Abstract
Objective
To examine the presentation characteristics of patients with kaposiform hemangioendothelioma (KHE) to describe the spectrum of disease and risk factors for Kasabach-Merritt phenomenon (KMP).
Study design
Retrospective review of 163 patients referred to the Vascular Anomalies Center at Children’s Hospital Boston for KHE between 1991 and 2009 identified 107 patients with sufficient data for inclusion.
Results
The prevalence of KHE in Massachusetts is approximately 0.91/100,000 children. KHE manifested in infancy in 93% of cases; 60% as neonates. Common presenting features included enlarging cutaneous lesion (75%), thrombocytopenia (56%), and musculoskeletal pain or decreased function (23%). Cutaneous KHE favored the extremities, especially overlying joints. In our cohort 71% developed KMP (11% after initial presentation), and 11% of patients lacked cutaneous findings. Retroperitoneal and intrathoracic lesions, though less common, were complicated by KMP in 85% and 100% of cases, respectively. Compared with superficial lesions, KHE infiltrating into muscle or deeper was 6.3 fold more likely to manifest KMP, and 18-fold higher if retroperitoneal or intrathoracic. KHE limited to bone or presenting after infancy did not manifest KMP.
Conclusion
An enlarging lesion is the most common presenting feature of KHE in infancy. Older patients with KHE or those lacking cutaneous manifestations present with musculoskeletal complaints or atypical symptoms. The risk of KMP increases dramatically when tumor infiltrates muscle or when KHE arises in the retroperitoneum or mediastinum.
Keywords: Kasposiform hemangioendothelioma, Kasabach-Merritt phenomenon, Vascular Anomaly, Vascular Tumor
Kaposiform hemangioendothelioma (KHE) is a rare vascular tumor typically first seen in infancy as a distinctive cutaneous lesion with ill-defined borders.[1] KHE may be confused with infantile hemangioma due to age of presentation and the presence of a vascular cutaneous lesion. Prenatal and adult-onset KHE have been described. Although infantile hemangioma has a predictable natural history of proliferation for several months followed by slow involution over several years, the evolution of infantile KHE results in smaller, fibrous remnants with microscopic evidence of residual tumor, usually with persistent cutaneous stain.[2] Infantile hemangioma may present with multifocal cutaneous lesions with or without hepatic lesions. In contrast, few cases of multifocal KHE have been reported; only one has shown KHE in multiple biopsy sites.[3] Biopsy-proven hepatic KHE has never been reported, although a single case involving the common bile duct was recently described.[4] KHE is described as a “rare” vascular tumor; no epidemiologic studies have reported incidence or prevalence data.
KHE is an infiltrative tumor that may cross tissue planes from dermis into subcutis, fascia, muscle, and bone. Characteristic T1–weighted MRI imaging reveals an ill-defined, hypo/isointense soft tissue thickening, often involving multiple tissue planes.[5] T2–weighted MRI imaging typically demonstrates a hyperintense mass with reticular stranding in subcutaneous fat. Histopathologic features of KHE include: infiltrating nodules/sheets of variably spindled endothelial cells, focal immunopositivity for lymphatic endothelial markers, slit-like vascular channels, absence of mitosis or nuclear atypia, microthrombi, hemosiderin deposition, edema, fibrosis, and abnormal lymphatic channels.[1, 5-8]
Kasabach-Merritt phenomenon (KMP) is a profound thrombocytopenia resulting from intralesional platelet trapping.[9] The first report in 1940 described “extensive purpura” as a complication of “capillary hemangioma.” With refined definition of the term “hemangioma” in recent decades, it is now clear that KMP occurs with KHE and tufted angioma, not with infantile or congenital hemangiomas.[5, 10] Overuse of the term KMP to describe any low platelet count or coagulopathy observed in a patient with a vascular anomaly has caused considerable confusion with respect to the underlying biology and outcomes of this phenomenon, including broadly reported mortality rates of 12-30% for KHE.[5, 11] Localized or disseminated coagulopathy is more commonly attributed to other vascular malformations.[12]
Given the challenging diagnostic and management considerations for KHE, this study was designed to retrospectively evaluate a large cohort of patients, defined by interdisciplinary consensus, to better understand the spectrum of this vascular tumor, including atypical presentations and predictors of KMP.
METHODS
We reviewed the medical records and database of the Vascular Anomalies Center at Children’s Hospital Boston from 1991 to 2009 using the search terms Kaposiform hemangioendothelioma, KHE, Kasabach-Merritt phenomenon, KMP, Kasabach-Merritt syndrome, and KMS to define a cohort of patients with probable KHE. Our Institutional Review Board approved this retrospective review. Our interdisciplinary team reviewed all cases and reached consensus on the diagnosis of KHE based on review of digital photos, imaging, clinical history, laboratory data and/or biopsy. Of the 163 patients in the initial search results, 118 patients carried a diagnosis of KHE, and 107 had sufficient clinical, imaging, and laboratory data for inclusion in this analysis. Data collected included: age of onset, presenting signs/symptoms, anatomic location, depth of infiltration, and platelet count to evaluate for KMP. KMP was broadly defined in this cohort as a platelet count of less than 100,000 per microliter. Depth of infiltration, as determined by radiographic findings or pathology, was designated as superficial or deep. Superficial lesions were those involving tissue layers from the dermis through subcutaneous tissue and involving the deep fascia. Deep lesions infiltrated muscle, bone, intrathoracic, or retroperitoneal sites. Histopathologic confirmation was not required for diagnosis. In 62 patients, a biopsy specimen was available and was reviewed by a pathologist with experience in vascular anomalies (HPK), confirming the diagnosis of KHE.
RESULTS
Epidemiology
This KHE cohort represented referrals from 30 states and 15 countries. We assume that our center was involved in the vast majority of cases from Massachusetts. Given the estimated 1.4 million children less than 18-years-old in Massachusetts in 2009[13] and 13 Massachusetts children with KHE, we estimate the prevalence of KHE as 0.91/100,000 children. Over the past decade there has been about one new case of KHE diagnosed in Massachusetts per year yielding an incidence of 0.071/100,000 children.
Demographics
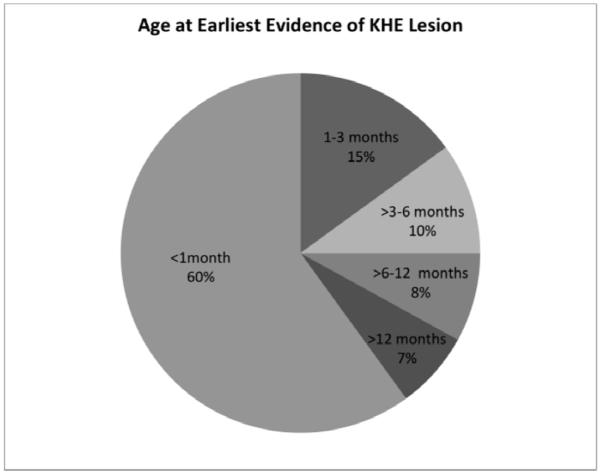
KHE manifested before one month of age in 60% of cases and during infancy in 93% of cases (Figure, A). The median age of initial presentation was two months (range birth to 49-years-old). One patient had a lesion on prenatal ultrasonography that was ultimately diagnosed as KHE. There was a slight male predominance in our cohort of 1.33:1 (61 male; 46 female).
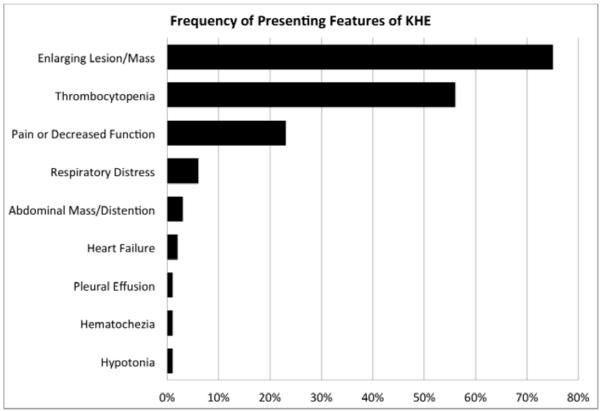
Figure 1.
KHE presentation characteristics. A, Age at first sign or symptom of KHE lesion. B, Frequency of presenting features of KHE. Individual patients commonly had more than one presenting sign or symptom.
Presenting Signs and Symptoms
Eighty-nine percent of patients had a cutaneous vascular lesion; no patients had multi-focal lesions. Cutaneous discoloration and progressive enlargement of the tumor occurred in 75% of cases. Other common presenting features included: thrombocytopenia (56%) and musculoskeletal dysfunction with decreased range of motion or pain (23%) (Figure, B). Analysis of musculoskeletal complaints by patient age at presentation revealed an increase from 19% of infant presentations to 71% of presentations after 1-year-old (data not shown).
Anatomic Distribution
Four anatomic regions were used to categorize the location of KHE: cervicofacial, upper extremity/shoulder, lower extremity/hip, and torso (including intrathoracic cavity and retroperitoneum). KHE most frequently involved an extremity, followed by torso, then the cervicofacial region. Twenty-six percent (27/107) of KHE lesions extend into more than one of these anatomic regions. Superficial lesions tended to arise in the extremities (10/16). The majority, 83%, of our KHE lesions were classified as deep lesions. Subgroups of deep lesions included: 3 bone only, 13 retroperitoneal and 9 intrathoracic lesions. KHE restricted to bone involved the femur, vertebrae or sacrum and presented with musculoskeletal pain without KMP.
Noncutaneous KHE
Eleven percent of our cohort did not have cutaneous involvement. Lesions arose in the torso (9/12) or the lower extremity (3/12). The median age at presentation of the noncutaneous KHE group was 6.5 months (range: birth to 6 years). Seven of twelve (58%) patients developed KMP. Five of these were retroperitoneal; all presenting in infants less than six-months-old. Presenting signs and symptoms in patients without cutaneous involvement are described in Table I. Pain and/or musculoskeletal dysfunction were more common in older patients.
Table 1.
Patients with KHE without cutaneous involvement.
| Age of Presentation |
Presenting Symptoms | Depth of Infiltration | KMP |
|---|---|---|---|
| Birth | Thrombocytopenia/congestive heart failure | Retroperitoneum | Yes |
| Birth | Petechiae/Thrombocytopenia | Retroperitoneum | Yes |
| 5 weeks | Ascites/respiratory distress | Retroperitoneum | Yes |
| 4 months* | Bloody stool | Retroperitoneum | Yes |
| 6 months | Abdominal mass/Thrombocytopenia | Retroperitoneum | Yes |
| 3 years | Abdominal pain/distention | Retroperitoneum (pancreas) | No |
| 4 months | Enlarging mass | Muscle (gluteus) | Yes |
| 7 months | Hypotonia | Muscle (paraspinal) | No |
| 10 months | Progressive lower extremity weakness | Muscle (paraspinal) | Yes |
| 2 years | Extremity pain/limp | Bone (femur) | No |
| 4 years | Hip pain/refusal to walk | Bone (sacrum) | No |
| 6 years | Back pain | Bone (vertebrae) | No |
Histopathology of all cases supported the KHE diagnosis, except one * without available histology.
Kasabach-Meritt phenomenon
Analysis of the frequency and risk factors for KMP was restricted to 96 patients with platelet counts. Although 56% of patients, overall, presented with symptoms of thrombocytopenia (bruising, petechiae, bleeding), subgroup analysis of the patients with known platelet counts revealed 71% of cases had KMP. Mean and median platelet nadirs for those the KMP were 17,300 and 11,500 platelets per microliter, respectively (data not shown). Anatomic location of cutaneous KHE lesions was not predictive of KMP; however, lesions large enough to involve more than one anatomic region did have increased KMP, odds ratio 7.93-15.90 (Table II). KHE superficial to muscle manifested KMP in only 36% lesions compared with 78% of lesions that invaded underlying muscle, bone, retroperitoneum or the thoracic cavity. The development of KMP in lesions involving the retroperitoneum or thoracic cavity was increased compared with superficial lesions, odds ratio 18.
Table 2.
KHE by anatomic site, depth of infiltration, with frequency of KMP and ORs for KMP.
| Lesion Frequency |
% Lesion Frequency |
KMP | % KMP | OR | 95% CI | |
|---|---|---|---|---|---|---|
| Anatomic Location | ||||||
| Extremity | 39/107 | 36% | 15/29 | 52% | 1 | |
| Truck | 21/107 | 20% | 15/20 | 75% | 2.80 | (0.80, 9.74) |
| Cervicofacial | 20/107 | 19% | 13/17 | 76% | 3.03 | (0.79, 11.54) |
| Truck & Extremity | 19/107 | 18% | 17/19 | 89% | 7.93 | (1.54, 40.74) |
| Cervicofacial & Truck +/− Extremity |
8/107 | 8% | 8/8 | 100% | 15.90 | (0.84, 301.03) |
| Depth | ||||||
| Superficial | 16/93 | 17% | 5/14 | 36% | 1 | |
| Deep | 77/93 | 83% | 56/72 | 78% | 6.30 | (1.85, 21.47) |
| Bone Only | 3/93 | 3% | 0/3 | 0% | 0.25 | (0.01, 5.72) |
| Muscle & Bone | 52/93 | 56% | 36/50 | 72% | 4.63 | (1.17, 25.01) |
| Retroperitoneal | 13/93 | 14% | 11/13 | 85% | 18 | (2.92, 110.96) |
| Intrathoracic | 9/93 | 10% | 9/9 | 100% |
Although most patients had KMP at time of presentation, 11% developed KMP later. The median interval to development of KMP for delayed cases was 6.5 weeks (range: 4 weeks to 2 years). Each of the eight patients who developed KMP subsequent to initial presentation was symptomatic at the time KMP was detected (enlarging lesion n=6; increased lesion firmness with change in cutaneous stain n=1, and respiratory distress n=1).
Twenty-eight cases in our cohort did not develop KMP, defined as a platelet count less than 100,000 per microliter (Table III). The median age for this group was 3.5 months (range: birth to 49 years). Eighty-five percent of these patients were offered treatment despite normal platelet levels. Novel presentation of KHE in patients older than 12 months did not include thrombocytopenia.
Table 3.
KHE lesions without KMP.
| Age of Presentation | Presenting Symptoms | Lesion Location | Treatment Offered |
|---|---|---|---|
| Birth* | Enlarging mass | Head | YES |
| 2 years | Swelling | Head/Neck | YES |
| 4 years | Swelling | Head | YES |
| 1 month | Enlarging mass/FTT | Neck | YES |
| < 4weeks | Enlarging mass | Upper Extremity | YES |
| 4 months‡ | Enlarging mass | Upper Extremity | NO |
| 5 months‡ | Enlarging mass | Upper Extremity /Trunk | YES |
| 7 months | Swelling/Cutaneous stain | Upper Extremity | YES |
| 13 months | Pain/Swelling | Upper Extremity | Information Not Available |
| 25 years* | Cutaneous stain/pain | Upper Extremity | YES |
| 48 years | Pain/Swelling | Upper Extremity | YES |
| Birth | Decreased arm movement/swelling |
Trunk | Information Not Available |
| Birth | Cutaneous stain | Trunk | NO |
| 1 week* | Enlarging mass | Trunk | YES |
| 1 month | Enlarging mass | Trunk | NO |
| 7 months | Hypotonia | Trunk/Retroperitoneum | Information Not Available |
| 14 months | Swelling/pain | Trunk | YES |
| 6 years | Back pain | Trunk | YES |
| 3 years | Abdominal pain/distention | Retroperitoneum (pancreas) | YES |
| Birth | Enlarging mass | Lower Extremity | YES |
| Birth | Enlarging mass | Lower Extremity | YES |
| Birth | Enlarging mass /pain | Lower Extremity | Information Not Available |
| 1 month | Enlarging mass | Lower Extremity | YES |
| 3 months | Ecchymotic stain | Lower Extremity | YES |
| 3 months* | Swelling/ Cutaneous stain n | Lower Extremity | YES |
| <5 months* | Enlarging mass | Lower Extremity | YES |
| 2 years | Extremity pain/limp | Lower Extremity | YES |
| 4 years | Hip pain/refusal to walk | Lower Extremity | YES |
Histology of all cases supported the KHE diagnosis, except two ‡ with “KHE-spectrum” and five * without available histology.
DISCUSSION
KHE involves a spectrum of lesions from small, superficial tumors without KMP to large, infiltrative lesions with life-threatening complications including KMP. We have characterized the largest cohort of KHE patients to date. Most patients have KMP and present in infancy with classic cutaneous lesions. Patients without cutaneous lesions present with atypical signs and symptoms and tend to be older. Numerous anatomic locations have been reported: cervicofacial region (sinuses,[14, 15] internal and external auditory canals,[16, 17] larynx,[18] thymus,[19] thyroid[3] and eyelid[20]), torso, extremities, mediastinum, and retroperitoneum[1]. Retroperitoneal tumors are frequently described in the literature because of their severity (publication bias); however, this location represented only 12% of tumors in our study, a lower proportion than previously published.[6, 21]
KMP is typically associated with more aggressive lesions and poorer outcomes.[22] No specific criteria have been established to risk-stratify patients with respect to the occurrence or recurrence of KMP. Previously, KHE lesions with a maximum cutaneous diameter greater than 8 centimeters or with infiltration of the retroperitoneum or mediastinum have been implicated as risk factors for KMP.[22] Our data suggest that KHE lesions extending into multiple anatomic regions confer an increased risk of KMP, though data available in our dataset did not permit for consistent analysis of maximal tumor dimensions.
The pathogenesis of KMP has yet to be elucidated. Clinically significant KMP is a severe thrombocytopenia, generally below 30,000 per microliter. We chose a generous platelet threshold of 100,000 per microliter to capture all cases and examine the distribution of platelet counts in KHE patients. Of 68 patients with platelet counts less than 100,000 per microliter, 47 had platelet count nadirs less than 30,000 per microliter. Mild thrombocytopenia may indicate a milder tumor, a quiescent tumor, partial response to successful therapy, or it may occur in other vascular lesions or have an etiology unrelated to vascular anomalies. KMP is refractory to transfused platelets, often causing painful tumor engorgement arguing against an intrinsic platelet defect. Abnormal platelet activation and aggregation may occur secondary to interaction with abnormal tumor endothelium resulting in localized trapping of platelets and consumption of clotting factors.[23] Others have hypothesized that the turbulent blood flow that results from the architecture of the small, convoluted capillaries seen in KHE triggers KMP.[8] The basal lamina of KHE endothelial cells has been shown to be discontinuous and poorly formed which may permit interaction of collagen with clotting factors.[1] In addition to the severe, persistent thrombocytopenia characteristic of KMP, patients often manifest elevated D-dimer and low fibrinogen. [5, 10, 24] Perturbations of prothrombin time, partial thromboplastin time, and hematocrit have been less uniformly described.[25, 26] Coagulopathy in addition to thrombocytopenia is associated with more aggressive presentations and may indicate concurrent infection or inflammation. Unfortunately, coagulopathy data were not routinely captured on patients in our cohort and were unavailable for retrospective review.
We have previously reported a series of patients without KMP [22] and have expanded from 10 to 28 cases in this study. Superficial tumors, tumors isolated to bone, or presentation at an older age are each characteristics associated with decreased frequency of KMP. Patients in our cohort with fascial involvement but not deeper invasion of muscle or bone had few complications of Kasabach-Merritt phenomenon, similar to “superficial” lesions involving skin and subcutaneous tissues alone. One theory explaining this is that repeated vessel microtrauma through muscle movement contributes to KMP. Anatomically, vascular lesions in bone may be physically constrained from expansion, altering their behavior compared with muscular lesions. Three cases of bone-only KHE in this study lacked KMP, and infiltrative lesions, including muscle and bone, developed KMP. Recent reports of adult-onset KHE also point to trauma as a possible trigger of the tumor proliferation.[27]
The cutaneous outcomes from KHE include three types of residuum: pseudo-port wine stains with papules, telangiectasias with swelling, and fibrotic subcutaneous infiltrates.[2] Importantly, patients with intramuscular, particularly peri-articular, KHE may develop decreased range of motion, contracture or chronic pain at the involved site over time.[2] Although fascial involvement may not predict KMP, it may conceivably promote scarring that may lead to long-term myofascial pain, this hypothesis warrants further investigation. Response to medical therapies, late effects of medical therapy and recurrence rates of KHE are not well delineated.
Referral bias at our center may under-represent adult patients and milder phenotypes, while over-representing severe, refractory or atypical patient presentations. Additionally, data collected through referrals depends on the accuracy and quantity of referral data. Due to the severity of disease in infancy and wide geographic spread of patients represented in our cohort, not all patients were seen in person. Therefore, response to therapy and overall outcomes data for KHE lesions are currently lacking and are the subject of continued investigation.
As a major referral center for vascular anomalies we anticipate referrals from the vast majority of Massachusetts patients with KHE, permitting the first estimate of incidence and prevalence for KHE. Extrapolating our prevalence calculation in Massachusetts using the nationwide 2009 U.S. Census data estimating 74.5 million children less than 18 years old[13], we estimate 678 cases of KHE in the United States.
Acknowledgments
We thank Kimberly Chalache, Mary Beth Sylvia, MS, FNP-BC, and Erin Spera, MS, CPNP for their assistance with data collection and their dedication to the care of the Vascular Anomaly Center patients.
Supported by Lovejoy Education and Research Grant and American Society of Hematology Trainee Research Grant (to S.C.) and National Institutes of Health/National Heart, Lung, and Blood Institute (K08 HL089509 to C.T.).
Abbreviations
- (KHE)
Kaposiform hemangioendothelioma
- (KMP)
Kasabach-Merritt phenomenon
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Contributor Information
Stacy E. Croteau, Division of Pediatric Hematology/Oncology, Children’s Hospital Boston.
Marilyn G. Liang, Department of Dermatology, Children’s Hospital Boston.
Harry P. Kozakewich, Department of Pathology, Children’s Hospital Boston.
Ahmad I. Alomari, Division of Interventional Radiology, Children’s Hospital Boston.
Steven J. Fishman, Department of Surgery, Children’s Hospital Boston.
John B. Mulliken, Department of Plastic Surgery, Children’s Hospital Boston.
Cameron C. Trenor, III, Division of Pediatric Hematology/Oncology, Children’s Hospital Boston.
REFERENCES
- [1].Zukerberg LR, Nickoloff BJ, Weiss SW. Kaposiform hemangioendothelioma of infancy and childhood. An aggressive neoplasm associated with Kasabach-Merritt syndrome and lymphangiomatosis. Am J Surg Pathol. 1993;17:321–8. doi: 10.1097/00000478-199304000-00001. [DOI] [PubMed] [Google Scholar]
- [2].Enjolras O, Mulliken JB, Wassef M, Frieden IJ, Rieu PN, Burrows PE, et al. Residual lesions after Kasabach-Merritt phenomenon in 41 patients. J Am Acad Dermatol. 2000;42:225–35. doi: 10.1016/s0190-9622(00)90130-0. [DOI] [PubMed] [Google Scholar]
- [3].Deraedt K, Vander Poorten V, Van Geet C, Renard M, De Wever I, Sciot R. Multifocal kaposiform haemangioendothelioma. Virchows Arch. 2006;448:843–6. doi: 10.1007/s00428-006-0177-6. [DOI] [PubMed] [Google Scholar]
- [4].Terui K, Nakatani Y, Kambe M, Fukunaga M, Hishiki T, Saito T, et al. Kaposiform hemangioendothelioma of the choledochus. J Pediatr Surg. 2010;45:1887–9. doi: 10.1016/j.jpedsurg.2010.05.031. [DOI] [PubMed] [Google Scholar]
- [5].Sarkar M, Mulliken JB, Kozakewich HP, Robertson RL, Burrows PE. Thrombocytopenic coagulopathy (Kasabach-Merritt phenomenon) is associated with Kaposiform hemangioendothelioma and not with common infantile hemangioma. Plast Reconstr Surg. 1997;100:1377–86. doi: 10.1097/00006534-199711000-00001. [DOI] [PubMed] [Google Scholar]
- [6].Mac-Moune Lai F, To KF, Choi PC, Leung PC, Kumta SM, Yuen PP, et al. Kaposiform hemangioendothelioma: five patients with cutaneous lesion and long follow-up. Mod Pathol. 2001;14:1087–92. doi: 10.1038/modpathol.3880441. [DOI] [PubMed] [Google Scholar]
- [7].Debelenko LV, Perez-Atayde AR, Mulliken JB, Liang MG, Archibald TH, Kozakewich HP. D2-40 immunohistochemical analysis of pediatric vascular tumors reveals positivity in kaposiform hemangioendothelioma. Mod Pathol. 2005;18:1454–60. doi: 10.1038/modpathol.3800444. [DOI] [PubMed] [Google Scholar]
- [8].Lyons LL, North PE, Mac-Moune Laiw F, Stoler MH, Folpe AL, Weiss SW. Kaposiform hemangioendothelioma: a study of 33 cases emphasizing its pathologic, immunophenotypic, and biologic uniqueness from juvenile hemangioma. Am J Surg Pathol. 2004;28:559–68. doi: 10.1097/00000478-200405000-00001. [DOI] [PubMed] [Google Scholar]
- [9].Kasabach H, Merritt K. Capillary Hemangioma with Extenstive Purpura: Report of a Case. Am J Dis Child. 1940;59:1063–70. [Google Scholar]
- [10].Enjolras O, Wassef M, Mazoyer E, Frieden IJ, Rieu PN, Drouet L, et al. Infants with Kasabach-Merritt syndrome do not have “true” hemangiomas. J Pediatr. 1997;130:631–40. doi: 10.1016/s0022-3476(97)70249-x. [DOI] [PubMed] [Google Scholar]
- [11].el-Dessouky M, Azmy AF, Raine PA, Young DG. Kasabach-Merritt syndrome. J Pediatr Surg. 1988;23:109–11. doi: 10.1016/s0022-3468(88)80135-0. [DOI] [PubMed] [Google Scholar]
- [12].Dompmartin A, Acher A, Thibon P, Tourbach S, Hermans C, Deneys V, et al. Association of localized intravascular coagulopathy with venous malformations. Arch Dermatol. 2008;144:873–7. doi: 10.1001/archderm.144.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].U.S. Census Bureau PD . Estimates of the Resident Population by Selected Age Groups for the United States, States, and Puerto Rico: July 1, 2009. U.S. Census Bureau, Population Division; 2010. [Google Scholar]
- [14].Lee CH, Jaw TS, Yang SF, Wu DK. Kaposiform hemangioendothelioma arising from the maxillary sinus: a case report. Kaohsiung J Med Sci. 2010;26:154–7. doi: 10.1016/S1607-551X(10)70023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Birchler MT, Schmid S, Holzmann D, Stallmach T, Gysin C. Kaposiform hemangioendothelioma arising in the ethmoid sinus of an 8-year-old girl with severe epistaxis. Head Neck. 2006;28:761–4. doi: 10.1002/hed.20414. [DOI] [PubMed] [Google Scholar]
- [16].Chang JM, Kwon BJ, Han MH, Kang HS, Chang KH. Kaposiform hemangioendothelioma arising from the internal auditory canal. AJNR Am J Neuroradiol. 2006;27:931–3. [PMC free article] [PubMed] [Google Scholar]
- [17].Hardisson D, Prim MP, De Diego JI, Patron M, Escribano A, Rabanal I. Kaposiform hemangioendothelioma of the external auditory canal in an adult. Head Neck. 2002;24:614–7. doi: 10.1002/hed.10074. [DOI] [PubMed] [Google Scholar]
- [18].Kim DW, Chung JH, Ahn SH, Kwon TK. Laryngeal kaposiform hemangioendothelioma: case report and literature review. Auris Nasus Larynx. 2010;37:258–62. doi: 10.1016/j.anl.2009.05.003. [DOI] [PubMed] [Google Scholar]
- [19].Wilken JJ, Meier FA, Kornstein MJ. Kaposiform hemangioendothelioma of the thymus. Arch Pathol Lab Med. 2000;124:1542–4. doi: 10.5858/2000-124-1542-KHOTT. [DOI] [PubMed] [Google Scholar]
- [20].Cho SH, Na KS. Haemangioendothelioma on the conjunctiva of the upper eyelid. Clin Experiment Ophthalmol. 2006;34:794–6. doi: 10.1111/j.1442-9071.2006.01320.x. [DOI] [PubMed] [Google Scholar]
- [21].Hu B, Lachman R, Phillips J, Peng SK, Sieger L. Kasabach-Merritt syndrome-associated kaposiform hemangioendothelioma successfully treated with cyclophosphamide, vincristine, and actinomycin D. J Pediatr Hematol Oncol. 1998;20:567–9. [PubMed] [Google Scholar]
- [22].Gruman A, Liang MG, Mulliken JB, Fishman SJ, Burrows PE, Kozakewich HP, et al. Kaposiform hemangioendothelioma without Kasabach-Merritt phenomenon. J Am Acad Dermatol. 2005;52:616–22. doi: 10.1016/j.jaad.2004.10.880. [DOI] [PubMed] [Google Scholar]
- [23].Hall GW. Kasabach-Merritt syndrome: pathogenesis and management. Br J Haematol. 2001;112:851–62. doi: 10.1046/j.1365-2141.2001.02453.x. [DOI] [PubMed] [Google Scholar]
- [24].Haisley-Royster C, Enjolras O, Frieden IJ, Garzon M, Lee M, Oranje A, et al. Kasabach-merritt phenomenon: a retrospective study of treatment with vincristine. J Pediatr Hematol Oncol. 2002;24:459–62. doi: 10.1097/00043426-200208000-00010. [DOI] [PubMed] [Google Scholar]
- [25].Mulliken JB, Anupindi S, Ezekowitz RA, Mihm MC., Jr Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 13-2004. A newborn girl with a large cutaneous lesion, thrombocytopenia, and anemia. N Engl J Med. 2004;350:1764–75. doi: 10.1056/NEJMcpc049002. [DOI] [PubMed] [Google Scholar]
- [26].Ryan C, Price V, John P, Mahant S, Baruchel S, Brandao L, et al. Kasabach-Merritt phenomenon: a single centre experience. Eur J Haematol. 2010;84:97–104. doi: 10.1111/j.1600-0609.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- [27].Karnes JC, Lee BT, Phung T, Alomari AI, Mulliken JB, Greene AK. Adult-onset kaposiform hemangioendothelioma in a posttraumatic site. Ann Plast Surg. 2009;62:456–8. doi: 10.1097/SAP.0b013e318184aafc. [DOI] [PubMed] [Google Scholar]