Abstract
Neuropeptide S (NPS) is a neuromodulatory peptide, acting via a G-protein-coupled receptor to regulate sleep, anxiety and behavioral arousal. Recent research has found that intracerebroventricular NPS can increase cocaine and alcohol self-administration in rodents, suggesting a key role in reward-related neurocircuitry. It is hypothesized that antagonism of the NPS system might represent a novel strategy for the pharmacological treatment of cocaine abuse. To this end, a small-molecule NPSR antagonist (RTI-118) was developed and tested in animal models of cocaine seeking and cocaine taking. Male Wistar rats (n=54) trained to self-administer cocaine and food under a concurrent alternating FR4 schedule exhibited specific dose-dependent decreases in cocaine intake when administered RTI-118. RTI-118 also decreased the reinstatement of extinguished cocaine-seeking behavior induced by conditioned cues, yohimbine and a priming dose of cocaine. These data support the hypothesis that antagonism of the neuropeptide S receptor may ultimately show efficacy in reducing cocaine use and relapse.
Keywords: cocaine, neuropeptide S, self-administration, relapse, reinstatement, yohimbine
1.1 - Introduction
Neuropeptide S (NPS) is a recently deorphanized peptide (ligand) and receptor system involved in modulating arousal, anxiety and feeding (Pape et al., 2010; Reinscheid et al., 2005; Vitale et al., 2008). Some of the first studies on NPS in rodents demonstrated that NPS not only increases arousal and locomotor activity, but also decreases anxiety-like behaviors (Xu et al., 2004). The administration of NPS into the ventricles of rats (ICV) increases time spent in the open arms of the elevated plus maze, indicating a decrease in anxiety-like behaviors. NPS administered ICV also increases horizontal activity in a locomotor activity assay, indicating a psychomotor stimulant effect. In combination, these data suggest that unlike cocaine, which stimulates motor activity and increases anxiety, and unlike benzodiazepines, which decrease locomotor activity and anxiety, NPS actually decreases anxiety while increasing locomotor activity (Leonard et al., 2008; Rizzi et al., 2008). Other researchers have also found that NPS increases physiological markers for stress and anxiety such as hypothalamo–pituitary-adrenal (HPA) axis activity while decreasing stress-induced hypothermia without changing immobility time in the tail-suspension test, suggesting a more important role for NPS in the selective regulation of anxiety over other affective disorders such as depression (Leonard et al., 2008; Okamura and Reinscheid, 2007; Smith et al., 2006). Thus, manipulation of the NPS system may provide a novel target for the treatment of anxiety- and stress-related psychiatric disorders.
Interestingly, preliminary reports have also described the influence of NPS on drug addiction. The most commonly abused psychomotor stimulants (i.e., caffeine and nicotine) modulate NPS and NPS receptor (NPSR) mRNA expression, which suggests a potential role for NPS in the effects of these drugs (Lage et al., 2006; Lage et al., 2007). Nicotine is also the only known drug that increases locomotor activity and decreases anxiety, which is strikingly similar to the behavioral profile of NPS receptor stimulation. These data suggest the NPS system could be a downstream effector of nicotine administration (Lage et al., 2007). Cao and colleagues (2011) found that ICV infusions of NPS modulate responses in a conditioned approach-avoidance paradigm. When low doses (100 pmol) of NPS were administered, rats responded by avoiding the side of the apparatus formerly paired with NPS, indicating an aversive response to NPS. High doses (1,000 pmol) produced a conditioned approach response, suggesting a conditioned preference for the NPS-paired side. These results indicate a role for NPS in the modulation of subjective hedonic states and that NPS can directly modulate aspects of reward and aversion. Cao et al. also found that rats would self-administer a very low dose (3.4 – 34 pmol/infusion) of NPS directly into the lateral ventricles, suggesting that NPS itself may have reinforcing properties. NPS also modulates the rewarding effects of drugs and drug-associated stimuli in the following ways. Central infusions of NPS increase cocaine-seeking behavior in the cue-induced reinstatement model (Kallupi et al., 2011; Paneda et al., 2009). NPS also increases the rate of cocaine self-administration in both cocaine-naïve and cocaine-dependent mice (Paneda et al., 2009). These results were selective for drug-seeking behavior and not a result of non-specific increases in activity. The NPS system has also been implicated in addiction-related behaviors such as the modulation of subjective behavioral responses pertaining to reward and aversion (Cao et al., 2011).
Taken together, these data suggest that the endogenous NPS system plays a facilitative role in addiction-related processes, including reward, reinforcement and the conditioned relapse to drug-seeking. Developing novel treatments that are focused on decreasing the activation of the NPS system may ultimately reduce the use of or relapse to drugs of abuse. Accordingly, we hypothesized that antagonism of the NPSR would decrease cocaine-related behaviors in rodent models of drug addiction.
To this end, our lab has now evaluated RTI-118 (hydrochloride salt of compound 1l from Zhang et al., 2008), an NPSR antagonist that has improved aqueous solubility over the previously described NPSR antagonist SHA-68 (Okamura et al., 2008). Compared to SHA-68, RTI-118 has slightly reduced receptor affinity as demonstrated in hNPSR expressed in CHO cells, but it has a lower partition coefficient (clog P and clog D), indicating better solubility in water (Okamura et al., 2008, Zhang et al 2008). The effects of RTI-118 and SHA-68 on intravenous cocaine self-administration in rats were determined and RTI-118 was further evaluated for its effects on the several models of extinguished cocaine seeking. Cocaine and food self-administration were used to test the efficacy of these antagonists in reducing ongoing self-administration to characterize their effects on cocaine taking. The reinstatement of extinguished cocaine seeking was employed to model cocaine seeking and was induced in three ways: using cues conditioned to cocaine infusions, following experimenter-administered cocaine and following injections of the pharmacological stressor, yohimbine.
Our hypothesis that RTI-118 would decrease cocaine self-administration and the reinstatement of extinguished cocaine-seeking behavior was confirmed in the studies reported below.
1.2 - Methods
Fifty-four male Wistar rats (330 grams) were housed in individual cages in a temperature- and humidity-controlled animal care facility on a reversed 12-hour light-dark cycle for the duration of the experiments. Rats were maintained at 85% of their free-feeding body weights by presentation of food pellets during the behavioral sessions and by supplemental post-session feeding and had free access to water. All procedures were approved by the LSUHSC-S Institutional Animal Care and Use Committee and were carried out in accordance with the NIH “Principles of laboratory animal care” (NIH publication No. 85–23).
Rats were implanted with chronic indwelling jugular catheters under pentobarbital anesthesia (50 mg/kg, i.p.) with methylatropine nitrate pretreatment (10 mg/kg, i.p.) according to previously reported procedures (e.g., Goeders and Guerin, 2008; Goeders et al., 2009). Catheters, constructed of silastic tubing (0.025 in i.d. × 0.12 in o.d.), were inserted into the right posterior facial vein and continued into the jugular vein, terminating outside the right atrium of the heart. The catheter was attached to the surrounding tissue with silk thread, guided under the skin around the forelimb and exited posterior to the scapulae through Marlex® mesh and a 22-guage guide cannula (Plastics One, Roanoke, VA, USA) that was sutured firmly under the skin. During experimental sessions this cannula was attached to Tygon tubing threaded through a stainless steel spring leash attached to a fluid swivel (Plastics One) suspended above the experimental chamber. Additional tubing connected the swivel to a 20-ml syringe in a motor-driven pump (Razel Scientific Instruments, Stamford, CT, USA) located outside the experimental chamber. When the experimental session was completed, the catheters were flushed with a solution of streptokinase (0.0067 mg/0.1 ml) and timentin (6.7 mg/0.1 ml) and a small piece of Tygon tubing, sealed on one end, was placed on the cannula to preserve catheter patency. An IBM-compatible personal computer and interface system (Med-Associates, St. Albans, VT, USA) was used to program the procedures and collect the experimental data.
1.2.1 - Cocaine and food self-administration
Experimental chambers (Med-Associates) within sound-attenuating enclosures were equipped with two retractable response levers, a stimulus light above each lever and a food pellet dispenser located on the front wall between the two levers. Following 5–7 days of recovery after surgery, rats (N = 24) were trained to respond under a multiple, alternating schedule of food (45 mg, Test Diets, Richmond, IN, USA) and cocaine (0.25 mg/kg/infusion delivered over 5.6 sec; Goeders and Guerin, 2008). Training began under a fixed-ratio 1 (FR1) schedule of reinforcement, and the requirement for food and cocaine reinforcement was gradually increased to FR4 over several sessions. Each two-hour daily self-administration session was divided into eight 15-minute bins during which either food or cocaine was available as indicated by the illumination of a stimulus light above the appropriate lever. A 1-min timeout period followed the completion of each bin. Once stable self-administration (i.e., less than 10% variability in the number of infusions over three consecutive sessions) was achieved, each rat was exposed to saline substitution (extinction) testing until reliable extinction responding (i.e., responses reduced by at least 80% for each active lever over 2 non-consecutive extinction sessions) was evident. These extinction sessions provided a low, stable baseline of responding in the absence of discrete reinforcers with which to compare the effects of the NPSR antagonists. The small molecule NPS receptor antagonists RTI-118 (5–30 mg/kg, i.p.) and SHA 68 (10–50 mg/kg, i.p.), or vehicle (5% Alkamuls EL-620 (Rhodia) in 0.9% saline) were administered in a random-dose order 30 minutes prior to the start of the self-administration session. Animals were retested several times with a range of doses of a single antagonist.
1.2.2 - Reinstatement of cocaine-seeking behavior
Three separate groups of rats were used for cue-, cocaine- and yohimbine-induced reinstatement of cocaine-seeking behavior. Rats were trained to self-administer cocaine (0.25 mg/kg/infusion over 5.6 sec) under an FR1 schedule of reinforcement. This requirement was gradually increased to FR4 as described above. During training for cue-induced reinstatement, each infusion was paired with the illumination of a house light and the sounding of a tone (66 db). These conditioned cues were specific for cue-induced reinstatement, and rats tested for the cocaine- and yohimbine-induced reinstatement of cocaine-seeking behavior were similarly trained but did not receive these cues during cocaine self-administration. Following at least ten days of stable FR4 self-administration, each animal was subjected to extinction training whereby responses on either lever produced no programmed consequences.
1.2.3 - Cue-induced reinstatement
When the criteria for extinction were met (as outlined above), rats (N = 12) were exposed to a single reinstatement session whereby each response on the previously cocaine-associated lever was recorded and resulted in the presentation of the compound conditioned stimulus of the house light and tone, but no cocaine was delivered. Thirty minutes prior to the reinstatement test session, each animal was injected with RTI-118 (1–20 mg/kg, i.p.) or vehicle, in a random-dose order.
1.2.4 - Cocaine-induced reinstatement
When the criteria for extinction were met, these rats (N = 9) were exposed to a single reinstatement session whereby each response on the previously cocaine-associated lever was recorded, but responding resulted in no programmed consequences. Prior to the start of the reinstatement session, each rat received two intraperitoneal injections: RTI-118 (1–20 mg/kg, i.p.) or vehicle 30 minutes before the session in a random-dose order, followed by an injection of cocaine (15 mg/kg) or saline (1 ml/kg, i.p.) 15 minutes before the session.
1.2.5 - Yohimbine-induced reinstatement
When the criteria for extinction were met, these rats (N = 9) were exposed to a single reinstatement session whereby each response on the previously cocaine-associated lever was recorded, but responding produced no programmed consequences. Prior to the start of the reinstatement session, each rat received two intraperitoneal injections: RTI-118 or vehicle 30 minutes before the session in a random-dose order followed by an injection of yohimbine (2.5 mg/kg) or saline (1 ml/kg, i.p.) 15 minutes before the start of the session (Buffalari and See, 2011; Kupferschmidt et al., 2009).
Following the reinstatement test session each rat was retrained on the FR4 schedule of cocaine self-administration until stable responding was observed. Self-administration behavior was extinguished and reinstated a maximum of four times testing different doses of RTI-118, with at least one vehicle-pretreatment session. This procedure allowed each animal to serve as its own control and decreased the total number of animals used in the experiment (Goeders et al., 2009).
1.2.6 - Statistical analyses
Since all experiments used a within-subjects design, data were analyzed using a two-way analysis of variance (ANOVA) with repeated measures analysis for the factor of dose/treatment. Dunnett’s multiple comparison tests were employed to determine significant differences among treatment groups and doses. Data were normalized to vehicle-treated responding (i.e., percent vehicle) in the cocaine and food self-administration experiment to account for the variability in baseline cocaine self-administration behavior among rats as well as the difference between the number of food pellets self-administered (~95 reinforcers/session) and the number of cocaine infusions delivered (~35 reinforcers/session) at baseline. Raw data are presented as well (Figures 1 and 2).
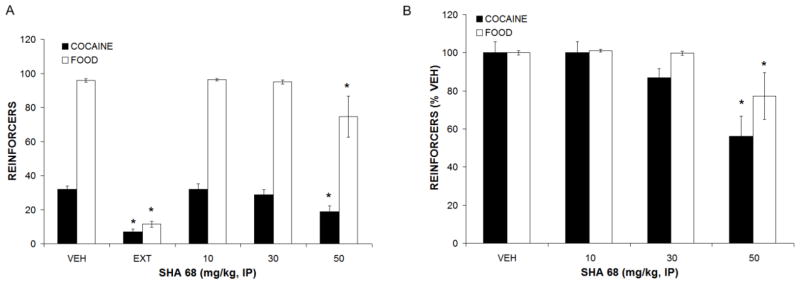
Figure 1.
Effects of SHA-68 pretreatment on cocaine and food self-administration. (A) Raw lever responses. (B) Normalized to vehicle responding. All data are means ± SEM, * p < 0.05 compared to vehicle. N = 12
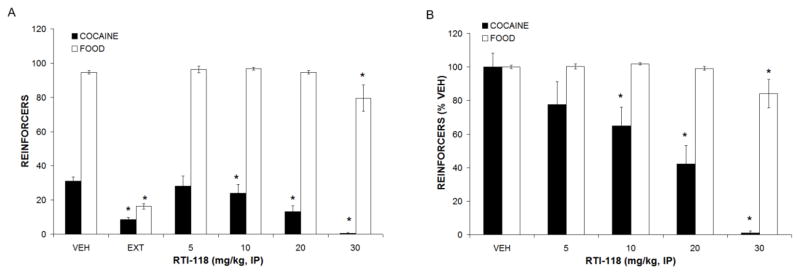
Figure 2.
Effects of RTI-118 pretreatment on cocaine and food self-administration. (A) Raw lever responses. (B) Normalized to vehicle responding. All data are means ± SEM, * p < 0.05 compared to vehicle. N = 12
1.3 - Results
1.3.1 - Cocaine and food self-administration
Rats rapidly and appropriately acquired both cocaine and food self-administration under an FR4 schedule of reinforcement over an average of five weeks. Following successful extinction training and vehicle treatment (an additional three weeks), administration of the NPSR antagonists, RTI-118 and SHA-68, was well tolerated with no significant outward signs of aversion, sedation or toxicity. Pretreatment with SHA-68 resulted in dose-related decreases (F3,30 = 3.935, p<0.05) in cocaine and food self-administration (Figure 1). These effects appeared to be non-selective and were only evident at the highest dose tested (50 mg/kg), suggesting a decrease in general motivation or locomotor activity rather than a selective effect in reducing cocaine reinforcement.
RTI-118 also produced a dose-related decrease (F4,38 = 5.012, p<0.05) in cocaine and food self-administration (Figure 2); however these effects were selective for cocaine at lower doses (e.g., 10 and 20 mg/kg). Since these doses did not affect food self-administration, the studies of the effects of RTI-118 on the reinstatement of extinguished cocaine-seeking behavior were restricted to doses of 20 mg/kg, i.p. or less.
1.3.2 - Reinstatement of cocaine-seeking behavior
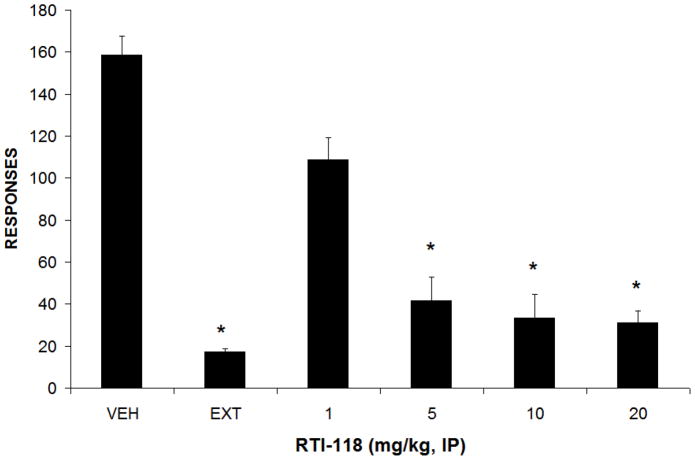
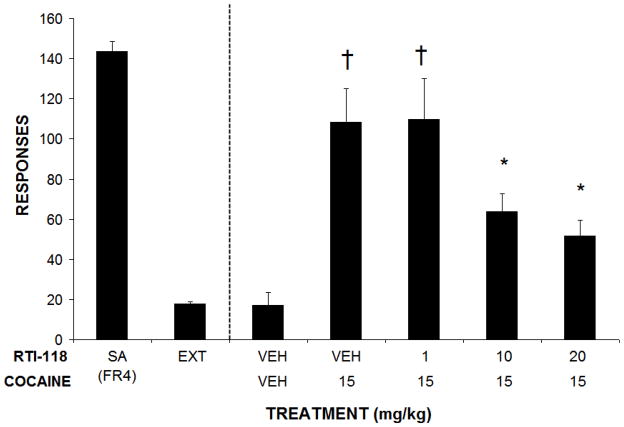
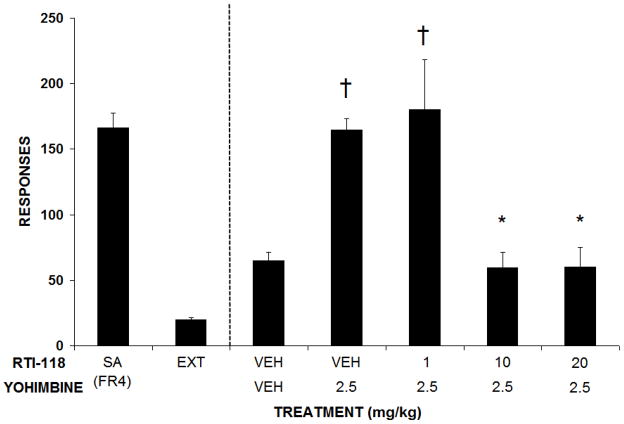
Rats attained stable cocaine self-administration following an average of three weeks of training under the FR4 schedule of reinforcement. All rats met the criteria for successful extinction (i.e., less than 20% of baseline responding for two consecutive extinction sessions) in an average of six sessions. Following successful extinction training, each rat was injected with RTI-118 (1–20 mg/kg, i.p.) or vehicle and responding on the previously cocaine-associated lever was analyzed. Administration of RTI-118 decreased reinstatement responding induced by conditioned cues in a dose-related fashion (F4, 23=4.73, p<0.05; Figure 3), with significant reductions following injections of 5, 10 and 20 mg/kg i.p. Intraperitoneal injections of cocaine (15 mg/kg) also robustly reinstated responding on the previously cocaine-paired lever as previously described (Mantsch and Goeders, 1999). Cocaine-induced reinstatement was also attenuated by pretreatment with RTI-118 (F4,28=3.124, p<0.05; Figure 4) with significant reductions in lever responding observed following 10 and 20 mg/kg, i.p. Rats injected with yohimbine (2.5 mg/kg i.p.) also increased responding on the previously cocaine-paired lever compared to vehicle as previously described (Buffalari and See, 2011; Kupferschmidt et al., 2009; Lee et al., 2004). This stress-induced reinstatement was also attenuated following pretreatment with RTI-118 (F4, 24=2.973, p<0.05, Figure 5), most markedly at doses of 10 and 20 mg/kg, i.p.
Figure 3.
Effects of RTI-118 pretreatment on cue-induced reinstatement responding. Lever responding under baseline self-administration and extinction conditions are also presented. Data are mean number of responses (± SEM), * p < 0.05 compared to vehicle. N = 12
Figure 4.
Effects of RTI-118 pretreatment on cocaine-induced reinstatement responding. Lever responding under baseline self-administration and extinction conditions are also presented. Data are the mean number of responses (± SEM).
† p< 0.05 compared to VEH/VEH treatment.
* p<0.05 compared to VEH/cocaine 15 mg/kg treatment. N = 9
Figure 5.
Effects of RTI-118 pretreatment on yohimbine-induced reinstatement responding. Lever responding under baseline self-administration and extinction conditions are also presented. Data are the mean number of responses (± SEM).
† p< 0.05 compared to VEH/VEH treatment.
* p<0.05 compared to VEH/yohimbine 2.5 mg/kg treatment. N = 9
1.4 - Discussion
RTI-118 hydrochloride is a new NPSR antagonist with slightly lower potency but improved aqueous solubility over SHA-68. Administration of SHA-68 resulted in a non-selective decrease in both cocaine and food self-administration at doses up to 50 mg/kg, i.p.. Our results with the reference compound SHA-68 are similar to those reported by Kallupi and colleagues (2011). In their hands, SHA-68 reduced both cocaine self-administration and food-maintained responding at doses up to 30 mg/kg, i.p. Interestingly, RTI-118 is able to selectively reduce cocaine self-administration at lower doses (10–20 mg/kg, i.p.) without affecting food self-administration. Combined with the fact that RTI-118 (109 nM) has a roughly 8-fold reduction in antagonist potency versus SHA-68 (13.9 nM) at the NPS 107I variant (Zhang et al., 2008), this clearly indicates that RTI-118 has better in vivo efficacy in reducing cocaine self-administration when compared to SHA-68. The observed difference in efficacy between the two antagonists may be attributed to differences in drug-like properties and not receptor potency. Calculated logD is a measure of lipophilicity (ChemAxon in MarvinSketch 5.8). At pH 7.4, the clogD of RTI-118 (2.16) is significantly lower than that of SHA-68 (4.34), indicating RTI-118 has significantly improved aqueous solubility at physiological pH. The selective effect of RTI-118 on reducing cocaine self-administration behavior is unique because SHA-68 (Kallupi et al., 2011) and RTI-118 both reduced cue-induced cocaine-seeking behavior at doses of 30 and 50 mg/kg, i.p. These data demonstrate NPS is a key mediator of several aspects of reward in rats and that RTI-118 and improved analogs might be useful for decreasing cocaine-taking behavior in humans.
Our current studies augment the proposed role of the NPS system in cocaine taking and now also include the relapse to cocaine-seeking behaviors. RTI-118 decreased the influence of three stimuli (conditioned cues, cocaine and yohimbine) on cocaine-associated lever responding during the reinstatement session. These studies indicate that NPSR antagonism may be a useful strategy in reducing relapse to cocaine use.
The ability of environmental stimuli to become conditioned to drug presentation and consequently drive relapse and craving is a well-documented phenomenon across many species (Epstein et al., 2006). As such, these associated cues are able to alter mesolimbic dopamine levels and drive cocaine-seeking responses in rats (Weiss et al., 2000). It has also been shown that ICV administration of NPS can potentiate the cue-induced reinstatement of both cocaine- and alcohol-seeking behavior (Cannella et al., 2009; Kallupi et al., 2011; Paneda et al., 2009). Results from the present study enhance those of previous research. While conditioned cues (a tone and light) were able to induce responding on the cocaine-associated lever, this effect was significantly reduced by pretreatment with the NPSR antagonist RTI-118.
Similarly, an experimenter-administered dose of cocaine can be used to prompt cocaine-seeking behavior (de Wit and Stewart, 1981; Shaham et al., 2003). Previous research in our laboratory has identified 15 mg/kg, i.p. as a dose which induces robust reinstatement behavior (Mantsch and Goeders, 1999), a result confirmed here. Pretreatment with RTI-118 reduced cocaine-induced reinstatement. These data demonstrate the role of NPS in cocaine-induced relapse.
Yohimbine, an α2-adrenergic receptor antagonist, is a pharmacological stressor that activates the HPA axis (Bouchez et al., 2012; Marinelli et al., 2007) and also induced cocaine-seeking behavior in both squirrel monkeys and rats. Yohimbine is commonly used as a pharmacological stressor to model the stress-induced reinstatement of drug-seeking behavior (Buffalari and See, 2011; Kupferschmidt et al., 2009; Lee et al., 2004). The administration of 2.5 mg/kg yohimbine resulted in a robust increase in responding on the cocaine-associated lever. This effect was blocked by pretreatment with RTI-118, extending the influence of NPS to stress- and cocaine-induced reinstatement in addition to the already-demonstrated role for NPS in cue-induced reinstatement. Taken together, the results of these studies demonstrate the efficacy of an NPSR antagonist in decreasing cocaine-seeking behavior induced by several different stimuli, suggesting that RTI-118 may be useful in decreasing relapse in a population of cocaine-dependent humans.
Early research into the anatomical distribution of the NPSR supports the hypothesis that this peptide is involved in modulating aspects of reward and aversion in rats. NPSR mRNA is expressed in several key addiction-related brain structures such as the amygdala, hippocampus, hypothalamus and cortex (Xu et al., 2007). Other researchers have studied the mechanism of these NPS-induced effects on drug-related behaviors and two other neuropeptide systems have been implicated as downstream effectors of NPS activity; corticotropin-releasing factor (CRF) and orexin. Microinjections of NPS into the lateral hypothalamus potentiate drug-seeking behavior in both alcohol and cocaine reinstatement models. This effect is blocked by the selective orexin-1 receptor antagonist SB-334867 (Canella et al., 2009; Kallupi et al., 2011). ICV NPS administration also activates c-Fos expression in hypocretin-1/orexin-1 neurons in the lateral hypothalamus, further supporting the interaction of orexins and NPS (Kallupi et al., 2011).
Of further importance is the interaction between NPS and the CRF system in the rat brain. Previous research has demonstrated a role for CRF in mediating NPS-related addictive behaviors. For example, NPS is unable to potentiate cocaine-seeking behavior in CRF1 knockout mice. However, these mice are sensitive to the anxiolytic effects of NPS administration, suggesting a selective interaction of CRF and NPS in cocaine-seeking models. This effect is also highlighted by the reduction of NPS-induced effects on drug-seeking behavior by the selective CRF1 receptor antagonist antalarmin (Paneda, 2009). This interaction is especially important in light of the crucial role of CRF in increasing addiction-related phenomena across many species and models (reviewed in George, Le Moal and Koob, 2012; Logrip et al., 2011; Goeders, 2002; Gurkovskya et al., 2005). Together, these data demonstrate an important role of both orexin and CRF systems as effectors of NPS administration.
Overall, these results demonstrate the utility and efficacy of small molecule NPSR antagonists in decreasing cocaine-reinforced behaviors in animal models of cocaine seeking and taking. These data suggest that the development of similar antagonists having suitable drug-like properties might ultimately have clinical value in decreasing cocaine dependence in human subjects.
HIGHLIGHTS.
Neuropeptide S can potentiate cocaine and ethanol self-administration suggesting the utility of neuropeptide S receptor antagonists in drug reinforcement.
We compared the effects of two neuropeptide S receptor antagonists in rat models of cocaine addiction.
RTI-118 selectively decreased cocaine self-administration when compared to SHA-68.
RTI-118 decreased cocaine-seeking behavior provoked by several different stimuli.
Acknowledgments
We would like to thank Dr. Rainer Reinscheid for thoughtful discussions and support. Research was financially supported by National Institutes of Health grant 1R01MH087826 from the National Institutes of Mental Health and from institutional funding from the department of Pharmacology, Toxicology & Neuroscience.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bouchez G, Millan MJ, Rivet JM, Billiras R, Boulanger R, Gobert A. Quantification of extracellular levels of corticosterone in the basolateral amygdaloid complex of freely-moving rats: A dialysis study of circadian variation and stress-induced modulation. Brain Res. 2012;3;1452:47–60. doi: 10.1016/j.brainres.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology (Berl) 2011;213(1):19–27. doi: 10.1007/s00213-010-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannella N, Economidou D, Kallupi M, Stopponi S, Heilig M, Massi M, Ciccocioppo R. Persistent increase of alcohol-seeking evoked by neuropeptide S: an effect mediated by the hypothalamic hypocretin system. Neuropsychopharmacology. 2009;34(9):2125–34. doi: 10.1038/npp.2009.37. [DOI] [PubMed] [Google Scholar]
- Cao J, de Lecea L, Ikemoto S. Intraventricular administration of neuropeptide S has reward-like effects. Eur J Pharmacol. 2011;658(1):16–21. doi: 10.1016/j.ejphar.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189(1):1–16. doi: 10.1007/s00213-006-0529-6. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Le Moal M, Koob GF. Allostasis and addiction: role of the dopamine and corticotropin-releasing factor systems. Physiol Behav. 2012;106(1):58–64. doi: 10.1016/j.physbeh.2011.11.004. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27(1–2):13–33. doi: 10.1016/s0306-4530(01)00034-8. Review. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Effects of the combination of metyrapone and oxazepam on cocaine and food self-administration in rats. Pharmacol Biochem Behav. 2008;91(1):181–9. doi: 10.1016/j.pbb.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE, Clampitt DM, Keller C, Sharma M, Guerin GF. Alprazolam and oxazepam block the cue-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology (Berl) 2009;201(4):581–8. doi: 10.1007/s00213-008-1326-1. [DOI] [PubMed] [Google Scholar]
- Gurkovskaya OV, Palamarchouk V, Smagin G, Goeders NE. Effects of corticotropin-releasing hormone receptor antagonists on cocaine-induced dopamine overflow in the medial prefrontal cortex and nucleus accumbens of rats. Synapse. 2005;57(4):202–12. doi: 10.1002/syn.20172. [DOI] [PubMed] [Google Scholar]
- Kallupi M, Cannella N, Economidou D, Ubaldi M, Ruggeri B, Weiss F, Massi M, Marugan J, Heilig M, Bonnavion P, de Lecea L, Ciccocioppo R. Neuropeptide S facilitates cue-induced relapse to cocaine seeking through activation of the hypothalamic hypocretin system. Proc Natl Acad Sci U S A. 2011;107(45):19567–72. doi: 10.1073/pnas.1004100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt DA, Tribe E, Erb S. Effects of repeated yohimbine on the extinction and reinstatement of cocaine seeking. Pharmacol Biochem Behav. 2009;91(3):473–80. doi: 10.1016/j.pbb.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Lage R, Diéguez C, López M. Caffeine treatment regulates neuropeptide S system expression in the rat brain. Neurosci Lett. 2006;410(1):47–51. doi: 10.1016/j.neulet.2006.09.064. [DOI] [PubMed] [Google Scholar]
- Lage R, González CR, Diéguez C, López M. Nicotine treatment regulates neuropeptide S system expression in the rat brain. Neurotoxicology. 2007;28(6):1129–35. doi: 10.1016/j.neuro.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29(4):686–93. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Leonard SK, Dwyer JM, Sukoff Rizzo SJ, Platt B, Logue SF, Neal SJ, Malberg JE, Beyer CE, Schechter LE, Rosenzweig-Lipson S, Ring RH. Pharmacology of neuropeptide S in mice: therapeutic relevance to anxiety disorders. Psychopharmacology (Berl) 2008;197(4):601–11. doi: 10.1007/s00213-008-1080-4. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Koob GF, Zorrilla EP. Role of corticotropin-releasing factor in drug addiction: potential for pharmacological intervention. CNS Drugs. 2011;25(4):271–87. doi: 10.2165/11587790-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Goeders NE. Ketoconazole does not block cocaine discrimination or the cocaine-induced reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav. 1999;64(1):65–73. doi: 10.1016/s0091-3057(99)00090-8. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Lê AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007;195(3):345–55. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- Okamura N, Reinscheid RK. Neuropeptide S: a novel modulator of stress and arousal. Stress. 2007;10(3):221–6. doi: 10.1080/10253890701248673. Review. [DOI] [PubMed] [Google Scholar]
- Okamura N, Habay SA, Zeng J, Chamberlin AR, Reinscheid RK. Synthesis and pharmacological in vitro and in vivo profile of 3-oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide (SHA 68), a selective antagonist of the neuropeptide S receptor. J Pharmacol Exp Ther. 2008;325(3):893–901. doi: 10.1124/jpet.107.135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pañeda C, Huitron-Resendiz S, Frago LM, Chowen JA, Picetti R, de Lecea L, Roberts AJ. Neuropeptide S reinstates cocaine-seeking behavior and increases locomotor activity through corticotropin-releasing factor receptor 1 in mice. J Neurosci. 2009;29(13):4155–61. doi: 10.1523/JNEUROSCI.5256-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Jüngling K, Seidenbecher T, Lesting J, Reinscheid RK. Neuropeptide S: a transmitter system in the brain regulating fear and anxiety. Neuropharmacology. 2010;58(1):29–34. doi: 10.1016/j.neuropharm.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinscheid RK, Xu YL, Civelli O. Neuropeptide S: a new player in the modulation of arousal and anxiety. Mol Interv. 2005;5(1):42–6. doi: 10.1124/mi5.1.8. Review. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Vergura R, Marzola G, Ruzza C, Guerrini R, Salvadori S, Regoli D, Calo G. Neuropeptide S is a stimulatory anxiolytic agent: a behavioural study in mice. Br J Pharmacol. 2008;154(2):471–9. doi: 10.1038/bjp.2008.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168(1–2):3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Smith KL, Patterson M, Dhillo WS, Patel SR, Semjonous NM, Gardiner JV, Ghatei MA, Bloom SR. Neuropeptide S stimulates the hypothalamo-pituitary-adrenal axis and inhibits food intake. Endocrinology. 2006;147(7):3510–8. doi: 10.1210/en.2005-1280. [DOI] [PubMed] [Google Scholar]
- Vitale G, Filaferro M, Ruggieri V, Pennella S, Frigeri C, Rizzi A, Guerrini R, Calò G. Anxiolytic-like effect of neuropeptide S in the rat defensive burying. Peptides. 2008;29(12):2286–91. doi: 10.1016/j.peptides.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci USA. 2000;97(8):4321–6. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeng J, Ly NK, Henriksen SJ, de Lecea L, Civelli O. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43(4):487–97. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol. 2007;500(1):84–102. doi: 10.1002/cne.21159. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gilmour BP, Navarro HA, Runyon SP. Identifying structural features on 1,1-diphenyl-hexahydro-oxazolo[3,4-a]pyrazin-3-ones critical for Neuropeptide S antagonist activity. Bioorg Med Chem Lett. 2008;18(14):4064–7. doi: 10.1016/j.bmcl.2008.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]