Abstract
A fundamental question for placebo research is whether such responses are a predisposition, quantifiable by brain characteristics. We examine this issue in chronic back pain (CBP) patients that participated in a double-blind brain imaging (fMRI) clinical trial. We recently reported that when the 30 CBP participants were treated, for two weeks, with topical analgesic or no drug patches, pain and brain activity decreased independently of treatment type, and thus were attributed to placebo responses. Here we examine in the same group brain markers for predicting placebo responses, that is for differentiating between post-treatment persistent (CBPp) and decreasing (CBPd) groups. At baseline, pain and brain activity for rating spontaneous fluctuations of back pain were not different between the two groups. However, based on brain activity differences after treatment, we identified that at baseline the extent of information shared (functional connectivity) between left medial prefrontal cortex and bilateral insula accurately (0.8) predicted post-treatment groups. This was validated in an independent cohort. Additionally, using frequency domain contrasts we observe that, at baseline, left dorsolateral prefrontal cortex high-frequency oscillations also predicted treatment outcomes and identified an additional set of functional connections distinguishing treatment outcomes. Combining medial and lateral prefrontal functional connections we observe a statistically higher accuracy (0.9) for predicting post-treatment groups. These findings show that placebo response can be identified a priori at least in CBP, and that neuronal population interactions between prefrontal cognitive and pain processing regions predetermine probability of placebo response in the clinical setting.
Introduction
Placebo conditioning studies show that placebo analgesia is a true antinociceptive effect with psychobiological origins [9,20,39,41]. These mechanisms interact with the effects of active drugs such as mu-opioids and can in turn interfere with a drug’s therapeutic effects [10–12,41,42,54]. Placebo responses in clinical populations are less clearly understood. A series of studies in IBS patients demonstrated that positive conditioning with a placebo cream reduced the intensity of clinical pain though mechanisms similar to those observed for placebo analgesia in healthy subjects [16,42,43]. However, further investigations into mechanisms that induce placebo analgesia in chronic pain are warranted, to potentially improve treatment strategies and also to be able to design more effective clinical trials.
In a recent placebo controlled, double blind, clinical trial for 5 % lidocaine topical patch treatment we reported that the active treatment was not significantly different from placebo in its effectiveness for treating chronic back pain [24]. A thorough examination of the data corroborated by several reports in the literature [13,24,28,29,35,49] demonstrated that 5% lidocaine relieves pain through a placebo effect. We also confirmed that the overall decrease in clinical pain was due to the use of the patch or treatment, and not a consequence of spontaneous remission in chronic pain because an untreated CBP group (observation only control) showed minimal change in back pain. The placebo patch treatment was effective in nearly half of the patients (independent of the type of treatment), while the remaining patients showed little or no change in back pain [24]. To investigate the mechanisms for this marked interindividual variability in pain relief with a placebo treatment, here we investigate brain functional connectivity differences between the two groups at baseline, testing the hypothesis that in CBP patients placebo responses are contingent on predispositions that may be captured with brain network properties.
The rationale for this investigation was examination of brain placebo mechanisms in the clinical population and for the clinical trial setting in which they were studied. Existing studies have demonstrated the predictive role of brain networks to placebo response only in healthy subjects and specifically in response to placebo conditioning [26,32,46,48]. However, there is no knowledge for brain based placebo prediction in clinical populations, and especially when tested during a clinical trial. Therefore, a secondary aim of the study was to demonstrate that, in chronic pain patients, the clinical trial setting (combination of presence of physicians, brain scanner, and therapy), with neutral instructions (“the treatment may or may not improve your pain”), is sufficient to evoke a placebo effect based on predisposing factors. Towards these goals, we focused on baseline brain activity to identify networks that predict placebo response using two separate approaches one targeting spontaneous back pain related networks and the other comparing BOLD oscillation properties in the whole brain, using a model free approach. We test that 1) specific brain network properties predispose chronic pain patients towards placebo analgesia before start of a clinical treatment, and 2) multiple networks synergistically interact and enhance ability to forecast placebo response.
Methods
The present study is a reanalysis of data presented regarding the effects of 5% lidocaine on spontaneous pain of CBP [24]. In the latter study we showed that active treatment was not different from the placebo arm. Here we regroup the CBP participants into placebo responders and non-responders and analyze brain network properties for predicting these groupings.
Subjects
Data from a total of 30 patients (16 males, 14 females, mean age 51.36 ± 9 years SD) with chronic back pain was used. All subjects were right-handed and gave informed consent to procedures approved by the Northwestern University Institutional Review Board. Patients were included if they had CBP for >1 year, and a pain score > 40/100 VAS at the baseline visit. For 72 hours prior to the first session, the subjects refrained from analgesic medications. During the 2 weeks of treatment period, subjects could take up to 2 regular strength acetaminophen tablets (325 mg) per day if needed. For more details and demographics see [24].
To validate the main finding we examined brain properties in a separate group of 7 CBP and 5 OA (osteoarthritis) subjects that we had studied in the past [7].
Subject groups and experimental sessions
The CBP patients were assigned to two groups based on the level of absolute change in pain. The patients that reported more than median decrease in pain were assigned to the CBP decreasing (CBPd) group and the remaining subjects were designated to the CBP persisting (CBPp) group.
Data acquisition and pain rating task
Data was collected from participants in three experimental sessions. The baseline session was conducted immediately before start of treatment and the post treatment session was performed after 2 weeks of daily administration of the patch.
Functional scans were acquired while the subjects rated spontaneous fluctuations in their back pain. In addition T1-weighted structural images were acquired. Scan parameters and image preprocessing details are described in [24].
Statistical analysis
GLM analysis for pain related activations in CBPp and CBPd groups
To assess spontaneous pain related activations, we used the procedure by Baliki et al., [5]. Briefly, the ratings obtained in the scanner were binarized relative to the mean [5]. The binarised vector was convolved with a canonical hemodynamic response function (gamma function: lag, 6 s; SD, 3 s) in FEAT. The significance of the model fit to BOLD signal in each voxel time was calculated, yielding statistical parametric maps for each subject and condition. All group level analyses were carried out using FEAT in a random effects analysis after the co-registration of individual scans to standard space [152 subject average Montreal Neurological Institute (MNI) space, http://www.bic.mni.mcgill.ca/cgi/icbm_view/]. The T1 images acquired for every patient was used for properly aligning and registering the functional data to the standard template. Average group activity map was generated for the CBPp and the CBPd groups from the baseline scan to ascertain the region that corresponds significantly with spontaneous pain ratings. Contrast maps were generated by comparing the CBPd and CBPp groups with a random effects un-paired t-test. To correct for multiple comparisons, cluster-based corrections was applied to at Z-scores > 2.3 and a cluster probability threshold of p < 0.05. All imaging analyses were corrected for confounds due to age and sex.
Functional connectivity analysis in regions activated by spontaneous pain
Functional connectivity between pain processing brain regions was tested for differences between the two groups at baseline. The regions that showed differences in spontaneous pain related activations between the CBPp and CBPd groups after treatment were selected as regions of interest (ROIs) (see Table 2 for list of regions). To assess baseline differences in the connectivity within the pain related network, masks (5 × 5 × 5 voxels) were selected and the average activity within the ROI extracted. These time series were z-transformed and correlated with corresponding time series of all other ROIs, on a per subject basis. Connectivity was measured as zero-lag Pearson correlations between pairs of regions, which is a measure of amount of information, or temporal synchrony, between shared pairs of regions (throughout the paper we refer to functional connectivity between a region A and a region B as A.B). Only mean correlations with R-values greater than 0.2 were studied (pairwise p-value <0.001). Next, mean connectivity for each pair of regions was calculated for each group (CBPp and CBPd), which quantifies the magnitude of information shared between pairs of brain regions. An unpaired t-test of R-values identified significant group differences in functional connectivity. Based on a Bonferroni correction for multiple comparisons (in this case 5 for 6 ROIs), a p-value of 0.01 was considered as significant.
Table.2.
Coordinates of brain regions activated for spontaneous ratings of CBP
| Brain Region |
Z-VALUE | co-ordinates | |||
|---|---|---|---|---|---|
| x | y | z | |||
| CBPd: baseline activity pain task |
RmPFC/ACC (BA 9, 32) |
3.66 |
10 |
58 |
16 |
| CBPp: baseline activity pain task |
RmPFC/ACC (BA 9, 32) |
3.78 | 12 | 38 | 18 |
| CBPp > CBPd pain task 2 weeks |
RaINS (BA 13) | 3.84 | 34 | 24 | 2 |
| LaINS (BA 13) | 2.90 | −34 | 26 | 2 | |
| RmINS (BA 13) | 3.49 | 40 | 8 | 0 | |
| LmINS (BA 13) | 3.40 | −38 | 8 | −4 | |
| RdmPFC (BA 8) | 3.5 | 4 | 42 | 36 | |
| RlPFC (BA 10) | 3.79 | 28 | 46 | 20 | |
| RACC (BA 32) | 3.71 | 2 | 20 | 38 | |
CBPp = CBP persisting, CBPd = CBP decreasing, R, right; L, left; MPFC, medial prefrontal cortex; ACC, anterior cingulated cortex; aINS, anterior insula; mINS, mid insula; dmPFC, dorsal medial prefrontal cortex; LPFC, lateral prefrontal cortex; Brodmann areas are shown in parenthesis.
Baseline spectral power differences
Spectral analysis was carried out using custom routines in MATLAB 7.9 (The MathWorks, 2009). Specifically, power of the BOLD signal in frequency space was determined voxel-wise using Welch’s method and normalized by total power [4]. The average power of each frequency band in BOLD response (low frequency: 0.01–0.05 Hz, mid frequency: 0.05–0.12 Hz and high frequency: 0.12–0.20 Hz) was calculated at each voxel. For each frequency band, individual subject whole-brain spectral power maps were generated. The subject maps were then transformed into standard space using FLIRT while making use of the T1 maps of each subject. Individual spectral power maps for each frequency band were submitted separately to a two-sample unpaired-t test for a between group comparison. Statistical differences between groups were computed using a random effects analysis (z-score > 2.3, cluster threshold P < 0.01 corrected for multiple comparisons).
Validation study
The findings were validated in a separate group of 7 CBP and 5 OA subjects that we had studied in the past [7]. The validation group had undergone treatment, testing and scanning procedures similar to those used in the double-blind study except this group had participated in an open-label trial (i.e., there was no placebo arm) and fMRI and pain measurements were obtained at baseline and after two weeks of treatment. fMRI scans were preprocessed using the same procedures as above and the resultant data was only used for ROI analysis using brain coordinates derived from results obtained in the double-blind study. The 12 chronic pain patients (7 CBP and 5 OA) were grouped by pain relief at 2-weeks of treatment based on median change in pain.
Differences between CBPd and CBPp in whole-brain LdlPFC functional connectivity
Based on the group comparison, dorsal lateral prefrontal cortex or LdlPFC showed significant differences in BOLD frequency. The BOLD signal time series was extracted from the LdlPFC and was used as a vector to measure LdlPFC whole brain connectivity. Correlation coefficients measured in every voxel were converted to normally distributed z scores using the Fischer's transform, i.e., dividing by the square root of the variance, estimated as 1/√(df-3), where df represents the degrees of freedom. Because the BOLD time course consecutive samples are not statistically independent, the df was corrected by a factor of 2.86, in accordance to Bartlett theory [51,52]. To compare between the CBPp and CBPd groups, a two-sample paired-t test was used to compare connectivity maps between the two groups (random effects analysis z-score > 2.3, cluster threshold P < 0.05 corrected for multiple comparisons). Brain regions identified as significantly differently connected in each group were then used in functional connectivity analysis, using the same approach as described above.
Principal components analysis
A multivariate PCA approach was used to identify interrelationships between networks. Prior to performing PCA (SPSS, Chicago, IL), the suitability of data for factor analysis was assessed with the Kaiser-Meyer-Olkin (KMO) test and significance testing with Barlett’s test of sphericity. The number of orthogonal components was limited according to a significance threshold determined by the Scree test. Components with eigenvalues greater than 1 were regarded as significant. Factors that loaded with values less than 0.4 were removed to determine only the most robust factors. The extracted components were then further analysed to study the demarcated relationships using linear regressions.
Results
Patient pain scores and grouping
At baseline, the mean pain intensity for the 30 CBP patients was 71.6 ± 4.3 SEM. We used a median split to categorize the placebo responders. The median of the absolute change in pain was 18.5 (NRS) after 2 weeks of treatment in the 30 subjects [24]. This value corresponds to a clinically significant value of 28.4% decrease in chronic pain [21,23]. Based on this classification, the subjects with more than median decrease were designated as CBP decreasing (CBPd) group. The remaining subjects were designated persisting or CBPp group. There were no significant differences between the two groups in demographics, disease parameters or medication use at baseline (Table 1).
Table 1.
Demographic parameters for CBPp and CBPp patients.
| CBPp | CBPd | t-test | |
|---|---|---|---|
| Number of subjects | 15 | 15 | |
| Age | 52.6 ±2.6 | 50.13 ±2.1 | 0.74 |
| Sex | 7 females (46.6%) | 7 females (46.6%) | 232 |
| Duration | 16.5±3.8 years | 11.5±2.2months | 1.1 |
| VAS | 7.9±0.4 | 7.6±0.3 | 0.62 |
| MPQ sensory | 16.5±2.2 | 16.5±1.4 | 0.81 |
| MPQ affective | 4.9±1.1 | 2.66±0.66 | 0.36 |
| BAI | 11.8±2.3 | 12.8±2.3 | −0.3 |
| BDI | 6.5±1.1 | 6.7±1.0 | −0.17 |
| NPS | 55.6±4.5 | 54.5±3.5 | 0.20 |
| MQS | 3.9±1.2 | 5.9±1.9 | 0.76 |
VAS=visual analogue scale; MPQ = McGill pain questionnaire; NPS = Neuropathic pain scale; BDI = Beck’s depression index. BAI = Beck’s anxiety index; MQS = Medication Quantification Scale. Data presented as Mean±SEM
Pain network connectivity at baseline predicts placebo response
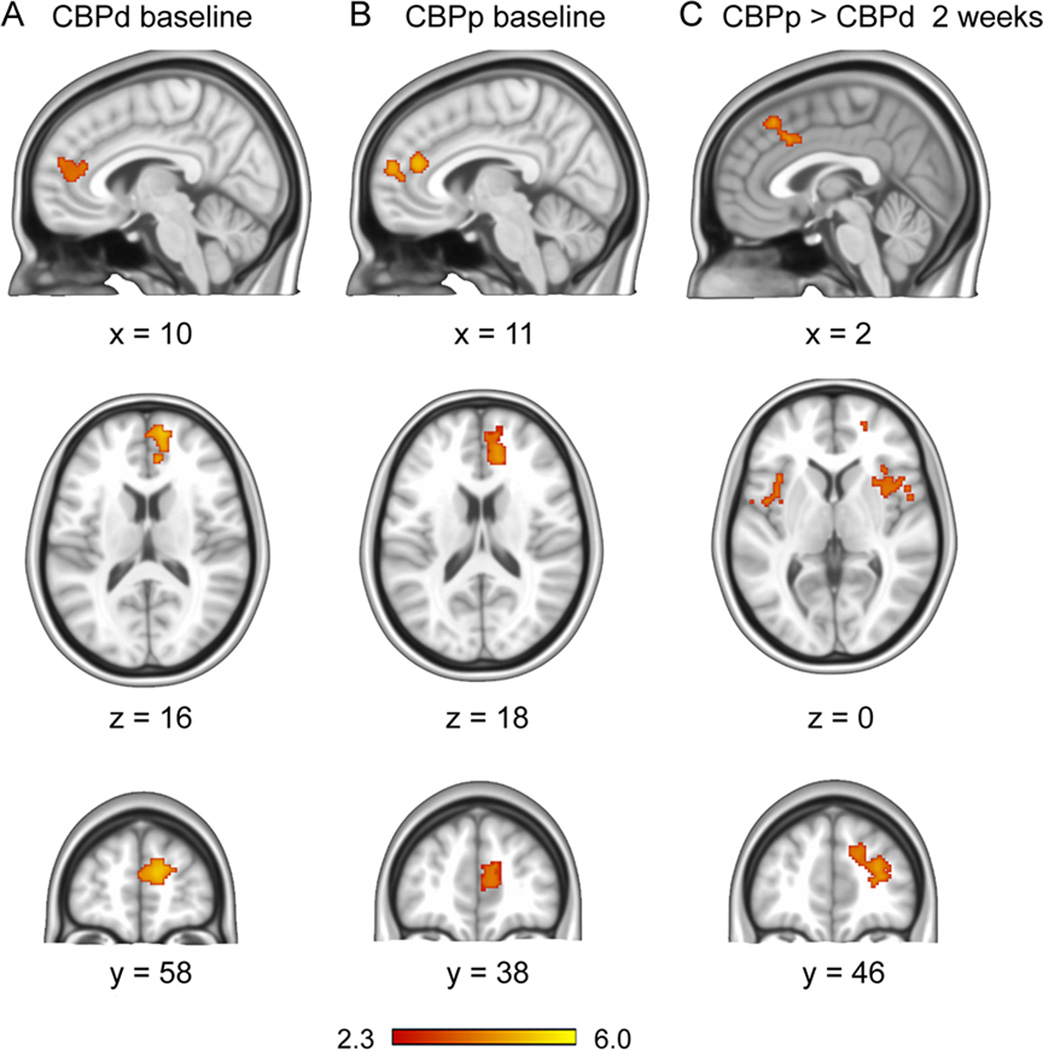
To identify brain parameters predictive of placebo response we first determined the difference between the CBPp and CBPd groups based on brain activity for spontaneous pain ratings. Brain activity related to the spontaneous pain ratings were determined using general linear modeling, corrected for age and sex. At baseline, mean activation associated with spontaneous pain fluctuations (Fig. 1A and 1B) were observed in the medial prefrontal and rostral anterior cingulate in the CBPd group and also in the CBPp group, and the contrast between groups was null. But after 2 weeks of treatment, there was a significantly greater activation in CBPp than in CBPd (Fig. 1C) (opposite contrast was null). This activation was localized to the bilateral aINS (anterior insula, BA 13), RmINS and LmINS (right and left mid insula, BA 13), mid cingulate (BA 32) extending into the RdmPFC (right dorsal medial prefrontal cortex, BA 8) and r LPFC (right lateral prefrontal cortex BA 10) (Table.2). The RdmPFC and ACC were contiguous with the peak activation in the dmPFC. Therefore, the dmPFC was chosen to represent this region.
Fig.1. Brain activity for spontaneous fluctuations of back pain, in persisting and decreasing CBP groups.
A, B. Group-averaged brain activity for rating back pain at baseline (prior to start of treatment), in CBPd (A) and CBPp (B) (n=15 subjects per group). In both groups brain activity was limited to the medial prefrontal cortex (BA 9) and the genual anterior cingulate cortex (BA 32), and the contrast between the groups was null. C. Two weeks after treatment brain activity contrast between the two groups (CBPp > CBPd) shows greater activation in CBPp in bilateral anterior insula (BA 13; horizontal slice at z=0), bilateral dorsal cingulate (BA 24 and 32; sagittal slice at x=2), right dorsal medial prefrontal cortex (RdmPFC, BA 8; coronal slice at y = 46), and lateral frontal pole (BA 10). Activity and contrast maps were generated using random-effects statistics with z score > 2.3 and cluster threshold p < 0.05, corrected for multiple comparisons.
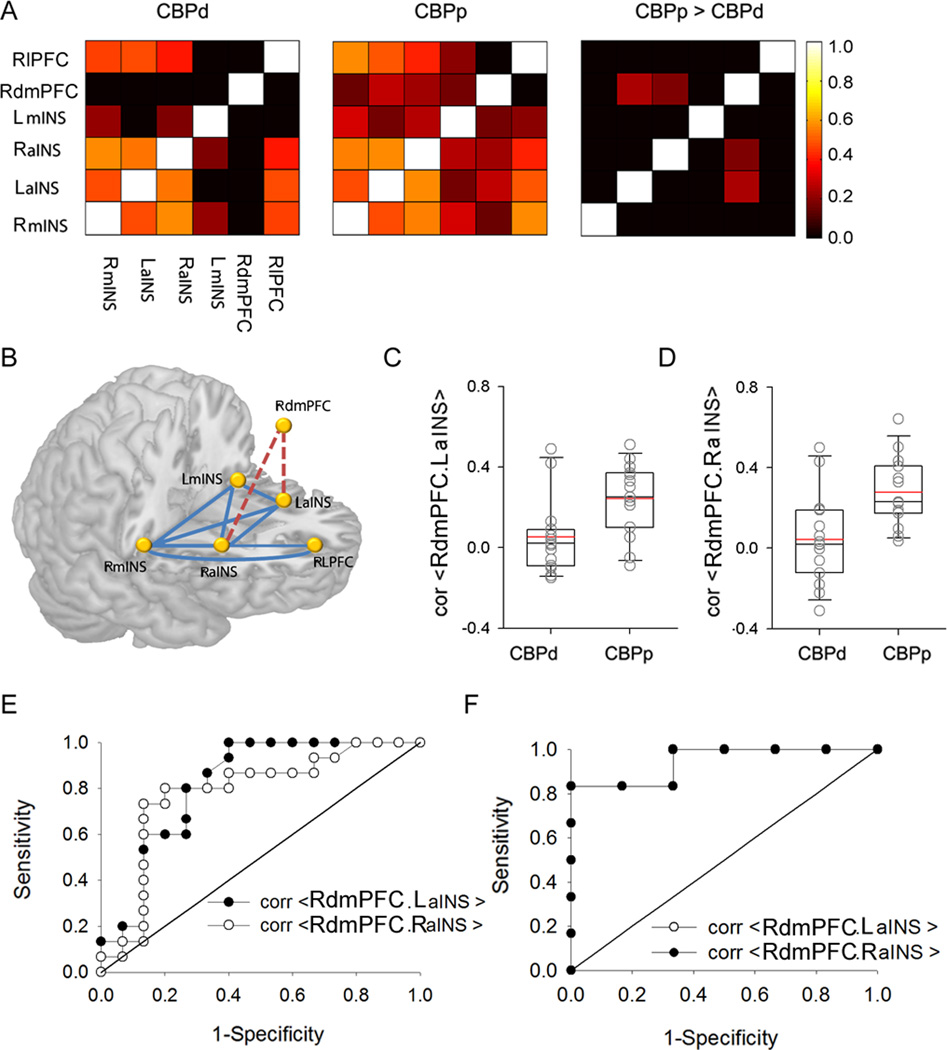
We hypothesised that the functional connectivity between these six regions differs between the two groups at baseline. The time series from these six regions was extracted, and pairwise correlations between them were calculated for the CBPp and CBPd group. The mean connectivity between these regions is shown in Fig. 2A. The CBPd group showed no connectivity above threshold (0.2) between the dmPFC and the insula regions, including right and left anterior and right and left mid insula (Fig. 2A), while the CBPp showed a greater than threshold connectivity between these regions (Fig. 2A, second column). Contrasting these functional connections between the two groups indicated two links significantly stronger in CBPp: RdmPFC connectivity with LaINS (p=0.0025), and with RaINS (p=0.01, borderline significant after Bonferroni correction for 5 comparisons) (Fig. 2A, third column, C and D). None of the functional connections was significantly stronger in the CBPd group. Thus, two functional connections are identified, from within the brain regions related to spontaneous pain, that differentiated the groups before treatment. As the connectivity strengths were higher for CBPp, this result suggests that these networks predispose the subjects to be less susceptible to placebo response. The dmPFC has a well-described role in affective states [22,25,36] and here it was discovered that affective MPQ scores correlated with RdmPFC.LaINS connectivity (R= 0.39, p=0.037) and marginally with RdmPFC.RaINS connectivity (R= 0.35, p=0.064). No relationship was observed for RdmPFC.LaINS connectivity with pain intensity (VAS) (R=−0.009, p=0.96) or sensory MPQ scores (R=0.21, p=0.26), implying that the RdmPFC.aINS connectivity is related to negative affective properties of back pain.
Fig.2. Brain functional connectivity strengths at baseline predict patients who will report persisting or decreasing back pain after a 2-week placebo treatment.
A. Adjacency matrices showing strengths of baseline functional connectivity between 6 regions of interest, in CBPd (left) and CBPp (middle). The contrast between the two groups (CBPp > CBPd) shows regions with significantly stronger connectivity in CBPp (right). B. Three dimensional schematic of the functional network examined at baseline in standard space. Connections in red are significantly stronger in CBPp. C. D. Functional connectivity strengths are distinct between CBPp and CBPd (individual values overlaid upon box plots) for between RdmPFC and LaINS (C), and RdmPFC and RaINS (D). E. Receiver operator curve (ROC) characteristics for discriminating between CBPp and CBPd at 2 weeks after treatment based on functional connectivities calculated at baseline between RdmPFC and LaINS (filled circles) and between RdmPFC and RaINS (open circles). Both functional connectivities significantly predict future outcomes at an accuracy of about 0.8. F. In a separate group of 12 chronic pain subjects ROC characteristics were measured to test validity of discriminating between persisting versus decreasing chronic pain, after 2 weeks of assumed inert treatment. Functional connectivities at baseline between RdmPFC and LaINS (filled circles) and between RdmPFC and RaINS (open circles, symbols are not seen as outcomes are identical) again strongly predict future outcomes (accuracy > 0.9).
ROC analysis for predicting placebo response by functional connectivity
To assess the strength of predictability of the two groups (identified after two weeks of treatment) from functional connectivity measures at baseline, we calculated the receiver operating characteristics (ROC). The ROC for discriminating between the two groups was 0.82 (chi2 test for logistic regression, p<0.002) for the connectivity strength between RdmPFC.LaINS (95% confidence interval [CI]: 0.65–0.98) and 0.78 (chi2 test, p = 0.006, 95% CI: 0.60–0.96) for connectivity between RdmPFC.RaINS. Thus, both pairs of functional connections measured at baseline distinguished the CBPp and CBPd subjects that were grouped based on placebo analgesia observed two weeks later (Fig. 2C–E).
Validating the prediction of placebo response by functional connectivity
The predictive capacity of the functional connectivity between the RdmPFC.LaINS and RdmPFC.RaINS were validated in a separate data set. This data set consisted of 7 CBP and 5 OA patients had participated in an open label trial for a 2 week treatment with the 5 % lidocaine patch (see [7] for details). We hypothesised that the functional connections identified in the 30 CBP subjects predicts interindividual differences in placebo response in this separate group of chronic pain patients, assuming that the analgesia observed in this new cohort is also a placebo effect.
As in the first cohort, subjects were segregated into two groups based on a median split of absolute change in pain. The patients that responded to the treatment were classified as the chronic pain decreasing group (CPd; n = 7), and the remaining were categorised as chronic pain persistent group (CPp, n = 5). BOLD time series was extracted from the RdmPFC, LaINS and RaINS in the baseline scans of the validation cohort, using co ordinates from the first cohort of 30 subjects. Functional connectivity was significantly greater in CPp between RdmPFC.RaINS (F1,11 =10.2, p=0.01) and also between RdmPFC.LaINS (F1,11 =12.9, p=0.005). Moreover, ROC analysis for the RdmPFC.LaINS connectivity (Fig. 2F) discriminated between CPp and CPd with accuracy of 0.97 (p=0.07; 95% CI: 0.88–1.0). The RdmPFC.RaINS connectivity also significantly discriminated the two groups with accuracy of 0.86 (p=0.04; 95% CI: 0.63–1.0). These results sufficiently validate the main observations discovered in the initial cohort.
BOLD oscillations at baseline discriminate between placebo responses
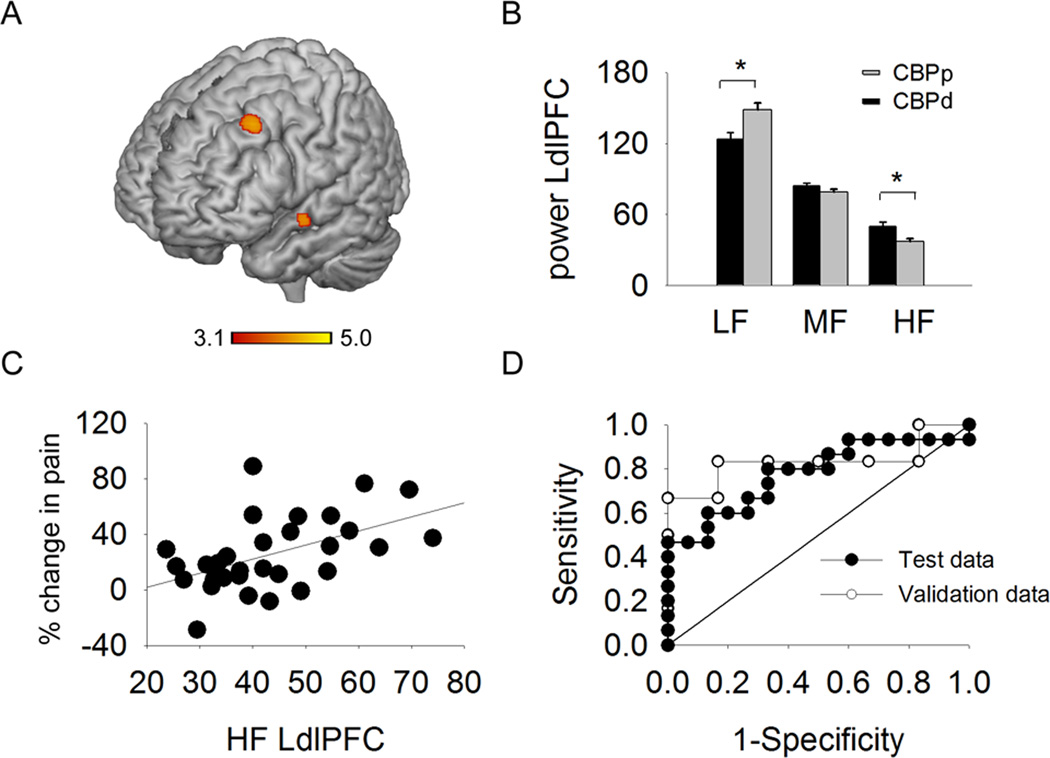
As we have recently demonstrated that analysis of BOLD oscillations in the frequency domain provides additional information regarding brain properties, above that detected by general linear modeling [8], we hypothesized that there are additional brain networks that might further enhance predictability of placebo responders and that these networks can be identified when searched for in the frequency domain. We assessed whole brain full bandwidth spectral properties of BOLD oscillations for 3 frequency bands (low: 0.01–0.05 Hz, mid: 0.05–0.12 Hz and high: 0.12–0.20 Hz), in CBPp and CBPd groups for baseline brain activity (Fig.3). The CBPd group had significantly greater high frequency power (HF) in the LdlPFC BOLD response than in the CBPp groups (x= −30, y=14, z=42; Table 1, (Fig.3B)). Other regions included the right dlPFC (x= 36, y=22, z=46) and the precuneus (x= 0, y=−56, z=18). No significant differences were detected for power in the mid frequency band (MF). The CBPp showed significantly greater lower frequency power (LF) than CBPd in the LdlPFC (same co-ordinates as the high frequency contrast) and also showed more LF power in the superior temporal gyrus. To constrain the analysis to the most pertinent finding, all subsequent analysis were directed to the LdlPFC because it has a well acknowledged role in placebo response [27,46,48,53].
Fig.3. Difference in power spectral density between CBPp and CBPd, during back pain rating task, measured at baseline predicts placebo reponse.
A. Whole brain voxel-wise differences in power for the high frequency band of BOLD oscillations, contrasted between CBPp and CBPd groups at baseline. Regions shown in red-yellow depict significantly greater power in in CBPd (un-paired t-test, random-effects model, z-core >2.3, cluster p<0.01, corrected for multiple comparisons), localized mainly to the left dorsal lateral prefrontal cortex (LdlPFC). B. Bar graphs depicting the mean ± SEM spectral power for LdlPFC, for three frequency bands (low, mid, high), in CBPp and CBPd groups (* p< 0.05). C. High frequency power in LdlPFC BOLD is related to extent of pain relief with placebo (delta pain = pain between baseline and 2 weeks post treatment). D. Receiver operating curve (ROC, filled circles) characteristics for discriminating between CBPp and CBPd at 2 weeks after treatment based on LdlPFC high frequency power in BOLD response calculated at baseline. Prediction was at an accuracy of 0.78 (p = 0.01). ROC for a separate group of 12 chronic pain subjects (grey circles) where only high frequency power in BOLD at LdlPFC was calculated, to test differences between CBPp and CBPd, after 2 weeks of placebo treatment. Prediction accuracy was 0.8 (p=0.078).
The BOLD time series was extracted from the LdlPFC, and power in the three frequency bands was measured in a post hoc test. The HF power was greater (F1,29 = 8.9, p = 0.006) in the CBPd when compared to the CBPp group (MANOVA main effect F1,29=4.3, p= 0.013, Fig. 3B). Conversely, LF power was significantly higher in the CBPp as compared to the CBPd group (F1,29 = 8.8, p=0.006,). There was no difference in the power for the mid frequency band between the two groups (F1,29=1.6, p = 0.22). In addition the LdlPFC power in HF band (at baseline) correlated significantly with the extent of change in pain by the treatment (two weeks later) (R29=0.5, p=0.005, see Fig. 3C).
ROC analysis for predicting placebo response by power of BOLD oscillations
The HF power within LdlPFC was evaluated as a predictive measure of placebo response using ROC analysis. The accuracy of this measure for discriminating between the two groups was 0.78 (p=0.01; 95% confidence interval [CI]: 0.61–0.95; Fig. 3D).
Validating the prediction of placebo response by power of BOLD oscillations
As with the pain network connectivity measures, the LdlPFC high frequency power in BOLD response was also evaluated for its predictive effectiveness by testing it in the 12 CP validation cohort. The HF measure (Fig. 3D) for discriminating of CP had an accuracy of 0.83, with borderline significance (p=0.062; 95% CI: 0.51–1.1).
CBPp and CBPd groups show distinct LdlPFC functional connectivity at baseline
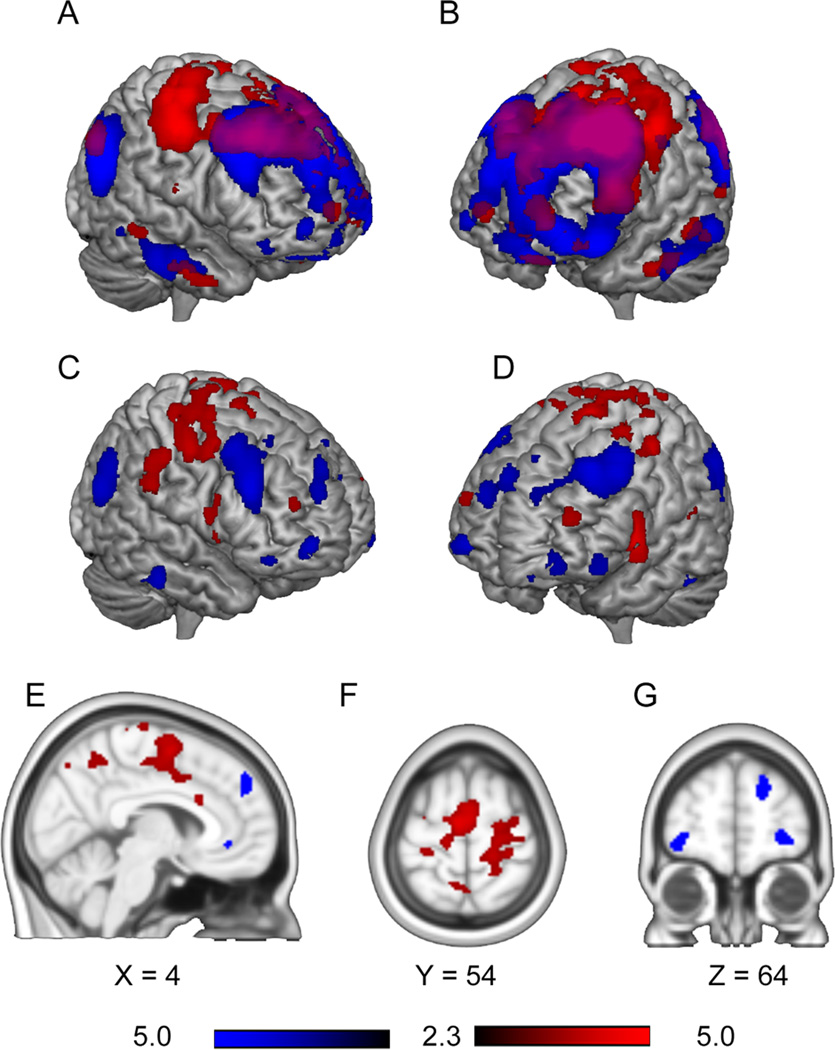
The LdlPFC has been established as an important contributor to the placebo response in multiple studies in healthy subjects [27,46,48,53], and its HF oscillations discriminate between CBPp and CBPd. We next hypothesized that the LdlPFC BOLD oscillations are shared between brain regions such that underlying functional connections would uniquely modulate different brain circuits in placebo responders and non responders in CBP. To identify these circuits we compared whole brain LdlPFC functional connectivity between the CBPp and CBPd groups. The LdlPFC showed connectivity within the frontal parietal attention network and with temporal cortices in both groups. The contrast between the two groups (Fig. 4 A–G) showed that the LdlPFC was more connected with sensorimotor regions and the mid cingulate in the CBPd group, while in the CBPp group LdlPFC connectivity was predominantly with prefrontal brain regions, such as parts of the lateral prefrontal, orbitofrontal, and dorsal medial prefrontal cortices, as well as with bilateral posterior parietal cortex (Table 3). Therefore, we observe that the CBPp and CBPd groups exhibit distinct networks linked with LdlPFC, and these differential interactions are based on sharing specific BOLD oscillations with LdlPFC.
Fig.4. Differences in LdlPFC whole brain connectivity between CBPp and CBPd groups at baseline.
A–B. Show mean connectivity for CBPp (blue) and CBPd (red) groups with LdlPFC. Purple represents regions with connectivity overlap of CBPp and CBPd groups. C–G. Difference between CBPp and CBPd in whole brain LdlPFC connectivity (contrast for CBPp > CBPd is blue, and for CBPd> CBPp is red; un-paired t-test, random-effects model, z-core >2.3, cluster p<0.05, corrected for multiple comparisons).
Table 3.
Brain regions showing greater connectivity with LdlPFC in the CBPp and CBPd groups.
| Brain region | z-max | x | Y | Z | |
|---|---|---|---|---|---|
| CBPr > CBPp | Bi SMA (6), mCC (24) | 3.74 | −6 | −10 | 66 |
| Bi S1, M1 (3, 4, 5, 7, 44) | 3.37 | 16 | −32 | 58 | |
| LIFG (22, 44) | 3.69 | −50 | 6 | 2 | |
| R S2 (2) | 2.97 | 62 | −26 | 44 | |
| CBPp > CBPd | Bi dlPFC (6, 8) | 3.88 | −34 | 26 | 42 |
| R dmpPFC (8) | 3.24 | 6 | 52 | 38 | |
| L aPPC (39) | 3.46 | −46 | −66 | 22 | |
| RaPPC | 3.23 | 52 | −60 | 34 | |
| RlPFC (9) | 2.98 | 18 | 56 | 26 | |
| LlFPC (10) | 2.68 | −34 | 60 | −8 | |
| RlFPC(10) | 2.7 | 18 | 58 | 26 | |
| LOFC(11) | 2.68 | −36 | 58 | −8 | |
| R caudate | 2.25 | 20 | 20 | 10 | |
CBPp = CBP persisting, CBPd = CBP decreasing, Bi = bilateral, L= left, R= right, SMA = supplementary motor area, mCC= mid cingulate cortex, S1= primary somatosensory area 1, M1= primary motor area 1, L IFG = left inferior frontal gyrus, S2= somatosensory area 2, dlPFC= dorsal lateral prefrontal cortex, dmPFC = dorsal medial prefrontal cortex, aPPC = angular posterior parietal cortex, LPFC = lateral prefrontal cortex, lFPC= lateral frontopolar cortex, OFC = orbital frontal cortex. Brodmann areas are shown in parenthesis.
Distinct BOLD frequencies are coupled with distinct brain connectivity patterns and behavioural measures
The next analysis determines the properties of LdlPFC linked networks relative to the networks identified in relation to spontaneous pain, their interactions with pain parameters and their combined influence on placebo response. With this aim, we combined representative measures from the three pertinent findings in a principal components analysis along with delta pain scores as a behavioural measure of placebo response.
First, RdmPFC.LaINS connectivity and affective MPQ scores were added to the PCA to represent the pain network connectivity that correlated with affect and discriminated placebo non responders from responders among CBP patients. The second sets of values added to the PCA represented LdlPFC connectivity differences between CBPp and CBPd groups. This included three regions that showed highest LdlPFC connectivity in the CBPd > CBPp contrast and were RS1 (right somatosensory area 1), RM1 (right primary motor area 1), and LmCC (mid cingulate cortex), see Table 3. Areas representing highest dlPFC connectivity with brain regions identified in the CBPp>CBPd contrast were right RdmPFC, LaPPC (left angular posterior parietal cortex) and RlPFC (right lateral prefrontal cortex). Then, dlPFC power in two frequency bands, the HF and MF, were added to the PCA. LF values were excluded since LF and HF were highly correlated with each other (for the LdlPFC, LF and HF R= −0.8, p<0.0001).
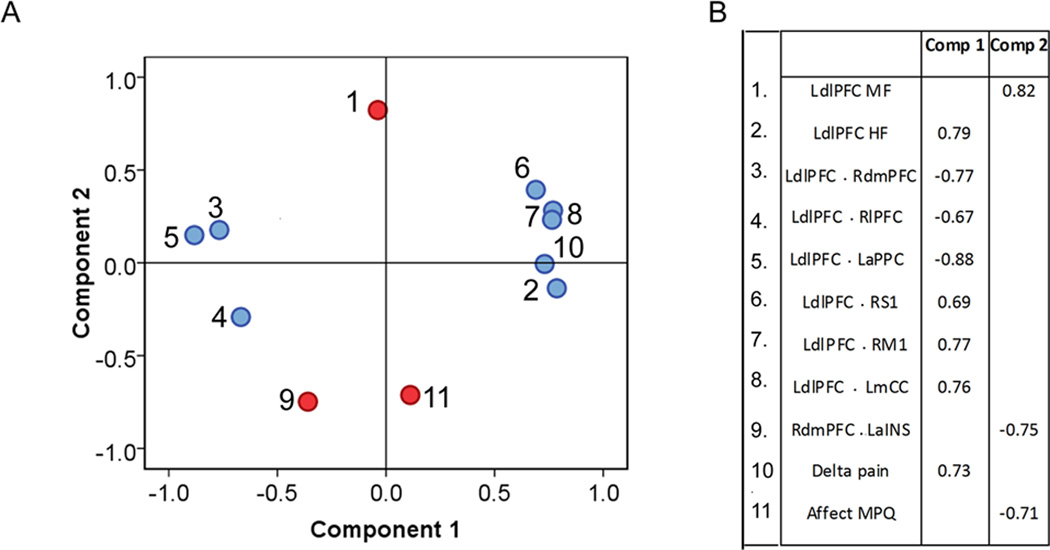
The PCA yielded two principal components (with eigenvalues > 1, Fig. 5A, B). The strength of the model was significant (KMO =0.69, and Barletts test of sphericity p<0.0001 with 55 degrees of freedom). After varimax rotation, the cumulative variance explained by the two components was 63%. Component 1 showed the variables that group with the placebo response measure (delta pain) and with the HF oscillations in LdlPFC, where some functional connections loaded positively (regions observed in the CBPd>CBPp contrast: LdlPFC.RM1, LdlPFC.RS1 and LdlPFC.LmCC), and others loaded negatively (regions observed in CBPp> CBPd contrast: LdlPFC.RlPFC, LdlPFC.RdmPFC and LdlPFC.LaPPC). The positive and negative loadings identify the networks facilitating or inhibiting the placebo response, which were related to the HF oscillations in LdlPFC as confirmed in the correlation results (Fig. 5C).
Fig.5. Distinct BOLD frequencies are coupled with distinct LdlPFC brain connectivity patterns and behavioural measures.
A. Loading plot for principal components analysis. Only two components were extracted. B. Factor loadings of each variable on principal component 1 and 2. HF= high frequency, MF=mid frequency, LdlPFC=left dorsal lateral prefrontal cortex; RdmPFC= right dorsal medial prefrontal cortex, LaPPC = left angular posterior partietal cortex, RlPFC= Right lateral prefrontal cortex, RM1=left primary motor area 1, RS1= right primary somatosensory area 1, LmCC = left mid cingulate cortex, LaINS= left anterior insula.
Component 2 grouped LdlPFC oscillations in MF band with affective MPQ and with the functional connection RdmPFC.LaINS (Fig. 5A, B), identifying that the LdlPFC oscillations in MF band is negatively correlated with RdmPFC.LaINS, and also negatively correlated with affective MPQ (Fig. 5D). Overall, the PCA analysis unravels that LdlPFC oscillations in different frequency bands underlie all the networks involved in predicting placebo response, with separate bands being involved in distinct behavioural outcomes.
Placebo prediction estimates based on combining brain networks
We tested whether combining the multiple functional connections would significantly improve prediction of placebo outcomes. First, we built a logistic multiple regression where all functional networks loading on component 1 were used as independent variables to predict CBPp and CBPd groupings. None of these networks showed a significant contribution, after correcting for the variance of the other networks, suggesting that all five networks provide similar and thus redundant information. Therefore, we selected connectivity values of one, LdlPFC.LmCC, that showed the greatest prediction accuracy for discriminating the two groups (accuracy= 0.81). When functional connectivity strength of LdlPFC.LmCC was partitioned into quartiles (increments of correlation coefficient of 0.12), an odds ratio of 9.1 was obtained (p <0.03, 95% CI 1.3–63.2) for predicting placebo response.
The functional connection, RdmPFC.LaINS, derived from component 2 of PCA analysis, on its own predicted responders with an accuracy of 0.82; with odds ratio of 0.34 for quartile partitioning (p<0.01, 95%CI 0.15–0.78). When the two functional networks derived from component 1 and component 2, LdlPFC.LmCC and RdmPFC.LaINS, were combined in a logistic multiple regression model prediction accuracy increased to 0.9 (with SEM 0.06 and 95%CI 0.78–1.0), which was significantly better than predictions by each network independently (chi-square = 9.2, p<0.01). Thus, the two functional connections synergistically predict placebo analgesia, where one (LdlPFC.LmCC) identifies responders and the other (RdmPFC.LaINS) non-responders.
Discussion
The primary observation of this study is that baseline brain functional connections predict future placebo response in CBP. These connectivity differences were observed before treatment, that is at a time when back pain properties were matched, and when brain activity for spontaneous fluctuations of back pain were similar between the groups. The treatment outcome grouping was determined in the experimental setting of a standard clinical trial, coupled with neutral instructions, and without the use of placebo conditioning or deception. We demonstrate that BOLD oscillations predict placebo responses, and that the localized oscillatory activity is closely related with functional connectivity suggesting that the two phenomena are different manifestations of a unitary mechanism. Placebo prediction by functional connectivity and by BOLD oscillatory properties were replicated in a separate chronic pain cohort, validating our main observations and implying that these results may be generalized, at least, to CBP at large.
The findings demonstrate that multiple brain measures predispose and thus effectively forecast placebo response in CBP. The predictive networks were derived from two different, and independent, analysis techniques that resulted in complementary findings. Both approaches, to our knowledge, are novel methodologies for identifying predictive brain functional biomarkers. We observe that the CBP patients with greater connectivity between brain regions that are known for their role in processing pain, emotion and self referential thinking have a decreased probability to respond to placebo treatment. Not only were the dmPFC/ACC and the bilateral anterior insula more functionally connected in the CBPp group, the strength of these connectivity measures were also positively correlated with negative affect. The dmPFC has a well described role in affective states, anxiety, fear, disgust, emotional appraisal, situations of uncertainty or ambiguity and conscious threat appraisal [22,25,36]. On the other hand, the anterior insula has been widely shown to be linked with self related processes such as self reflection and appraisal of one’s own internal states [18]. Both regions show greater activation and increased connectivity in a number of pathological conditions involving stress and emotional dysregulation, for example in post traumatic stress disorder [30], phobia [40], and panic disorder [15]. The fact that neuronal populations across these two regions are more synchronised with each other and the extent of this synchrony correlates with negative affect suggests that heightened emotional processes prevent these CBP patients from responding to placebo treatment.
Our lab has previously shown brain anatomical/functional organization for BOLD oscillations as a function of frequency [8], and delineated an association between high frequency in BOLD and chronic pain intensity, thus demonstrating an association between the BOLD energy content and behaviour [4]. Similarly, an association between BOLD frequency and functional connectivity has been demonstrated in related brain regions in patients suffering with diabetic neuropathy [14] and in chronic pain [4,33]. Thus, the role of BOLD oscillations is extended in the present study to demonstrate that local BOLD oscillations reflect behavioural predispositions and can be used to quantify prediction of future outcomes.
Our frequency based approach suggests a central role of bandwidth specific BOLD oscillations in the LdlPFC in predicting placebo analgesia. We demonstrate that the coupling between frequency and brain connectivity was a strong determinant of placebo response. The LdlPFC functional connections seem to broadcast similar information to a multiplicity of brain regions, positively and negatively related to HF. In particular, HF mediated LdlPFC connectivity to regions that are known to respond to acute noxious stimuli such as mid cingulate, S1, S2 and M1 stimuli [1,19] predict greater placebo analgesia. The LdlPFC is strongly linked with pain modulation and placebo response. For instance, Zubeita et al. have shown that opioid binding is significantly elevated in LdlPFC in placebo responders after placebo conditioning [55]. Other studies also show that increased activity in LdlPFC is coupled with greater placebo responses [45,46,48]. In addition, a series of placebo conditioning studies in IBS patients suggest a top down influence of LdlPFC in placebo effect [16,17,38],[3,31,44]. Moreover, temporary lesions of the LdlPFC, induced with repetitive transcranial magnetic stimulation (rTMS), blocks placebo analgesia [27] suggesting a necessary and sufficient modulatory role of LdlPFC in experimental placebo responses.
In addition, we show evidence that the two complementary set of networks, the dmPFC centered and the LdlPFC centered, interact with each other to set the direction of placebo response. The level of MF oscillations in LdlPFC correlated negatively with dmPFC-insula connectivity and with affective MPQ scores suggesting that LdlPFC oscillations in this frequency band is involved in regulation of emotional processes that facilitate placebo response. In non responders on the other hand, the LdlPFC was more synchronized with the dmPFC and other higher order associative regions involved in cognition and in processing emotion. Taken together, these findings suggest that increased LdlPFC interaction with other prefrontal regions and the dmPFC connectivity with insula reflects a heightened emotional state that interferes with and diminishes the capacity of cognitive and pain modulatory networks in inducing expectation based analgesia. The specifics of these links need systematic investigation and are possibly associated with experience and conditioning in CBP patients.
Placebo response is inextricably linked to the context in which an inert treatment is administered [20,37,54] but very little has been known about placebo mechanisms in chronic pain. Chronic pain alters brain circuitry, induces maladaptive brain plasticity, and is associated with cognitive abnormalities [2,5,6,50]. Since treatment expectations and beliefs are altered in chronic pain due to each patient’s particular clinical history, and list of successful or failed treatments [11,41], it is likely that brain circuitry for placebo analgesia in chronic pain patients differs from that in healthy subjects. A recent elegant study examined predictability of placebo for thermal pain in healthy subjects using a pattern-based regression technique where estimates of whole-brain activity were generated for placebo predictability [46,47]. Brain regions involved in emotional appraisal predicted placebo analgesia, and this finding agrees with our results. On the other hand the predictive power of their approach was 2–8 times weaker than ours. The latter may be due to the use of placebo conditioning that result in placebo analgesia in a high proportion of subjects and thus reduces the spread of differences between groups. A more important difference was the subjects (healthy vs. CBP), and our success rate of accurate prediction may be a direct consequence of studying chronic pain patients.
An important limitation of this study is that it nearly half of the subjects in the CBPp and CBPd groups had received 5% Lidocaine patches. Yet, this near equal distribution of type of treatment in the two groups also insures minimal bias, especially regarding brain markers, by the type of treatment. Importantly, in the initial study we showed that the 5% lidocaine had no independent drug effect [24]. This was further confirmed by observing that only the patch treated subjects reported changes in pain when compared to a separate untreated group of CBP patients. Additionally, here we validated the primary outcomes of our placebo prediction in the CBP in a second independent cohort. Another limitation of this study is that the participants were allowed to use rescue medication (acetaminophen), the use of which was not quantified. As we did not document individual subject use of rescue medication, it is possible that the CBPd group consumed more rescue medication (up to 2 regular strength acetaminophen tablets per day). However, even in that situation, given the low efficacy of acetaminophen against chronic back pain, it is unlikely that this difference would account for a greater than 60% decrease in back pain. The marked analgesia was arguably linked with psychoneurobiological parameters that were found in this study and corroborate previous studies [17,27,34,48]. Moreover, it should be kept in mind that the predictive mechanisms discovered in this study are specific to CBP patients and may not necessarily generalize to other patient groups.
Overall, this study provides the first evidence for brain based placebo prediction in clinical populations. The evidence suggests that placebo response can be accurately predicted and is mediated by competing networks, one set of functional connections increases and another set decreases the probability of response. If these results can be replicated, and expanded into other chronic pain conditions, they should reveal brain mechanisms of placebo specific to chronic pain conditions, and also pave the way for developing more efficient clinical trial designs and for understanding the biological underpinnings of clinical trial outcomes.
Acknowledgments
We thank Judy L. Paice for help in instructing participants for proper use of therapy. We thank all participants in the study, and Apkarian lab personnel for help in various aspects of the study and insightful discussions. The study was funded by Endo Pharmaceuticals and in part by National Institutes of Health R01 NS35115. Endo Pharmaceuticals provided financial aid, lidocaine and placebo patches, but had no involvement in other aspects of the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest to declare.
References
- 1.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152:S49–S64. doi: 10.1016/j.pain.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arntz A, van Eck M, de Jong P. Avoidance of pain of unpredictable intensity. Behav Res Ther. 1991;29:197–201. doi: 10.1016/0005-7967(91)90048-8. [DOI] [PubMed] [Google Scholar]
- 4.Baliki MN, Baria AT, Apkarian AV. The cortical rhythms of chronic back pain. J Neurosci. 2011;31:13981–13990. doi: 10.1523/JNEUROSCI.1984-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baliki MN, Geha PY, Jabakhanji R, Harden N, Schnitzer TJ, Apkarian AV. A preliminary fMRI study of analgesic treatment in chronic back pain and knee osteoarthritis. Mol Pain. 2008;4:47. doi: 10.1186/1744-8069-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baria AT, Baliki MN, Parrish T, Apkarian AV. Anatomical and functional assemblies of brain BOLD oscillations. J Neurosci. 2011;31:7910–7919. doi: 10.1523/JNEUROSCI.1296-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beauregard M. Mind does really matter: evidence from neuroimaging studies of emotional self-regulation, psychotherapy, and placebo effect. Prog Neurobiol. 2007;81:218–236. doi: 10.1016/j.pneurobio.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Benedetti F. Mechanisms of placebo and placebo-related effects across diseases and treatments. Annu Rev Pharmacol Toxicol. 2008;48:33–60. doi: 10.1146/annurev.pharmtox.48.113006.094711. [DOI] [PubMed] [Google Scholar]
- 11.Benedetti F, Carlino E, Pollo A. How placebos change the patient's brain. Neuropsychopharmacology. 2011;36:339–354. doi: 10.1038/npp.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3001244. 70ra14. [DOI] [PubMed] [Google Scholar]
- 13.Campbell JN. How does topical lidocaine relieve pain. Pain. 2012;153:255–256. doi: 10.1016/j.pain.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Cauda F, D'Agata F, Sacco K, Duca S, Cocito D, Paolasso I, Isoardo G, Geminiani G. Altered resting state attentional networks in diabetic neuropathic pain. J Neurol Neurosurg Psychiatry. 2010;81:806–811. doi: 10.1136/jnnp.2009.188631. [DOI] [PubMed] [Google Scholar]
- 15.Chechko N, Wehrle R, Erhardt A, Holsboer F, Czisch M, Samann PG. Unstable prefrontal response to emotional conflict and activation of lower limbic structures and brainstem in remitted panic disorder. PLoS One. 2009;4:e5537. doi: 10.1371/journal.pone.0005537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craggs JG, Price DD, Perlstein WM, Verne GN, Robinson ME. The dynamic mechanisms of placebo induced analgesia: Evidence of sustained and transient regional involvement. Pain. 2008;139:660–669. doi: 10.1016/j.pain.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craggs JG, Price DD, Verne GN, Perlstein WM, Robinson MM. Functional brain interactions that serve cognitive-affective processing during pain and placebo analgesia. Neuroimage. 2007;38:720–729. doi: 10.1016/j.neuroimage.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 19.DaSilva AF, Becerra L, Makris N, Strassman AM, Gonzalez RG, Geatrakis N, Borsook D. Somatotopic activation in the human trigeminal pain pathway. J Neurosci. 2002;22:8183–8192. doi: 10.1523/JNEUROSCI.22-18-08183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diederich NJ, Goetz CG. The placebo treatments in neurosciences: New insights from clinical and neuroimaging studies. Neurology. 2008;71:677–684. doi: 10.1212/01.wnl.0000324635.49971.3d. [DOI] [PubMed] [Google Scholar]
- 21.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanley MA, Jensen MP, Ehde DM, Robinson LR, Cardenas DD, Turner JA, Smith DG. Clinically significant change in pain intensity ratings in persons with spinal cord injury or amputation. Clin J Pain. 2006;22:25–31. doi: 10.1097/01.ajp.0000148628.69627.82. [DOI] [PubMed] [Google Scholar]
- 24.Hashmi J, Baliki M, Chanda M, Huang L, Parks E, Schnitzer T, Apkarian V. Lidocaine patch (5%) is no more potent than placebo in treating chronic back pain when tested in a randomised double blind placebo controlled brain imaging study. Molecular Pain. 2012;8:29. doi: 10.1186/1744-8069-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalisch R, Wiech K, Critchley HD, Dolan RJ. Levels of appraisal: a medial prefrontal role in high-level appraisal of emotional material. Neuroimage. 2006;30:1458–1466. doi: 10.1016/j.neuroimage.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Kong J, Kaptchuk TJ, Polich G, Kirsch I, Vangel M, Zyloney C, Rosen B, Gollub R. Expectancy and treatment interactions: a dissociation between acupuncture analgesia and expectancy evoked placebo analgesia. Neuroimage. 2009;45:940–949. doi: 10.1016/j.neuroimage.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krummenacher P, Candia V, Folkers G, Schedlowski M, Schonbachler G. Prefrontal cortex modulates placebo analgesia. Pain. 2010;148:368–374. doi: 10.1016/j.pain.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 28.Krumova EK, Zeller M, Westermann A, Maier C. Lidocaine patch (5%) produces a selective, but incomplete block of Adelta and C fibers. Pain. 2012;153:273–280. doi: 10.1016/j.pain.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 29.Lam VY, Wallace M, Schulteis G. Effects of lidocaine patch on intradermal capsaicin-induced pain: a double-blind, controlled trial. J Pain. 2011;12:323–330. doi: 10.1016/j.jpain.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- 31.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 32.Lu HC, Hsieh JC, Lu CL, Niddam DM, Wu YT, Yeh TC, Cheng CM, Chang FY, Lee SD. Neuronal correlates in the modulation of placebo analgesia in experimentally-induced esophageal pain: a 3T-fMRI study. Pain. 2010;148:75–83. doi: 10.1016/j.pain.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Malinen S, Vartiainen N, Hlushchuk Y, Koskinen M, Ramkumar P, Forss N, Kalso E, Hari R. Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proc Natl Acad Sci U S A. 2010;107:6493–6497. doi: 10.1073/pnas.1001504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Napadow V, Dhond RP, Kim J, LaCount L, Vangel M, Harris RE, Kettner N, Park K. Brain encoding of acupuncture sensation--coupling on-line rating with fMRI. Neuroimage. 2009;47:1055–1065. doi: 10.1016/j.neuroimage.2009.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicolaou A, Nicholson B, Hans G, Brasseur L. Outcome predictors for treatment success with 5% lidocaine medicated plaster in low back pain with neuropathic components and neuropathic pain after surgical and nonsurgical trauma. J Pain Res. 2011;4:25–38. doi: 10.2147/JPR.S15534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 37.Pollo A, Amanzio M, Arslanian A, Casadio C, Maggi G, Benedetti F. Response expectancies in placebo analgesia and their clinical relevance. Pain. 2001;93:77–84. doi: 10.1016/S0304-3959(01)00296-2. [DOI] [PubMed] [Google Scholar]
- 38.Price DD, Craggs J, Verne GN, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127:63–72. doi: 10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- 40.Straube T, Mentzel HJ, Miltner WH. Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biol Psychiatry. 2006;59:162–170. doi: 10.1016/j.biopsych.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat Med. 2010;16:1277–1283. doi: 10.1038/nm.2229. [DOI] [PubMed] [Google Scholar]
- 42.Vase L, Robinson ME, Verne GN, Price DD. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients. An empirical investigation. Pain. 2003;105:17–25. doi: 10.1016/s0304-3959(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 43.Vase L, Robinson ME, Verne GN, Price DD. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain. 2005;115:338–347. doi: 10.1016/j.pain.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 44.Villemure C, Bushnell MC. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95:195–199. doi: 10.1016/S0304-3959(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 45.Wager TD. Expectations and anxiety as mediators of placebo effects in pain. Pain. 2005;115:225–226. doi: 10.1016/j.pain.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 46.Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J Neurosci. 2011;31:439–452. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 48.Watson A, El-Deredy W, Iannetti GD, Lloyd D, Tracey I, Vogt BA, Nadeau V, Jones AK. Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain. 2009;145:24–30. doi: 10.1016/j.pain.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wehrfritz A, Namer B, Ihmsen H, Mueller C, Filitz J, Koppert W, Leffler A. Differential effects on sensory functions and measures of epidermal nerve fiber density after application of a lidocaine patch (5%) on healthy human skin. Eur J Pain. 2011;15:907–912. doi: 10.1016/j.ejpain.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Weissman-Fogel I, Moayedi M, Tenenbaum HC, Goldberg MB, Freeman BV, Davis KD. Abnormal cortical activity in patients with temporomandibular disorder evoked by cognitive and emotional tasks. Pain. 2011;152:384–396. doi: 10.1016/j.pain.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 51.Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 52.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 53.Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zubieta JK, Stohler CS. Neurobiological mechanisms of placebo responses. Ann N Y Acad Sci. 2009;1156:198–210. doi: 10.1111/j.1749-6632.2009.04424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zubieta JK, Yau WY, Scott DJ, Stohler CS. Belief or Need? Accounting for individual variations in the neurochemistry of the placebo effect. Brain Behav Immun. 2006;20:15–26. doi: 10.1016/j.bbi.2005.08.006. [DOI] [PubMed] [Google Scholar]