of central spindle assembly: microtubules, motors and MAPs. Nat Rev Mol Cell Biol. 2009;10:9–20. doi: 10.1038/nrm2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover DM, Leibowitz MH, McLean DA, Parry H. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 1995;81:95–105. doi: 10.1016/0092-8674(95)90374-7. [DOI] [PubMed] [Google Scholar]
- Green MR, Woolery JE, Mahadevan D. Update on Aurora Kinase Targeted Therapeutics in Oncology. Expert Opin Drug Discov. 2011;6:291–307. doi: 10.1517/17460441.2011.555395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman MJ, Sisi P, Banbury DN, Byrne C. Patterned acquisition of skin barrier function during development. Development. 1998;125:1541–52. doi: 10.1242/dev.125.8.1541. [DOI] [PubMed] [Google Scholar]
- Honda K, Mihara H, Kato Y, Yamaguchi A, Tanaka H, Yasuda H, et al. Degradation of human Aurora2 protein kinase by the anaphase-promoting complex-ubiquitin-proteasome pathway. Oncogene. 2000;19:2812–9. doi: 10.1038/sj.onc.1203609. [DOI] [PubMed] [Google Scholar]
- Karthigeyan D, Prasad SB, Shandilya J, et al. Biology of Aurora A kinase: implications in cancer manifestation and therapy. Med Res Rev. 2011;31:757–793. doi: 10.1002/med.20203. [DOI] [PubMed] [Google Scholar]
- Kashatus DF, Lim KH, Brady DC, Pershing NL, Cox AD, Counter CM. RALA and RALBP1 regulate mitochondrial fission at mitosis. Nat Cell Biol. 2011;13:1108–15. doi: 10.1038/ncb2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenbach AN, Schweppe DK, Faherty BK, Pechenick D, Pletnev AA, Gerber SA. Quantitative phosphoproteomics identifies substrates and functional modules of Aurora and Polo-like kinase activities in mitotic cells. Sci Signal. 2011;4:rs5. doi: 10.1126/scisignal.2001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A, Krug K, Pengelley S, Macek B, Hauf S. Mitotic substrates of the kinase aurora with roles in chromatin regulation identified through quantitative phosphoproteomics of fission yeast. Sci Signal. 2011;4:rs6. doi: 10.1126/scisignal.2001588. [DOI] [PubMed] [Google Scholar]
- Koster MI, Roop DR. Mechanisms regulating epithelial stratification. Annu Rev Cell Dev Biol. 2007;23:93–113. doi: 10.1146/annurev.cellbio.23.090506.123357. [DOI] [PubMed] [Google Scholar]
- Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–80. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, Fuchs E. Desmoplakin: an unexpected regulator of microtubule organization in the epidermis. J Cell Biol. 2007;176:147–54. doi: 10.1083/jcb.200609109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebig T, Erasmus J, Kalaji R, Davies D, Loirand G, Ridley A, et al. RhoE Is required for keratinocyte differentiation and stratification. Mol Biol Cell. 2009;20:452–63. doi: 10.1091/mbc.E07-11-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlepage LE, Ruderman JV. Identification of a new APC/C recognition domain, the A box, which is required for the Cdh1-dependent destruction of the kinase Aurora-A during mitotic exit. Genes Dev. 2002;16:2274–85. doi: 10.1101/gad.1007302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LY, Wood JL, Ye L, Minter-Dykhouse K, Saunders TL, Yu X, et al. Aurora A is essential for early embryonic development and tumor suppression. J Biol Chem. 2008;283:31785–90. doi: 10.1074/jbc.M805880200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee AI, Lytton NA, Watt FM. Calcium-induced changes in cytoskeleton and motility of cultured human keratinocytes. Experimental cell research. 1987;172:43–53. doi: 10.1016/0014-4827(87)90091-7. [DOI] [PubMed] [Google Scholar]
- Manfredi MG, Ecsedy JA, Meetze KA, Balani SK, Burenkova O, Chen W, et al. Antitumor activity of MLN8054, an orally active small-molecule inhibitor of Aurora A kinase. Proc Natl Acad Sci U S A. 2007;104:4106–11. doi: 10.1073/pnas.0608798104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalit A, Vlcek S, Gruenbaum Y, Foisner R. Breaking and making of the nuclear envelope. Journal of cellular biochemistry. 2005;95:454–65. doi: 10.1002/jcb.20433. [DOI] [PubMed] [Google Scholar]
- Marumoto T, Honda S, Hara T, Nitta M, Hirota T, Kohmura E, et al. Aurora-A kinase maintains the fidelity of early and late mitotic events in HeLa cells. J Biol Chem. 2003;278:51786–95. doi: 10.1074/jbc.M306275200. [DOI] [PubMed] [Google Scholar]
- Mori D, Yamada M, Mimori-Kiyosue Y, Shirai Y, Suzuki A, Ohno S, et al. An essential role of the aPKC-Aurora A-NDEL1 pathway in neurite elongation by modulation of microtubule dynamics. Nat Cell Biol. 2009;11:1057–68. doi: 10.1038/ncb1919. [DOI] [PubMed] [Google Scholar]
- Poulson ND, Lechler T. Robust control of mitotic spindle orientation in the developing epidermis. J Cell Biol. 2010;191:915–22. doi: 10.1083/jcb.201008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poumay Y, Roland IH, Leclercq-Smekens M, Leloup R. Basal detachment of the epidermis using dispase: tissue spatial organization and fate of integrin alpha 6 beta 4 and hemidesmosomes. J Invest Dermatol. 1994;102:111–7. doi: 10.1111/1523-1747.ep12371742. [DOI] [PubMed] [Google Scholar]
- Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–63. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Maiato H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell. 2004;7:637–51. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Roop DR, Cheng CK, Titterington L, Meyers CA, Stanley JR, Steinert PM, et al. Synthetic peptides corresponding to keratin subunits elicit highly specific antibodies. J Biol Chem. 1984;259:8037–40. [PubMed] [Google Scholar]
- Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- Sasai K, Parant JM, Brandt ME, Carter J, Adams HP, Stass SA, et al. Targeted disruption of Aurora A causes abnormal mitotic spindle assembly, chromosome misalignment and embryonic lethality. Oncogene. 2008;27:4122–7. doi: 10.1038/onc.2008.47. [DOI] [PubMed] [Google Scholar]
- Simpson CL, Patel DM, Green KJ. Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nat Rev Mol Cell Biol. 2011;12:565–80. doi: 10.1038/nrm3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart IH. Variation in the plane of cell cleavage during the process of stratification in the mouse epidermis. Br J Dermatol. 1970;82:276–82. doi: 10.1111/j.1365-2133.1970.tb12437.x. [DOI] [PubMed] [Google Scholar]
- Tapia C, Kutzner H, Mentzel T, Savic S, Baumhoer D, Glatz K. Two mitosis-specific antibodies, MPM-2 and phospho-histone H3 (Ser28), allow rapid and precise determination of mitotic activity. The American journal of surgical pathology. 2006;30:83–9. doi: 10.1097/01.pas.0000183572.94140.43. [DOI] [PubMed] [Google Scholar]
- Torchia EC, Boyd K, Rehg JE, Qu C, Baker SJ. EWS/FLI-1 induces rapid onset of myeloid/erythroid leukemia in mice. Mol Cell Biol. 2007;27:7918–34. doi: 10.1128/MCB.00099-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchia EC, Caulin C, Acin S, Terzian T, Kubick BJ, Box NF, et al. Myc, Aurora Kinase A, and mutant p53(R172H) co-operate in a mouse model of metastatic skin carcinoma. Oncogene. 2012;31:2680–90. doi: 10.1038/onc.2011.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchia EC, Chen Y, Sheng H, Katayama H, Fitzpatrick J, Brinkley WR, et al. A genetic variant of Aurora kinase A promotes genomic instability leading to highly malignant skin tumors. Cancer Res. 2009;69:7207–15. doi: 10.1158/0008-5472.CAN-09-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vader G, Lens SM. The Aurora kinase family in cell division and cancer. Biochim Biophys Acta. 2008;1786:60–72. doi: 10.1016/j.bbcan.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci U S A. 1999;96:8551–6. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuspa SH, Kilkenny AE, Steinert PM, Roop DR. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 1989;109:1207–17. doi: 10.1083/jcb.109.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, et al. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–93. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.