Abstract
Bioengineered spider silk-like proteins can serve as biomaterials for various biomedical applications. These proteins can be assembled in several morphological forms such as films, microcapsules, spheres, fibers, gels and scaffolds. However, crucial points for recombinant spider silks for human use are toxicity and immunogenicity. To assess this issue two bioengineered spider silk proteins composed of different numbers of repetitive motifs of the consensus repeats from spidroin-1 from Nephila clavipes (15X and 6X) were cloned and expressed in E. coli. The proteins were free of tag-sequence and were purified using two methods based on (i) thermal and (ii) organic acid resistance of the spider silks. The soluble spider silk proteins were not cytotoxic and did not activate macrophages over a wide range of concentrations, except when present at the highest concentration. Films made of the different silk variants supported the growth of the cells. Based on these data, and since the biodegradation rate of silk is very slow, the bioengineered spider silks are presumed safe biomaterials for biomedical applications.
Keywords: spider silk, bioengineering, tag-free purification, cytotoxicity, immunogenicity
Introduction
The unique properties of silks such as mechanical strength, toughness, biocompatibility and biodegradability make them excellent materials for biomedical applications 1. In nature silks are generated in fiber form, while after processing in vitro they can be assembled into several morphological forms such as films, hydrogels, fibers, scaffolds, microcapsules, micro- and nanospheres 2,3. Tissue engineering and regeneration, controlled drug release, medical imaging and biochemical sensors are examples of potential applications for silks in medicine. The textile industry has provided silkworm silk as an accessible biomaterial. In contrast, spider silks are limited in supply mainly due to difficulties in breeding (short lifespan, territoriality and cannibalism of spiders) and complexity in collecting pure silk (since multiple silk fibers are produced from spinnerets). The identification of partial cDNA sequences encoding spider silk proteins triggered efforts to generate recombinant silk production 4,5. Initially the expression of fragments of native cDNA did not result in satisfactory yield. However, recent development with synthetic spider silk gene designs opened up new horizons to explore spider silks for medical needs 4,6.
The sequences of bioengineered spider silks are based on the consensus motifs of the corresponding natural equivalents. The consensus motif is reversely transcribed into oligonucleotides compatible to the host organism codon usage. Codon optimization renders higher protein expression in the host. Next, the double-stranded oligonucleotides are repeatedly ligated to generate larger sized genetic constructs. Using synthetic oligonucleotides, various DNA modules can be generated and their combinatorial ligation allows for the design of genes encoding bioengineered spider silk proteins with different properties. Moreover, the bioengineered silk proteins may be further modified in order to gain new functions. This strategy of hybrid protein construction at the DNA level combines the sequence of bioengineered spider silk, which is responsible for biomaterial structure, with sequences of polypeptides for functionalization. The cell-adhesive sequence of the fibronectin (RGD - Arg-Gly-Asp), silica formation sequence of silaffin (R5 peptide), hydroxyapatite nucleation and crystallization sequence (fragment of dentin matrix protein 1), cell penetrating peptide and DNA biding poly(L-lysine), and antimicrobial peptides have all been combined via genetic engineering with spider silk sequences as examples of such hybrid or fusion proteins 7–11. The technology of hybrid protein construction, to provide the desired function to biomaterials, expands the potential applications for spider silks as designable protein-based biomaterials.
The technology for synthetic spider silk production enabled the required amounts of spider silk proteins to be generated, with affinity chromatography often used for purification 7,10,12. However, the tag-sequence needed for this type of purification might affect the secondary structure of the silk, or the solubility or antigenicity. The heat resistance of silk proteins was successfully used to purify spider silk variants 13–15. The majority of the proteins from the expression host were denatured at high temperature, while silk proteins were not. Other methods based on organic acid spider silk solubility were exploited 16,17. The expressing cells were lysed in organic acid resulting in the extraction of silk and hydrolysis of contaminating host proteins. Moreover, since currently the most efficient and cost effective expression host is E. coli, a Gram negative bacterium, possible contamination of endotoxin is an issue to consider. Despite these expression and purification challenges, bioengineered spider silk biomaterials were generated and shown to be biocompatible and supported the growth of various cell types 4.
The objective of the present work was to purify spider silks generated by recombinant DNA methods, and to assess cytotoxicity and macrophage activation. To achieve this goal, the bioengineered spider silks, referred as 15X and 6X (39.5 kDa and 16 kDa, respectively), were prepared. These proteins were composed of different numbers of repetitive motifs (15 and 6, respectively) derived from the natural sequence of the major ampullate spidroin 1 (MaSp1) of Nephila clavipes and were used in number of prior studies 7–11. The sequence of the monomeric unit was SGRGGLGGQGAGAAAAAGGAGQGGYGGLGSQGT. In previous studies, for purification purposes, the His-Tag/Thrombin/S-Tag/enterokinase sequence was linked with the spider silk polymers. Since adjacent sequences might influence silk properties, in the present study the vector expressing 15X and 6X was constructed without additional purification sequences. Protein expression was carried out using a fed-batch bioreactor and the proteins were purified via two alternative protocols, thermal and organic acid resistance. The silk proteins obtained from the two processes were studied in terms of cytotoxicity and macrophage activation.
Materials and Methods
Construction of expression plasmids pETNX-15X and pETNX-6X
The expression vector pET30(a)+ (Novagen, Madison, WI) was modified with a linker NX. The linker NX with cohesive ends complementary to those generated by NdeI and XhoI was created by annealing two synthetic oligonucleotides and then insertion into complementary sites of pET30(a)+ plasmid. Constructed plasmid, named pETNX, possessed new restriction sites NheI and SpeI for cloning silk genes and a stop codon up to C-terminal His-Tag sequence. The two complementary oligonucleotide sequences for the linker were: NXF 5′ TATGGCTAGCGGTGACCTGAATAACACTAGTTAAAC and NXR 5′ CTCGAGTTTAACTAGTGTTATTCAGGTCACCGCTAGCCA. The restriction sites NheI and SpeI are underlined and the stop codon is italicized. Next, the constructs pETNX-15X and pETNX-6X were obtained by subcloning the NheI-SpeI fragment of pET30-15X and pET30-6X, respectively, into the pETNX vector. Plasmids pET30-15X and pET30-6X carried the genes 15X and 6X, respectively, and the construction of spider silk multimer genes was described previously 7. The sequences of obtained plasmids were confirmed by sequencing at the University of Adam Mickiewicz Core Facility in Poznan. Enzymes for digestions and ligation were supplied from Promega (Madison, WI).
Protein expression
The expression plasmids pETNX-15X and pETNX-6X were transformed into E. coli BLR (DE3) (Novagen, Madison, WI). Bacteria were routinely cultivated in LB broth at 37°C. For large scale expression a fermentor, Bioflo 3000 (New Brunswick Scientific, Edison, NJ), was used. Cells were grown in minimal medium 18 supplemented with 1% yeast extract and 50 μg/ml kanamycin. The pH was maintained at 6.8 by addition of ammonia water except for periods when the pH increased above 6.88 due to glucose depletion, whereby the feed solution (50% glucose, 10% yeast extract, 2% MgSO4, 50 μg/ml kanamycin) was added. The level of dissolved oxygen was sustained at 40% by automatically increasing the agitation speed to 850 rpm and by supplying compressed air. Cells were grown at 37°C to OD 600 of 9–11 and then induced with 1 mM IPTG (A&A Biotechnology, Gdynia, Poland). After 4 hours of incubation, cells were harvested by centrifugation at 3500g. Reagents were supplied from Sigma (St. Louis, MO) otherwise specified.
Protein purification
For protein purification two methods were used. The first method, named 80/20, has been described previously 15. Cells were added with 20 mM HEPES pH 7.5, 100 mM NaCl and lysed with lysozyme (Thermo Scientific, Rockford, IL), sonicated, DNaseI treated and then centrifuged at 50000g in 4°C for 30 min. Soluble bacterial proteins were precipitated by heat denaturation (80°C for 20 min) and removed by sedimentation at 50000g for 30 min. Next, the soluble silk proteins were precipitated with 20% ammonium sulfate, centrifuged at 10000g for 15 min, rinsed with 20% ammonium sulfate and then dissolved in 6M guanidinium thiocyanate. The proteins obtained were dialyzed against 10 mM Tris pH 7.5 and precipitates formed during dialysis were removed by sedimentation at 125000g for 30 min. At the second purification method, named PA, the lysis of bacteria and purification of silk proteins were in propionic acid 17. One gram of bacterial wet pellet was combined with 1 ml of 13.3N propionic acid, diluted to 2.3N acid with ultrapure water and agitated 1 hour at room temperature. The precipitated bacterial proteins were clarified by centrifugation at 50000g for 30 min, and then the clarified supernatant was dialyzed extensively into 10 mM Tris pH 7.5. Aggregates formed during dialysis were removed by sedimentation at 125000g for 30 min. Next, the soluble silk proteins were applied to a strong anion exchange resin, Q Sephacel XL (Amersham Bioscences, Uppsala, Sweden) that had been equilibrated with 10mM Tris pH 7.5. After 1 hour agitation the resin was transferred to the column and silk proteins were rinsed. The column was washed with 10mM Tris pH 7.5 to recover any remaining silk protein.
Regardless of the purification method, the silk proteins were subsequently concentrated by ultrafiltration (Millipore Centrifugal Filter Units, Millipore, Jaffrey, NH) and any remaining endotoxins were removed using Detoxi-Gel Endotoxin Removing Gel Kit (Thermo Scientific, Pierce, Rockford, IL) according to manufacturer instructions. The silk solution was sterilized by filtration using an Ultrafree-MC sterile 0.22 μm filter unit (Millipore, Jaffrey, NH). The content of the endotoxin in the protein solution was measured using Limulus Amebocyte Lysate (LAL) Kinetic-QCL quantitative, kinetic assay (Lonza, Walkersville, MD) as recommended by the supplier, at the Institute of Biotechnology and Antibiotics in Warsaw. Protein concentration was determined from absorbance at 280nm. The quality of proteins was analyzed by separation on 15% SDS-PAGE gel and staining with Roti-Blue (Carl Roth, Karlsruhe, Germany). Fluorescence spectra were recorded on F-4500 FL Spectrophotometer (Hitachi, Japan) at Poznan University of Technology for proteins at concentration of 100μg/ml in 10mM Tris pH 7.5 and at the excitation of 280 and 295nm. Reagents were supplied from Sigma (St. Louis, MO) otherwise specified.
In vitro cytotoxicity/cell viability studies
LDH leakage/mitochondrial activity
For in vitro cytotoxicity/cell viability studies the murine fibroblasts NIH 3T3 cell line was used. Cells were maintained in DMEM (Sigma, St. Louis, MO) medium supplemented with 10% FCS (Gibco Invitrogen, Grand Island, NJ) and 80 mg/l gentamycin (KRKA, Novo Mesto, Slowenia). Cells were grown at 37°C in an atmosphere of 95% air - 5% CO2. For assays, 5 × 104 cells/well (1.43 × 105/cm2) were plated in 96 well plates and incubated overnight. The next day, different concentrations of silk proteins were added to the microtiter wells. The medium was used as a negative control, while 1%Triton-100X (Sigma, St. Louis, MO) was a positive control. The cells were incubated for 24 and 48 hours. At desired time points, plates were centrifuged at 600g for 10 min and 10 μl of media was transferred to another plate on ice. This plate was used immediately for the LDH assay according to manufacturer instructions (LDH Cytotoxicity Assay Kit II, BioVision Mountain View, CA). The cytotoxicity (%) was calculated by (test sample − negative control)/(positive control − negative control) × 100. For the MTT assay, to each well 50 μl (5 mg/ml) MTT reagent (Sigma, St. Louis, MO) was added. After 4 hours of incubation, the medium was discarded and the insoluble purple formazan was dissolved with 200 μl DMSO. The absorbance of the colored solution was measured at 570nm with a microplate reader ELX808IV (Bio-Tek Instruments, Winooski, VT). The mitochondrial function and by extension the relative cell viability (%) related to the negative control were calculated by test sample/negative control × 100. Each experiment was run three times (each in triplicate).
Apoptosis assay
Apoptosis was assessed by measurement of caspase 3 activity using Colorimetric Caspase 3 Assay Kit (Sigma, St. Louis, MO). NIH 3T3 cells were seeded into 6-well plates at a density of 1 × 106 cells/well (1.05 × 105/cm2) and cultured for 24 h. Next, cells were treated with 100 μg/ml and 1000 μg/ml silk sample. The medium without stimulator was used as negative control. After 24 h cells were collected, washed in PBS and resuspended in chilled Lysis Buffer. After incubation on ice for 20 min, lysed cells were centrifuged at 14000g for 15 min. The protein concentration at supernatant was calculated using the standard BCA Protein Assay Kit (Thermo Scientific, Pierce, Rockford, IL) as recommended by the supplier. Caspase Assay mixture was prepared in a 96-well plate by mixing the test sample, assay buffer and caspase substrate (Ac-DEVD-pNA). After overnight incubation at 37°C, the absorbance of released p-nitroaniline was measured at 405nm. The Total Protein Normalized Caspase Activity was calculated by (Test Sample/Total Sample Lysate Protein) / (Control/Total Control Lysate Protein) × 100 where Test Sample and Control were the absorbance of the test and control sample, respectively, and Total Sample/Control Lysate Protein was the total amount of protein (mg) of test/control sample, respectively. The Caspase Assay was performed in duplicates in two independent experiments.
Macrophage stimulation and determination of TNF-α release
The murine macrophage cell line J774 was grown in DMEM medium containing 10% FCS and 80 mg/l gentamycin at 37°C in an atmosphere of 95% air - 5% CO2. J774 cells were plated at 1 × 105 cells per well (2.86 × 105/cm2) in 96-well plate and incubated overnight. The next day, different concentrations of silk proteins were added to microtiter wells. The medium without stimulator was used as negative control, while lipopolysaccharide (LPS – Escherichia coli- 0111:B4, Sigma, St. Louis, MO) was a positive control. After 5 hours of incubation cells were centrifuged at 600g and medium was collected to assay for tumor necrosis factor alpha (TNF-α) release. The level of TNF-α was measured by ELISA as recommended the producer (Ready-Set-Go! Mouse TNF-α, eBioscence, San Diego, CA). The experiment was replicated three times in duplicates.
Film preparation and cell culture
In order to prepare a films, 50 μl of silk solution (1 mg/ml) was added to the single well of the 96-well cell culture polystyrene (PS) plate destined for cell suspension cultures (Sarstedt, Newton, NC). The hydrophobic surface of the plates prevents cells attachment to the plastic. Silk solution was allowed to dry overnight at room temperature in a biohazard hood. Films were washed twice with sterile PBS, pre-incubated with DMEM supplemented with 10% FCS for 1h at 37°C and then NIH 3T3 cells were plated at a density of 1 × 103 cells/well (2.86 × 103/cm2). In order to examine cell phenotype, cells were observed at different time points by using phase contrast microscopy (Leica DMI3000B, Wetzlar, Germany) at 10 × magnification. The hydrophobic wells without silk films were used as negative controls (PS), while tissue culture treated plates (TCT) (Sarstedt, Newton, NC) were used as positive controls.
Statistics
To calculate the significance of the observed effects, two-tail one sample t-tests were used. To test for significant differences between different experimental groups one-way ANOVA was used. In the case of significance ANOVA (p<0.05) post hoc tests with Bonferroni correction were performed. Differences were considered significant when p < 0.05.
Results
Expression and purification of recombinant spider silk proteins
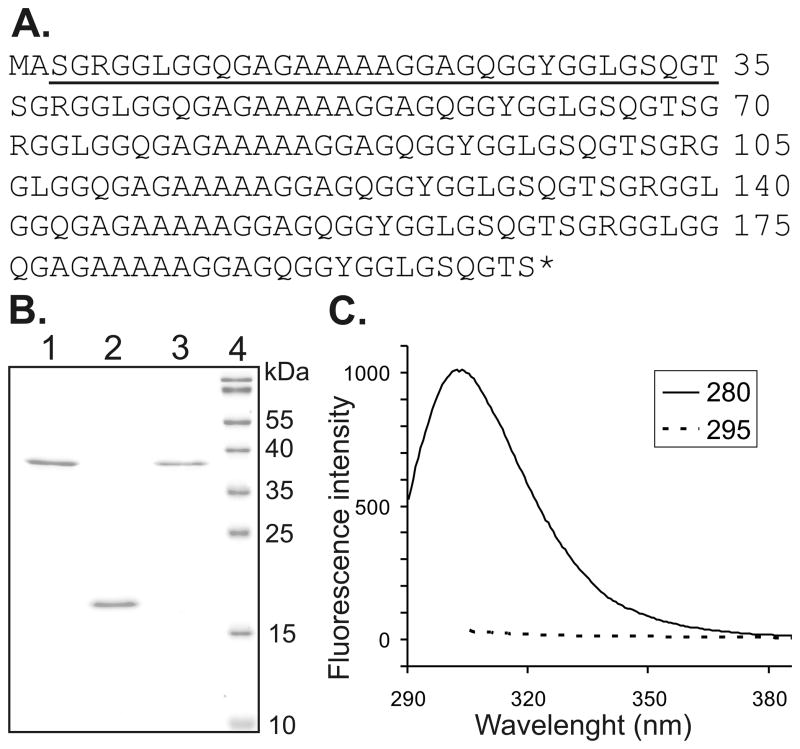
The expression vector pET30(a)+ was modified in order to obtain spider silk proteins without any artificial sequences or purification tags. Since the recombinant proteins did not carry a Tag sequence, it was not possible to determine yield in the crude extract. The proteins were purified using two different methods. Using 80/20 method we were able to purify one of the protein variants: 15X (15X80/20). However, the 6X required a higher percentage of ammonium sulfate for precipitation. Unfortunately, under these conditions the 6X co-precipitated with other, unknown bacterial proteins (data not shown). Using PA method both variants of spider silk proteins (15XPA and 6XPA) were purified. In SDS-PAGE analysis, the proteins showed no inpurities, what was confirmed by measuring fluorescence emission (Fig. 1). Excitation with 280nm leads to fluorescence emission of tyrosines and tryptophans, while excitation with 295nm excites tryptophans only. In the analyzed silk proteins tyrosine occurs abundantly, while tryptophan does not occur, thus the fluorescence emission upon excitation with 295nm would indicate contamination with E. coli proteins. Analysis of emission spectra for three silk variants revealed fluorescence only upon excitation with 280nm (the example of 6XPA shown in Fig. 1C), which indicated the purity of the silk proteins. The apparent molecular weight of the 15X and 6X were in good agreement with expected values (39.5 kDa and 16 kDa, respectively). Yields of the two proteins differed depending on the purification protocol (Table I). The bacterial wet pellet obtained from the same fermentation gave four times more 15X protein when the PA method was applied. However, the PA method resulted in higher endotoxin levels and additional steps of purification were necessary. The application of anion exchange chromatography lowered the LPS concentration about 2–3 times. After concentration and final polishing, both 15X protein samples indicated endotoxin level below 1 EU/ml, while for the 6X 1.35 EU/ml what corresponded to 2.5EU per milligram of protein.
Figure 1.
Amino acid sequence of bioengineered spider silk protein (6X) and analysis of purified silk proteins. (A) The monomeric unit of bioengineered spider silk protein is underlined and represents the consensus sequence derived from the MaSp1 of Nephila clavipes. The sequence of 15X is the same except the monomer unit is repeated 15 times. (B) Purified proteins were separated on 15% SDS-PAGE gel and stained with Roti-Blue. 1) 15XPA, spider silk 15-mer purified using the PA method, 2) 6XPA, spider silk 6-mer purified by the PA method, 3. 15X80/20 spider silk 15-mer purified using the 80/20 method, 4) Molecular weight markers (PageRuler, Fermentas). (C) Fluorescence emission spectra of purified 6XPA are shown with an excitation wavelength of 280 or 295nm.
Table 1.
Comparison of protein purification methods: yield and endotoxin level. Protein yield was calculated spectrophotometrically at A280 and indicated as the purified protein amount per liter of culture medium. Endotoxin level was measured using LAL Kinetic-QCL assay. 1 EU=Endotoxin Unit corresponds 100 pg of endotoxin/lipopolysaccharide (LPS). ND- not determined.
| 15X80/20 | 15XPA | 6XPA | |
|---|---|---|---|
| Protein yield (mg/L) | 45.8 | 200 | 96.8 |
| Endotoxin after dialysis | <1 EU/ml | 3.48 EU/ml | 2.44 EU/ml |
| Endotoxin after IEX | ND | <1 EU/ml | 1.30 EU/ml 4 EU/mg |
| Endotoxin after polishing | <1 EU/ml | <1 EU/ml | 1.35 EU/ml 2.5 EU/mg |
In vitro cell cytotoxicity / viability studies
Cytotoxicity of the two variants of spider silk–like proteins which differed in molecular weight but were purified by the same method (proteins 15XPA and 6XPA) and spider silk variants of the same molecular weight but purified by two different methods (15XPA versus 15X80/20) were compared.
LDH leakage
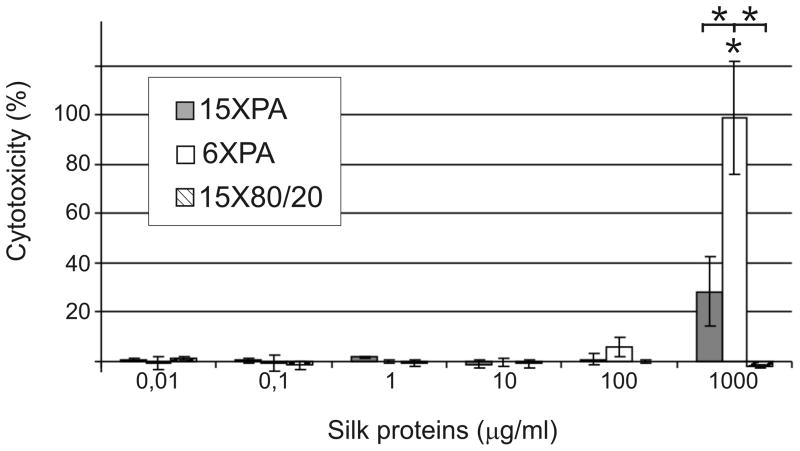
Mouse fibroblasts (NIH 3T3) were cultured in the presence of the spider silk proteins for 24h. Cytotoxicity was calculated from absorbance of test and control samples (Fig. 2). The 15X80/20 protein did not affect the integrity of the plasma membrane at any of the concentrations tested. Proteins purified by PA method did not cause LDH leakage, only the highest dose of PA purified proteins (1000 μg/ml) demonstrated some cytotoxicity. The significantly increased cytotoxicity of 6X compared to 15X suggests that the cytotoxicity might be associated with molecular weight. Cytotoxicity of 6XPA differed significantly when compared with the highest dose of 15XPA and 15X80/20.
Figure 2.
Cytotoxicity of spider silk proteins evaluated by LDH released into culture medium. NIH 3T3 cells were maintained in medium supplemented with different concentrations of spider silk proteins for 24h. The amount of LDH released was measured by spectrophotometric analysis and cytotoxicity was calculated as described in the Materials and Methods. Results are expressed as the means of three independent experiments and error bars show the standard deviation.
Mitochondrial activity
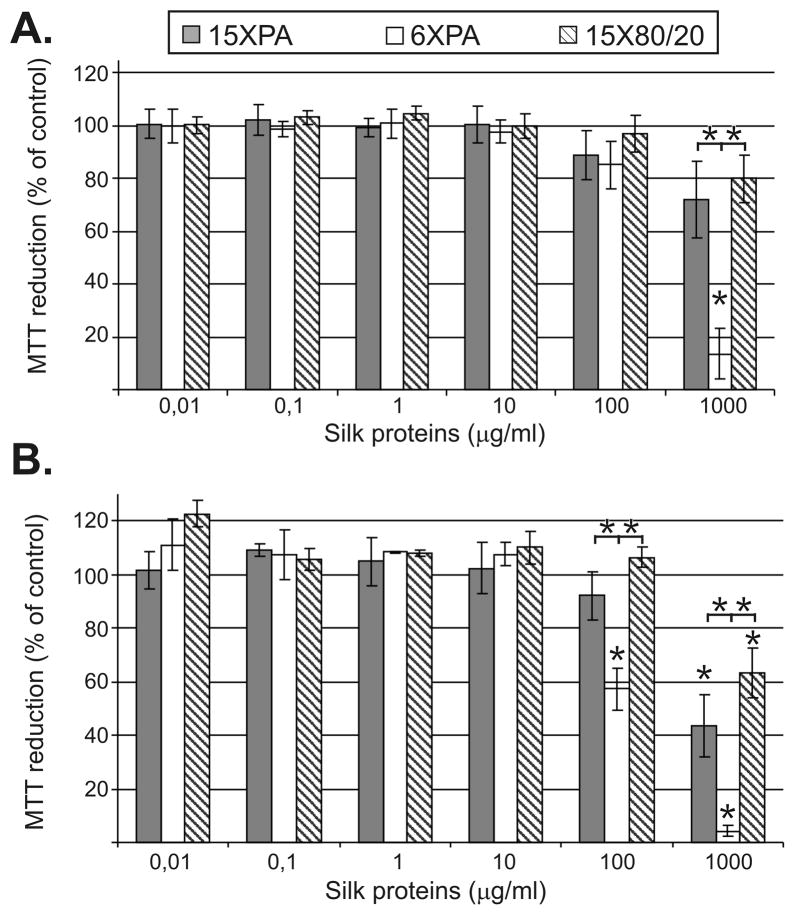
The mitochondrial function and by extension cell viability was measured using MTT. NIH 3T3 cells were maintained in medium supplemented with different concentrations of the spider silk proteins for 24 and 48h. The percentage of MTT reduction was calculated from absorbance readings of test and control samples. The bioengineered spider silk proteins did not reduce cell viability over the range of concentrations tested, but were found to do so at the highest concentration. After 24h of incubation, only the highest concentration (1000 μg/ml) of all tested proteins caused various degrees of reduction in mitochondrial function. The 15XPA, 6XPA and 15X80/20 reduced mitochondrial activity up to 28%, 86% and 20%, respectively (Fig. 3A), however only the 6XPA effect was significant. Longer incubation of the cells in the presence of the highest dose of spider silk proteins decreased cell viability. After 48h, 1000 μg/ml of 15XPA, 6XPA and 15X80/20 caused significant reduction in mitochondrial activity up to 56%, 96% and 36%, respectively (Fig. 3B). At 100 μg/ml, only 6XPA significantly reduced cell viability to 42%. Moreover, the effect of the 6XPA sample differed significantly when compared with reduction of mitochondrial activity triggered by 15XPA and 15X80/20.
Figure 3.
Mitochondrial activity assessed using MTT. NIH 3T3 cells were cultured in the presence of the spider silk proteins for 24h (A) and 48h (B). The % of the MTT reduction was calculated as described in the Materials and Methods. Results are expressed as means of three independent experiments and error bars show the standard deviation.
Apoptosis assay
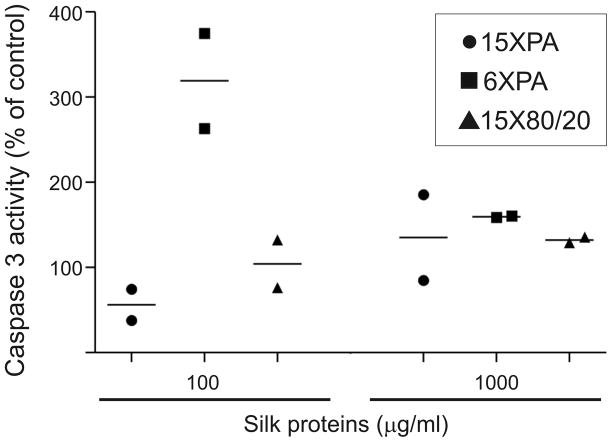
Since for the 15X80/20 protein the plasma membrane remained intact while the mitochondrial activity was affected, the possibility of apoptosis was assessed. The caspase 3 activity of NIH 3T3 cells was measured after 24h incubation in the presence of 100 μg/ml and 1000 μg/ml spider silk samples. The moderate enhancement of the relative caspase 3 activity (about 130% of the control) was observed for 15X spider silk proteins when tested at 1000 μg/ml (Fig. 4). The highest dose of 6XPA moderately increased activity of caspase. However, at the concentration of 100 μg/ml 6XPA caused more than a 3-fold increase in caspase 3 activity, while the 15X spider silk proteins had no effect.
Figure 4.
Effect of spider silk proteins on caspase 3 activity in NIH 3T3 cells. Cells were cultured in medium supplemented with different concentrations of spider silk proteins for 24h. After cell lysis, the same amount of cell lysates were used for caspase 3 assay. The normalized caspase 3 activity was calculated as described in the Materials and Methods. Graph shows results and means of two independent experiments.
Macrophage activation and determination of TNF-α release
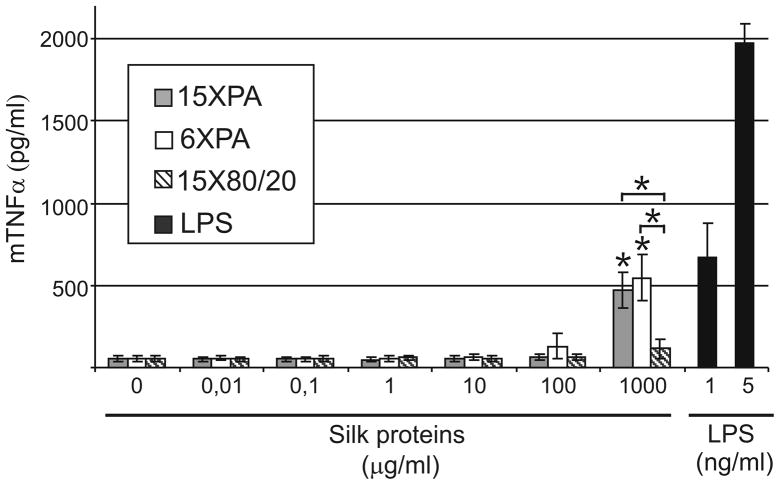
Monocytes and macrophages are phagocytes, regulating the immune responses and the development of inflammation. They are the primary source of pro-inflammatory mediators like interleukin 1 (IL-1) and tumor necrosisfactor alpha (TNF-α). The abilityof spider silk proteins to stimulate the production of TNF-α was investigated in a murine macrophage-like cell line (J774). The 15X purified by 80/20 method did not induce the secretion of TNF-α (Fig. 5). Macrophages stimulated with 15XPA and 6XPA released about 10 times more TNF-α than cells without stimulation. However, this effect was only observed at the highest concentration of the tested PA spider silk protein.
Figure 5.
TNF-α release induced by spider silk proteins from the macrophage cell line J774 determined by ELISA. The macrophages were incubated in media containing different concentrations of spider silk proteins for 5h. LPS was used as a positive control. The results are expressed as means of three independent experiments and error bars show the standard deviations of the means.
Growth of NIH 3T3 cells on spider silk films
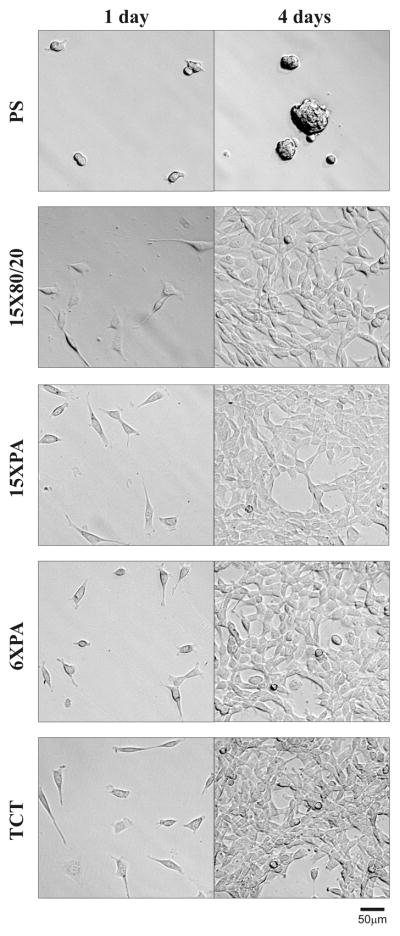
In order to assess the potential toxicity of solid biomaterial made of the different variants of the recombinant spider silk, the silk proteins were cast into film, NIH 3T3 cells were seeded on the silk films and control wells, and then the growth and morphology of the cells were observed under phase contrast microscopy. After 24h of incubation cells plated on the spider films displayed the same morphology as cells seeded on a positive control plate (TCT). The negative control plate (hydrophobic polystyrene plate - PS) did not support cell attachment and growth. Silk films were prepared on PS in order to distinguish false positive results. After 4 days of incubation, all silk films and TCT supported the growth of the fibroblasts, in contrast to the PS plate.
Discussion
The bioengineered spider silk proteins used in this study were based on the consensus repeat derived from MaSp1 of Nephila clavipes 7. The new constructed expression vector pETNX did not carry an N-terminal His-Tag/Thrombin/S-Tag/enterokinase configuration, to avoid impacting spider silk properties or to avoid additional steps for tag removal. The spider silk proteins obtained were purified according protocols that take advantage of the properties of natural silk. Using a thermal method one protein variant (15X) was successfully purified, while using propionic acid extraction, proteins 15X and 6X were obtained. The organic acid based method was therefore more universal and can be used for obtaining pure spider silk-like proteins independent on their molecular weight. Moreover, this acid method was more efficient. We were able to obtain about four times more 15XPA protein than 15X80/20. At Mello et al. study, spider silk-like proteins have been purified using formic acid and formic/propionic acid containing Gdn-HCl, which caused lysis and decreased purity of spider silk proteins 16. However, following nickel affinity or ion exchange steps contaminating bacterial proteins were effectively removed. Protein yields at their study were also increased relative to their previously reported data about 4–5-fold 16. In general, the application of organic acid increased the quantity of purified spider silk proteins. The yield of the majority of bioengineered spider silk proteins purified by affinity chromatography ranged between 5–40 mg/L 4. Utilizing propionic acid purification we obtained 100–200 mg/L of bioengineered spider silk. However, it should be noted that high density fermentation (OD600 of 40–50) and purification with temperature based method also resulted at high spider silk protein recovery (up to 140–360 mg/L) 15.
Although the organic acid extraction provided higher protein recovery, this method resulted in higher endotoxin contamination comparing to the 80/20 method. Q Sepharose XL as a strong anion exchanger bound negatively charged endotoxins at pH above 2. Application of Q Sepharose XL reduced LPS contamination below the detection limit in 15XPA preparations, however the 6XPA variant still indicated the presence of LPS. Moreover, after additional polishing, 6XPA still was contaminated with LPS. Recently new cell washing procedures which reduce LPS content were described 12. Since spider silk protein recovery was higher using the organic acid extraction method, exploration of inexpensive and simple techniques to reduce LPS contamination would be reasonable next steps.
The bioengineered spider silk proteins obtained demonstrated self-assembling properties shaping into various structural forms like films and microspheres (data not shown). Beyond these properties the clinical applications of biomaterials require compatibility with living organisms. A few studies have demonstrated that spider silk materials of natural or bioengineered proteins were biocompatible. Natural dragline silk implanted subcutaneously in pigs was immunotolerated comparably to polyurethane, collagen or gauze 19. Later, these results were supported by a study by Lewis et al. 20. Enzymatic treatment with trypsin or trypsin/Proteinase K enhanced biocompatibility of subcutaneously implanted spider egg sac silks at a level comparable to polyglactin 21. Schwann cell seeded dragline silk was well tolerated and promoted peripheral nerve regeneration when tested in sciatic nerve replacements 22. These in vitro studies indicated that natural spider silks supported the growth of various cells: Schwann, NIH 3T3 fibroblasts, chondrocytes 23–25. Hakimi et al. used different methods to measure cell metabolism, viability and DNA content to quantify the effect of silk samples on the respiration and proliferation of endothelial cells 26. Spider egg sac silk as well as spider silk conditioned medium caused inhibition of the proliferation of endothelial cells, however the inhibitory effect was less than that caused by silkworm silk 26.
Since natural spider silk is not easily accessible, recombinant bioengineered spider silk variants are being explored. However, recombinant proteins can pose new challenges with respect to biocompatibility. Since bacterial expression is frequently used for silk-like protein production, contamination by the pyrogenic (endotoxic) agent LPS is always a concern. Furthermore, the method used for protein purification can also introduce some toxic additives. Thus, in vitro and in vivo recombinant spider silk toxicity evaluations are important. Films of bioengineered spider silk and their hybrid variants supported the growth of human bone marrow mesenchymal stem cells (hMSCs) 7,27. The cytotoxicity results for osteosarcoma cells indicated that one of four films prepared from different spider silk variants had a cell number significantly lower than tissue culture plastic 11. Nano-assemblies and nano-fibrils of spider silk peptides from Araneus ventricosus showed slight cytotoxicity to neuronal cells directly after culturing (0h), while the cytotoxicity effect was eliminated during incubation 28. Fibers of low pyrogenicity (less than 1 EU/mg) made of recombinant spider silk originated from MaSp1 of Euprosthenops australis and supported the growth of fibroblast cells 12. Moreover, these fibers implanted subcutaneously in rats did not cause any negative systemic or local reactions 29. Another in vivo study focused on wound dressings made of recombinant spider silk of unspecified origins. The results showed that wound healing in spider silk treated rats was much better than in nontreated aminals and the level of healing was comparable to collagen treatments 30.
All of these studies indicated good biocompatibility of the tested spider silks. However, it should be noted that the reported spider silks represented large diversity in terms of source (natural versus recombinant), type (dragline versus egg sac silk), origin (Nephila clavipes, Nephila edulis, Araneus diadematus, Araneus ventricosus, Euprosthenops australis), protein purification processes and format of biomaterials (films, fibers). Our bioengineered spider silk was based on MaSp1 sequence from Nephila clavipes. In terms of silk repeat unit, this was the same sequence as our prior bioengineered variants 7,11,27, however, it did not contain the 5.56 kDa sequence needed for purification. Since we are interested in application of the bioengineered spider silk variants in the biomedical field, the analysis of their cytotoxicity and immunogenicity were needed, particularly without potential interference from the purification tag.
We chose to test soluble forms of silk as essential starting point for biomaterials evaluation. Combining in vitro cell culture and soluble forms of biomaterials, we could achieve high sensitivity because the polymer dose per cell was maximized. Although the silk biomaterial degradation products seem more proper for toxicity study, it should be noted that the degradation process needs careful standardization for reproducibility. Moreover, material degradation in vitro may not necessary generate degradation products mirroring those occurring in vivo. For cytotoxicity study we have used an NIH 3T3 murine fibroblast cell line, the standard cells for in vitro toxicology study.
The present analyses demonstrated that spider silk-like proteins were not toxic and did not activate macrophages over a wide range of concentrations. Only an extremely high level of spider silk protein demonstrated some effects. The 15X80/20 variant did not affect the integrity of plasma membranes even at the highest concentration tested (1000 μg/ml), while 15XPA moderately but not significantly caused leakage of LDH. The difference between the 15X samples was significant. Since the LPS content in both protein preparations was the same, indicating that organic acid contamination was the source of cytotoxicity. At the same dose the 6XPA protein induced extensive LDH release. Since the same concentration of 15XPA protein did not display a similar effect, this suggests that silk-like proteins of lower molecular weight might be relatively more toxic.
In contrast, the highest dose of both 15X proteins slightly increased caspase-3 activity and reduced mitochondrial function. There were no significant differences between 15X proteins purified by the different methods. However, both proteins caused effects which differed significantly when compared with more severe effect triggered by 6XPA. Moreover, initially the lower concentration of 6XPA (100 μg/ml) did not affect metabolic function but enhanced caspase-3 activity, and later (after 48h) also decreased cell metabolic activity. It is not likely that endotoxin contamination of 6XPA could influence the metabolic activity of cells. E. coli LPS when tested at a concentration of 50 ng/ml and above generally stimulated proliferation of fibroblasts (as measured by MTT assay), while the lower concentrations had no effect 31. Since endotoxin level in the highest concentration of tested 6XPA was much lower than this value, it should not affect NIH 3T3 proliferation.
Macrophages are cells responsible for numerous homeostatic, immunological, and inflammatory processes. They are the first cells to recognize invading pathogens. Following contact with foreign elements, macrophages release cytokines - mediators of inflammation such as IL-1, TNF-α, or IL-6. Only the highest concentration of silk-like proteins purified by the PA method moderately induced J774 macrophages to produce TNF-α. Since 15X purified by the 80/20 method did not induced the secretion of TNF-α, the activation of macrophage was probably caused by contamination from the purification chemicals. LPS or residues of propionic acid could be mediators of macrophage activation. In the study 1 ng/ml LPS E. coli–0111:B4 induced TNF-α secretion at the level comparable to the highest concentrations of 6XPA and 15XPA. Since endotoxin level in silk samples was much lover than LPS controls, the observed effect of TNF-α release was probably not due the LPS. These observations indicated that the organic acid may be the culprit. Moreover, in contrast to the cytotocixity studies, the molecular weight of silk proteins did not affect macrophage activation.
The results obtained suggest that silk-like proteins of lower molecular weight might be more toxic or that the purification process was more problematic for the lower molecular weight version of the otherwise same protein. Contrary to 15XPA sample, the residue of LPS was present at 6XPA preparation even after additional polishing process. However, the similar activation of macrophages with highly divergent results in terms of cytotoxicity triggered by the proteins purified by acid extraction (15XPA versus 6XPA) suggests that molecular weight of the protein might be critical.
There are four major findings from this study: (1) bioengineered spider silk proteins (without additional Tag sequences) can be manufactured in a bacterial expression system and be purified utilizing methods based on the properties of natural spider silk, (2) bioengineered soluble spider silk proteins are not toxic and did not activate natural immune reactions, (3) very high doses of soluble spider silk show some toxicity in vitro, associated with the number of repeats in polymer and method of purification, and (4) despite the toxicity at very high doses of soluble protein, films made of different silk variants supported the in vitro growth of cells. These findings indicate that bioengineered spider silk proteins can be manufactured in high quantities and easily purified on a large scale for biomedical material needs. Moreover, the results of the study suggest that bioengineered spider silks are safe for medical applications. The toxicity and activation of macrophages, independent of the source (silk or contamination from purification chemicals), were observed at the spider silk concentration that would be unrealistic in vivo. Since the biodegradation of silk materials is extremely slow and also results in amino acid degradation products, silk concentrations expected at the site of biomaterial application would be biocompatible.
Figure 6.
3T3 NIH fibroblasts cultured on recombinant spider silk films. Silk proteins were cast into films on polystyrene plates (PS) destined for cell suspension cultures. 1 × 103 cells/well (2.86 × 103/cm2) were seeded onto films and observed up to 4 days. Photos were taken in phase contrast microscopy at 10 × magnification. The PS plate without silk films were used as negative controls, while tissue culture treated (TCT) plates were used as positive controls. Scale bar represents 50μm.
Acknowledgments
We thank the staff of Department of Biotechnology and Food Microbiology, Poznan University of Life Sciences for allowing us use of their bioreactor and particular Ryszard Stefanski for technical assistance. We thank Alina Dudkowiak and Barbara Jurzyk from the Institute of Physics, Poznan University of Technology for help with fluorescence spectra recording. The BioContract, the NIH Tissue Engineering Resource Center and the AFOSR supported this work.
References
- 1.Vepari C, Kaplan DL. Silk as a Biomaterial. Prog Polym Sci. 2007;32(8–9):991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24(3):401–16. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 3.Spiess K, Lammel A, Scheibel T. Recombinant spider silk proteins for applications in biomaterials. Macromol Biosci. 2010;10(9):998–1007. doi: 10.1002/mabi.201000071. [DOI] [PubMed] [Google Scholar]
- 4.Vendrely C, Scheibel T. Biotechnological production of spider-silk proteins enables new applications. Macromol Biosci. 2007;7(4):401–9. doi: 10.1002/mabi.200600255. [DOI] [PubMed] [Google Scholar]
- 5.Rising A, Widhe M, Johansson J, Hedhammar M. Spider silk proteins: recent advances in recombinant production, structure-function relationships and biomedical applications. Cell Mol Life Sci. 2010;68(2):169–84. doi: 10.1007/s00018-010-0462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kluge JA, Rabotyagova O, Leisk GG, Kaplan DL. Spider silks and their applications. Trends Biotechnol. 2008;26(5):244–51. doi: 10.1016/j.tibtech.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Bini E, Foo CW, Huang J, Karageorgiou V, Kitchel B, Kaplan DL. RGD-functionalized bioengineered spider dragline silk biomaterial. Biomacromolecules. 2006;7(11):3139–45. doi: 10.1021/bm0607877. [DOI] [PubMed] [Google Scholar]
- 8.Wong Po Foo C, Patwardhan SV, Belton DJ, Kitchel B, Anastasiades D, Huang J, Naik RR, Perry CC, Kaplan DL. Novel nanocomposites from spider silk-silica fusion (chimeric) proteins. Proc Natl Acad Sci U S A. 2006;103(25):9428–33. doi: 10.1073/pnas.0601096103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Wong C, George A, Kaplan DL. The effect of genetically engineered spider silk-dentin matrix protein 1 chimeric protein on hydroxyapatite nucleation. Biomaterials. 2007;28(14):2358–67. doi: 10.1016/j.biomaterials.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Numata K, Kaplan DL. Silk-Based Gene Carriers with Cell Membrane Destabilizing Peptides. Biomacromolecules. 2010;11(11):3189–95. doi: 10.1021/bm101055m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomes SC, Leonor IB, Mano JF, Reis RL, Kaplan DL. Antimicrobial functionalized genetically engineered spider silk. Biomaterials. 2011;32(18):4255–66. doi: 10.1016/j.biomaterials.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedhammar M, Bramfeldt H, Baris T, Widhe M, Askarieh G, Nordling K, Aulock S, Johansson J. Sterilized recombinant spider silk fibers of low pyrogenicity. Biomacromolecules. 2010;11(4):953–9. doi: 10.1021/bm9014039. [DOI] [PubMed] [Google Scholar]
- 13.Fahnestock SR, Bedzyk LA. Production of synthetic spider dragline silk protein in Pichia pastoris. Appl Microbiol Biotechnol. 1997;47(1):33–9. doi: 10.1007/s002530050884. [DOI] [PubMed] [Google Scholar]
- 14.Scheller J, Guhrs KH, Grosse F, Conrad U. Production of spider silk proteins in tobacco and potato. Nat Biotechnol. 2001;19(6):573–7. doi: 10.1038/89335. [DOI] [PubMed] [Google Scholar]
- 15.Huemmerich D, Helsen CW, Quedzuweit S, Oschmann J, Rudolph R, Scheibel T. Primary structure elements of spider dragline silks and their contribution to protein solubility. Biochemistry. 2004;43(42):13604–12. doi: 10.1021/bi048983q. [DOI] [PubMed] [Google Scholar]
- 16.Mello CM, Soares JW, Arcidiacono S, Butler MM. Acid extraction and purification of recombinant spider silk proteins. Biomacromolecules. 2004;5(5):1849–52. doi: 10.1021/bm049815g. [DOI] [PubMed] [Google Scholar]
- 17.Mello C, Arcidiacono S, Butler M. Patent No US 7335739 B2. Methods for the purification and aqueous fiber spinning of spider silks and other structural proteins. 2008
- 18.Huang J, Valluzzi R, Bini E, Vernaglia B, Kaplan DL. Cloning, expression, and assembly of sericin-like protein. J Biol Chem. 2003;278(46):46117–23. doi: 10.1074/jbc.M307792200. [DOI] [PubMed] [Google Scholar]
- 19.Vollrath F, Barth P, Basedow A, Engstrom W, List H. Local tolerance to spider silks and protein polymers in vivo. In Vivo. 2002;16(4):229–34. [PubMed] [Google Scholar]
- 20.Lewis RV. Spider silk: ancient ideas for new biomaterials. Chem Rev. 2006;106(9):3762–74. doi: 10.1021/cr010194g. [DOI] [PubMed] [Google Scholar]
- 21.Gellynck K, Verdonk P, Forsyth R, Almqvist KF, Van Nimmen E, Gheysens T, Mertens J, Van Langenhove L, Kiekens P, Verbruggen G. Biocompatibility and biodegradability of spider egg sac silk. J Mater Sci Mater Med. 2008;19(8):2963–70. doi: 10.1007/s10856-007-3330-0. [DOI] [PubMed] [Google Scholar]
- 22.Allmeling C, Jokuszies A, Reimers K, Kall S, Choi CY, Brandes G, Kasper C, Scheper T, Guggenheim M, Vogt PM. Spider silk fibres in artificial nerve constructs promote peripheral nerve regeneration. Cell Prolif. 2008;41(3):408–20. doi: 10.1111/j.1365-2184.2008.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allmeling C, Jokuszies A, Reimers K, Kall S, Vogt PM. Use of spider silk fibres as an innovative material in a biocompatible artificial nerve conduit. J Cell Mol Med. 2006;10(3):770–7. doi: 10.1111/j.1582-4934.2006.tb00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhbier JW, Allmeling C, Reimers K, Hillmer A, Kasper C, Menger B, Brandes G, Guggenheim M, Vogt PM. Interactions between spider silk and cells--NIH/3T3 fibroblasts seeded on miniature weaving frames. PLoS One. 2010;5(8):e12032. doi: 10.1371/journal.pone.0012032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gellynck K, Verdonk PC, Van Nimmen E, Almqvist KF, Gheysens T, Schoukens G, Van Langenhove L, Kiekens P, Mertens J, Verbruggen G. Silkworm and spider silk scaffolds for chondrocyte support. J Mater Sci Mater Med. 2008;19(11):3399–409. doi: 10.1007/s10856-008-3474-6. [DOI] [PubMed] [Google Scholar]
- 26.Hakimi O, Gheysens T, Vollrath F, Grahn MF, Knight DP, Vadgama P. Modulation of cell growth on exposure to silkworm and spider silk fibers. J Biomed Mater Res A. 2010;92(4):1366–72. doi: 10.1002/jbm.a.32462. [DOI] [PubMed] [Google Scholar]
- 27.Mieszawska AJ, Nadkarni LD, Perry CC, Kaplan DL. Nanoscale control of silica particle formation via silk-silica fusion proteins for bone regeneration. Chem Mater. 2010;22(20):5780–5785. doi: 10.1021/cm101940u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Numata K, Kaplan DL. Differences in cytotoxicity of beta-sheet peptides originated from silk and amyloid beta. Macromol Biosci. 2010;11(1):60–4. doi: 10.1002/mabi.201000250. [DOI] [PubMed] [Google Scholar]
- 29.Fredriksson C, Hedhammar M, Feinstein R, Nordling K, Kratz G, Johansson J, Huss F, Rising A. Tissue Response to Subcutaneously Implanted Recombinant Spider Silk: An in Vivo Study. Materials. 2009;2(4):1908–1922. [Google Scholar]
- 30.Baoyong L, Jian Z, Denglong C, Min L. Evaluation of a new type of wound dressing made from recombinant spider silk protein using rat models. Burns. 2010;36(6):891–6. doi: 10.1016/j.burns.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Yang H, Kaneko M, He C, Hughes MA, Cherry GW. Effect of a lipopolysaccharide from E. coli on the proliferation of fibroblasts and keratinocytes in vitro. Phytother Res. 2002;16(1):43–7. doi: 10.1002/ptr.912. [DOI] [PubMed] [Google Scholar]