Abstract
Cellular adaptive response to certain low level genotoxic stresses including the exposure to low dose ionizing radiation (LDIR) shows promise as a tool to enhance radioprotection in normal cells but not in tumor cells. Manganese superoxide dismutase (MnSOD), a fundamental mitochondrial antioxidant in mammalian cells plays a key role in LDIR-induced adaptive response. In this study, we aim to elucidate the signaling network associated with the MnSOD-induced radiation protection. A MnSOD-interacting protein profile was established in LDIR-treated human skin cells. Human skin keratinocytes (HK18) were irradiated with a single dose LDIR (10 cGy x-ray) and the cell lysates were immunoprecipitated using α-MnSOD and applied to two different gel-based proteomics followed by mass spectrometry for protein identification. Analysis of the profiles of MnSOD interacting partners before and after LDIR detected different patterns of MnSOD protein-protein interactions in response to LDIR. Interestingly, many of the MnSOD interacting proteins are known to have functions related to mitochondrial regulations on cell metabolism, apoptosis and DNA repair. These results provide the evidence indicating that in addition to the enzymatic action detoxifying superoxide, the antioxidant MnSOD may function as a signaling regulator in stress induced adaptive protection through cell survival pathways.
Keywords: MnSOD, protein interaction, low dose radiation, adaptive response, human keratinocytes
Introduction
Humans are repeatedly exposed to low doses of ionizing radiation (LDIR) present in the environment and through use of medical and industrial radioactive materials [1, 2]. Accumulating evidence suggests that exposure to LDIR induces an adaptive radioresistance response to subsequent more severe genotoxic conditions in healthy cells [3–5]. Interestingly LDIR does not show the same adaptive response in tumor cells [6], probably due to low or lack of manganese superoxide dismutase (MnSOD, SOD2) activity in many human cancers. Reconstitution of MnSOD expression in the MnSOD-deficient tumor cells results in the suppression of malignant phenotype [7–10]. Thus, it is reasonable to propose that LDIR activates specific proteins in normal cells, which provide increased cellular tolerance to more severe genotoxic damage such as high-dose irradiation.
MnSOD is the primary antioxidant enzyme in the mitochondria of mammalian cells to detoxify superoxide generated as byproducts of oxidative phosphorylation and oxidative stress conditions [11]. MnSOD has been shown to play a key role in providing this radioresistant effect by reducing the number of toxic superoxide (reactive oxygen species, ROS) formed after radiation [5, 12]. MnSOD helps to eliminate free radicals in the mitochondria by converting superoxide anions to H2O2, which can then be detoxified by the catalase activity of peroxidase [13]. Loss of MnSOD expression or its enzymatic activity leads to a high risk of tumorigenesis [14, 15] and high sensitivity of cells to genotoxic condition including ionizing radiation [16]. In addition, mice with MnSOD haploinsufficiency have abnormal mitochondrial function correlated with increased oxidative damage [17]. In addition, MnSOD gene expression and enzymatic activity can be induced by LDIR in mouse skin epithelial cells [5] and in the irradiated heart and intestine tissues [18]. Overexpression of MnSOD protects mouse epithelial tissues from radiation injury [19], enhances protection of the cells from apoptosis by maintaining the mitochondrial membrane [20], TNF signaling [21] and also inhibits the malignant phenotype of several human tumor cell lines [8, 22]. Moreover, targeting MnSOD has been suggested in cancer growth control [23]. All this evidence has led to the concept that MnSOD plays a fundamental role in regulation of cell proliferation [14, 23–25]; whereas the exact mechanism underlying MnSOD-mediated cell growth alteration, especially under stress-induced adaptive response remains to be elucidated.
To further elucidate MnSOD-mediated homeostatic function, in this study, we aim to decipher potential networks of MnSOD in LDIR-induced adaptive protection. We demonstrate a unique MnSOD interactome using human skin keratinocytes treated with a single dose 10 cGy x-ray. MnSOD is found to be able to interact with a large scale of cellular and mitochondrial proteins with varied functions involved in cellular metabolism, apoptosis and DNA repair. Thus, in addition to its primary function as superoxide dismutase, MnSOD may act as central signaling molecule in regulation of cellular response under LDIR-induced adaptive radioprotection.
Materials and methods
Cells and LDIR
Human keratinocytes immortalized by infection with HPV18 genome (HK18) [26, 27] were cultured in DMEM with 10% fetal bovine serum until 80% confluent. Designated cells were irradiated with 10 cGy ionizing radiation at room temperature using a Cabinet X-ray System Faxitron Series (dose rate: 0.028 Gy/min; Hewlett Packard, McMinnville, OR) and incubated for 6 hr before being harvested. Cells were collected by centrifugation (2000 rpm, 5 min) and resuspended in lysis buffer (20 mM Tris, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 μg/ml leupeptin, 1 mM PMSF). After sonication for 30 sec, samples were centrifuged at 14,000 rpm for 10 min to sediment cellular debris. Protein concentrations for the supernatants were obtained using Precision Red Advanced Protein reagent (Cytoskeleton, CO).
Clonogenic survival assay
When cells reached 70% confluency they were subjected to sham or radiation treatment. Cells were then rinsed with phosphate buffered saline, trypsinized, and counted in a single cell suspension. Different cell numbers (for 0 Gy and 10 cGy treatment: 500, 1000, 1500 cells; for 10 cGy + 5 Gy and 5 Gy treatment: 2000, 4000, 8000 cells) were seeded into a 60mM plates in triplicate. After incubation for 12 days, growth medium was removed and cells were fixed and stained with 2 ml Coomassie blue solution for 5 min. Then plates were rinsed with distilled water, all plates were counted for total colony number per plate. Clonogenic survival was calculated by normalizing the radiation treatment groups to the plating efficiency of sham treated cells. Data is an average three independent biological replicates, each with three technical replicates.
Immunoprecipitation (IP)
Total protein lysate (300 μg) was subjected to 1 hr pre-clearing with mouse normal IgG-AC (Santa Cruz, CA), followed by overnight incubation with 2 μg α-MnSOD antibody (Santa Cruz) and 50 μl Protein A beads. The beads were washed with PBS, collected by centrifugation and protein eluted by adding 30 μl SDS-PAGE loading buffer (0.3 M Tris pH 6.8, 50% glycerol, 5% SDS). IP products in SDS-PAGE loading buffer were heated to 100°C for 5 min and separated on pre-cast 4–20% Tris-glycine SDS-PAGE gel (Invitrogen, CA). The gel was stained with Sypro Ruby protein stain (Invitrogen) and the fluorescent image was detected on Typhoon 9410 laser scanner.
2D-Difference gel electrophoresis (2D-DIGE)
IP products were eluted in DIGE labeling buffer (7 M urea, 2 M thiourea, 2% CHAPS, 30 mM Tris) were separated in 2 dimensions according to GE Life Sciences Ettan DIGE system protocol. Briefly, each sample was minimally labeled with 1 μl of 1 mM Cy3 or Cy5 for 30 min. Labeling reactions were stopped by the addition of 1 μl of 1 mM lysine. The samples were pooled together and added to rehydration buffer (7 M urea, 2 M Thiourea, 2% CHAPS, 1.2% destreak, 1% pharmalytes). A final volume of 185 μl sample was loaded onto 11 cm ph3-10NL Bio-rad IEF strip and focused by passive overnight rehydration, followed by active isoelectric focusing for a total of 35,000 Vhrs. Strips were equilibrated with 10 mg/ml DTT for 15min followed by 25 mg/ml for 15 min with iodoacetamide, then applied to 8–16% Tris-glycine gel for 2nd dimension separation. The resulting CyDye labeled protein gel was imaged on Typhoon 9410. The gel was post-stained with sypro ruby protein stain for visualization during spot picking.
In-gel digestion and MALDI-TOF/TOF
Protein bands/spots of interest were robotically excised, using an EXQuest Spot Cutter (Bio-Rad), from Sypro Ruby stained gels. Gel plugs were destained in 100 mM ammonium bicarbonate for 1 hr and dehydrated with 100% acetonitrile (ACN). For the SDS-PAGE gel only, gel plugs were reduced by rehydrating plugs with 1 mM DTT and applying 56°C heat for 30 min, alkylated with 55 mM iodoacetamide at RT for 20 min and dehydrated again with 100% ACN. All gel plugs were rehydrated with 10 ng/μl trypsin to allow for overnight digestion. Tryptic peptides were extracted by washing the plugs 3 times for 5 min 10 μl with 0.1% TFA in 60% ACN and subsequently speed vacuumed down to 5 μl. One μl sample was mixed with 1 μl α-cyano-4-hydroxycinnamic acid (CHCA) matrix and analyzed in reflector mode by an ABI 4700 MALDI-TOF/TOF. The MS and MS/MS data was acquired and searched against the IPI human database using MASCOT server with the following search criteria: 100 ppm MS tolerance, 0.8 Da MS/MS tolerance, up to 2 missed cleavages, fixed modification (carbamidomethyl), and variable modifications (phospho (ST), phosphor (Y), oxidation (M), pyro-glu (N-term-E), pyro-glu (N-term-Q)). Identified proteins were determined to localize in the mitochondria according to the human mammalian protein localization online database (mitochondrial matrix, inner and outer membranes were included).
MnSOD enzymatic activity assay
Exponentially growing HK18 cells were irradiated with LDIR (10 cGy X-ray) or sham irradiation (control) and cell lysate was prepared 6 hr after irradiation with 50 mmol/L potassium phosphate buffer containing 1.34 mmol/L diethylenetriaminepentaacetic acid and protein concentration was determined. MnSOD activity was determined following the procedure as previously described [28].
Co-Immunoprecipitation (Co-IP)
Cells treated with 10 cGy radiation and collected at 6 hr after were immunoprecipitated with MnSOD antibody (mouse or rabbit, Santa Cruz). Precipitants were further subjected to Western Blot analysis. Antibodies used are BCL2 (Santa Cruz), RAD51 (Santa Cruz), NDUFA10 (GeneTex, TX), HK2 (Millipore, MD), CDK11A (AbCam, MA). Normal IgG was used as a negative control. Whole cell lysate without IP was used as positive control.
Function and interactional database analysis
To create the functionally categorized pie chart, UniProt ID’s from the list of 82 putative interacting partners, generated from the mass spectrometry data, were presented to the online tool GORetriever to get their GO annotations. The online plugin tool, CateGOrizer, used these annotations to categorize the proteins according to a custom classification list (Supplemental Table S1). For the protein-protein interaction map, the 82 interacting proteins were submitted to the online STRING database and a resulting protein network was created. This network is derived from published literature describing experimentally studied interactions and genome wide analysis that uses gene neighboring models and genetic profiling.
Results and discussion
Potential effectors of MnSOD in LDIR-induced radioprotection
In order to determine all the potential proteins that may interact with MnSOD, we performed α-MnSOD IP experiments with cellular lysates of control and LDIR treated HK18 cells. A flow chart of the experimental design and sample correction are illustrated in Fig. 1A. Briefly, cells received either no treatment or LDIR (10 cGy) that has been identified to induce an adaptive radioprotection in the HK18 cells [29] (Fig. 4C) were collected 6 hr after radiation. Cell lysates were immunoprecipitated using α-MnSOD and the IP products were collected and analyzed using gel-based proteomic experiments. Firstly, the MnSOD-IP product was separated by SDS-PAGE and stained with Sypro Ruby protein stain (Fig. 1B). Ten distinct bands in each lane were observed, excluding IgG bands, and excised for protein identification by mass spectrometry. A total of 71 proteins were identified from these bands. Secondly, the MnSOD-IP product was examined by 2-D DIGE (Fig. 1C). A total of 321 protein spots were identified in the gel and 30 differential spots were excised for identification by MALDI-TOF/TOF. These 30 spots yielded 11 unique proteins (~20 spots were identified as the same protein, arginine and glutamate-rich protein 1).
Fig. 1.
Experimental design for MnSOD interactome analysis. Human skin keratinocytes line HK18 was cultured to 80% confluence and subjected to no treatment (control) or LDIR (a single dose 10 cGy x-ray). Total protein lysate was collected 6 hr after LDIR and separated by SDS-PAGE gel (“Experiment #1”) and by 2D-DIGE (“Experiment #2”). Gel bands/spots were robotically excised, trypsin digested and analyzed for identification by MALDI-TOF/TOF. Proteins identified from both experiments were used for further analysis.
Fig. 4.
Validation of LDIR-activated MnSOD enzymatic activation and interactions with key effectors. (A) HK18 cells were either left untreated (0 hr) or exposed to LDIR of 10 cGy of x-ray and MnSOD enzymatic dismutase activity was measured (n = 3; **p<0.01). (B) Clonogenic survival assay. HK18 cells were subjected to the following radiation treatment: sham, 10 cGy, 10 cGy followed by 5 Gy at 6 hr later, only 5 Gy. Clonogenic survival was measured on day 12 after last irradiation. Three individual experiments were conducted; each with 3 technical replicates; the average clonogenic surviving fraction was used for graphing. The counts were normalized with the clone numbers observed in sham-irradiated cells (*p=0.02). (C) Key MnSOD-protein interactions were detected and confirmed by IP assays conducted with cell lysate 6 hr after LDIR followed by Western immunoblotting with antibodies to 5 indicated proteins selected from the MnSOD interactome; including effectors in different cellular function in both mitochondrial and non-mitochondrial categories. Whole cell lysate without IP was used as a positive control and IgG was used as a negative control.
In total, 82 distinct proteins were identified as putative partners that can interact with MnSOD (71 identified via SDS-PAGE, 11 identified via 2D-DIGE). The MnSOD interacting effector proteins are listed in Tables 1–3 according to their relative abundance in control samples compared to LDIR treated cells. Table 1 shows 16 proteins (19.5%) that were found to have similar expression between the sham and LDIR-treated cells (−1.5<fold change>1.5). A majority of the proteins showed increased or decreased expression which correlates to differences in the amount of interaction with MnSOD before/after LDIR treatment. Table 2 displays a total of 32 unique proteins (39%) which showed a decrease in the amount of MnSOD interaction after LDIR, whereas a similar number (34 proteins, 41.5%) showed an increased level of interaction after LDIR (Table 3). These findings provide the evidence indicating that MnSOD is capable of interacting with an array of cellular and mitochondrial proteins. In addition, cells are able to shift such MnSOD-interactome profiles under LDIR-induced adaptive response, suggesting that MnSOD is able to function as a signaling protein in adaptive response.
Table 1.
Proteins were identified from both gel-based proteomic experiments to reveal putative binding partners.
| IPI # | Gene name | Description | Number of peptides/spot volume in Control | Number of peptides/spot volume in LDIR treated | Mitochondrial proteins |
|---|---|---|---|---|---|
| IPI00911038 | ACIN1 | Apoptotic chromatin condensation inducer in the nucleus | 22 | 16 | |
| IPI00021439 | ACTG1 | actin | 33140000 | 23367000 | |
| IPI00413671 | BCLAF1 | BCL2 associating transcription factor 1 | 17 | 23 | |
| IPI00746232 | CCDC112 | Coiled coil domain containing protein 112 | 1077000 | 1540000 | |
| IPI00939566 | CCDC123 | Centrosomal protein of 89kDa | 11 | 15 | |
| IPI00010218 | CYP20A | Cytochrome P450 monooxygenase | 1 | 9 | |
| IPI00654605 | MAP7D1 | MAP7 domain containing protein 1 | 19 | 25 | |
| IPI00395973 | MFN1 | Mitofusin-1 | 16 | 18 | X |
| IPI00921950 | MRRF | Ribosome recycling factor | 25 | 17 | X |
| IPI00029561 | NDUFA10 | NADH dehydrogenase 1 | 1 | 10 | X |
| IPI00032201 | RAD51 | DNA repair protein RAD 51 | 6 | 10 | X |
| IPI00470906 | PHACTR2 | phosphotase and actin regulator 2 | 19240000 | 22770000 | |
| IPI00021743 | PhLOP2 | 6178000 | 8045000 | ||
| IPI00969099 | SRRM5 | serine/arginine repetitive matrix protein 5 | 23 | 34 | |
| IPI00552909 | THOC2 | THO complex 2 | 12 | 10 | |
| IPI00964265 | TNIP3 | TNFAIP3 interacting protein 3 | 12 | 36 |
This table shows proteins with similar expression levels after LDIR (cut off 1.5 fold changes).
Proteins ID’s originated from 2D-DIGE, therefore spot volumes are used for comparison.
Table 3.
Proteins were identified from both gel-based proteomic experiments to reveal putative binding partners.
| IPI # | Gene name | Description | Number of peptides/spot volume in Control | Number of peptides/spot volume in LDIR treated | Mitochondrial proteins |
|---|---|---|---|---|---|
| IPI00217193 | ANKRD11 | Medulloblastoma antigen | 15 | 23 | |
| IPI00478834 | ARGLU1 | arginine and glutamate-rich protein 1 | 0 | 16 | |
| IPI00909493 | CBARA1 | Isoform 5 of calcium uptake protein 1 | 0 | 12 | |
| IPI00217357 | CCAR1 | Isoform 1 of cell division cycle and apoptosis | 0 | 33 | |
| IPI00216511 | CDC25B | M-phase inducer phosphotase | 0 | 15 | |
| IPI00301923 | CDK9 | Isoform 1 of cyclin dependent kinase 9 | 0 | 12 | |
| IPI00966223 | CDK11A | cyclin dependent kinase 11A | 6960000 | 10780000 | |
| IPI00784201 | CEP290 | Centrosomal protein of 290kDa | 21 | 32 | |
| IPI00815832 | CNTLN | Centlein | 19 | 97 | |
| IPI00376004 | COX8 | Cytochrome c oxidase subunit 8C | 0 | 4 | X |
| IPI00966243 | CYB5B | 0 | 1 | ||
| IPI00148820 | DTX3 | Protein deltex-3 | 0 | 4 | |
| IPI00181684 | ERC1 | ELKS/Rab6-interacting/CAST family member 1 | 26 | 79 | |
| IPI00003921 | EPB41 | Isoform 1 of Protein 4.1 | 0 | 1 | |
| IPI00006217 | GRIN2D | Glutamate receptor subunit | 0 | 1 | |
| IPI00029557 | GRPEL1 | GrpE protein homolog 1 | 0 | 1 | X |
| IPI00217469 | HIST1H1A | Histone H.1 | 0 | 10 | |
| IPI00102864 | HK2 | Hexokinase 2 | 0 | 13 | X |
| IPI00023029 | IDH1 | NADP specific isocitrate dehydrogenase | 0 | 1 | |
| IPI00031485 | MRP63 | Ribosomal protein 63 | 0 | 4 | X |
| IPI00414431 | NF2 | Isoform 1 of Merlin | 0 | 12 | |
| IPI00166807 | OXR1 | Isoform 2 of Oxidation resistance protein | 0 | 1 | X |
| IPI00954590 | PANK2 | Isoform 4 of Panthothenate kinase 2 | 0 | 1 | |
| IPI00915357 | PDK3 | Pyruvate dehydrogenase kinase isozyme 3 | 0 | 43 | X |
| IPI00554660 | PIPOX | Peroximal sarcosine oxidase | 0 | 12 | |
| IPI00789417 | PPP2R2A | Protein phosphotase 2 regulatory subunit A | 0 | 1 | |
| IPI00376974 | PPP2R2B | Protein phosphotase 2 regulatory subunit B | 0 | 11 | |
| IPI00305282 | RAD50 | DNA repair protein RAD50 | 0 | 82 | |
| IPI00783594 | RNPS1 | Isoform 3 of RNA-binding protein with serine rich domain 1 | 0 | 9 | |
| IPI00007004 | RRP15 | RRP15 like protein | 821100 | 1635000 | |
| IPI00022302 | SNX24 | sorting nexin 24 | 427900 | 859800 | |
| IPI00399264 | TPD52 | Tumor protein D54 | 0 | 12 | |
| IPI00018744 | TRADD | Tumor necrosis factor receptor | 0 | 1 |
This table shows the proteins with increased expression levels after LDIR treatment (cut off < 1.5 fold change) with enhanced interaction after LDIR.
Table 2.
Proteins were identified from both gel-based proteomic experiments to reveal putative binding partners.
| IPI # | Gene name | Description | Number of peptides/spot volume in Control | Number of peptides/spot volume in LDIR treated | Mitochondrial proteins |
|---|---|---|---|---|---|
| IPI00217193 | ANKRD11 | Medulloblastoma antigen | 5795000 | 1954000 | |
| IPI00478834 | ARGLU1 | arginine and glutamate-rich protein 1 | 18830000 | 8090000 | |
| IPI00007266 | ALAS1 | 5-aminoevulinase synthase | 23 | 12 | X |
| IPI00032450 | ASPH | aspartyl/asparaginyl beta-hydroxylase | 8 | 0 | |
| IPI00456747 | ATP5F1 | ATP synthase | 15 | 0 | X |
| IPI00031653 | BRMS1L | breast cancer metastasis supressor 1 like protein | 8 | 0 | |
| IPI00966223 | CDK11A | cyclin dependent kinase 11A | 14 | 9 | X |
| IPI00887632 | CEP110 | centriolin | 35 | 0 | |
| IPI00889534 | CPS1 | carbamoyl phosphate synthetase variant | 25 | 0 | X |
| IPI00006725 | DDX23 | probable ATP dependent RNA helicase DDX23 | 12 | 0 | |
| IPI00007052 | FIS1 | Mitochondrial fission 1 protein | 1 | 0 | X |
| IPI00008552 | GLRX3 | glutaredoxin-3 | 20 | 0 | X |
| IPI00642320 | HMGN5 | nucleosomal binding protein 1 | 2319000 | 1714000 | |
| IPI00021363 | KDM5A | Lysine specific demethylase 5A | 20 | 0 | |
| IPI00965617 | MAGED4B | melanoma associated antigen D4 | 1 | 0 | |
| IPI00943073 | IK | Protein Red | 14 | 0 | |
| IPI00172591 | MRPL17 | 39S ribosomal protein L17 | 8 | 0 | X |
| IPI00797306 | MRPL25 | 39S ribosomal protein L22 | 7 | 0 | X |
| IPI00644704 | MRPL55 | 39S ribosomal protein L55 | 8 | 0 | X |
| IPI00292709 | PCK1 | phosphoenolpyruvate carboxykinase | 1 | 0 | X |
| IPI00646074 | PIK3C2B | phophoinositide-3-kinase, class 2 | 8 | 0 | |
| IPI00021363 | PNKD | Lysine specific demethylase 5A | 5 | 0 | |
| IPI00646374 | PRPF38B | pre-mRNA splicing factor 38B | 10 | 0 | |
| IPI00383449 | RAB15 | Ras-related protein Rab-15 | 22 | 0 | |
| IPI00375358 | RFC1 | Replication factor C subunit 1 | 1342000 | 716100 | |
| IPI00328293 | SRRM1 | serine/arginine repetitive matrix protein 1 | 29 | 19 | |
| IPI00867672 | SRSF5 | serine/arginine rich splicing factor 5 | 9 | 0 | |
| IPI00640515 | TAOK2 | 36 | 0 | ||
| IPI00104050 | THRAP3 | thyroid hormone receptor associated protein 3 | 24 | 0 | |
| IPI00514206 | TIMM23 | Translocase of inner mitochondrial membrane 23 | 9 | 0 | X |
| IPI00855862 | TRAF3IP1 | TRAF3 interacting protein 1 | 19 | 0 | |
| IPI00301719 | TRNT1 | tRNA nucleotidyltransferase 1 | 14 | 0 | X |
This table shows proteins with decreased expression levels after LDIR treatment (cut off < −1.5 fold change) and less interaction with MnSOD after LDIR.
MnSOD interaction proteins with metabolic functions
The UniProt identification numbers of all 82 MnSOD-interacting proteins (Tables 1–3) were submitted to a functional genomics resource tool, GORetriever, to obtain their current GO (gene ontology) annotations. These annotations were categorized by molecular functions using the web tool CateGOrizer according to a custom classification list (Supplemental Table S1). The pie chart with protein functional categories (Fig. 2) shows a wide variety of cellular and molecular functions for the identified proteins. Interestingly, the category containing the largest number of effectors is linked to cellular metabolism (GO: 0008152, 30.77%), including ALAS1, HK2, RNPS1, DDX23, RBMX, CCAR1, PCK1, IDH1, NDUFA10 and MFN1. HK2, a glycolytic enzyme, has been shown to promote a shift in ATP production from oxidative phosphorylation to aerobic glycolysis in tumor cells [30], which increases tumor cell resistance to apoptosis and promotes cell growth [31]. Several environmental factors can cause increased cellular expression of HK2. For example, the presence of insulin can cause HK2 to localize from the cytosol into the mitochondria and this location is more favorable for aerobic glycolysis [32]. Therefore the following chain of events is reasonable, specific unknown cellular triggers cause HK2 to relocate to the mitochondria, which causes a shift in ATP production from oxidative phosphorylation to aerobic glycolysis, helping tumor cells to resist apoptosis after radiation. It is plausible that a similar sequence of events occurs in healthy cells. LDIR could be the trigger for increased localization of HK2 in the mitochondria, where our data confirms its encounter with MnSOD after LDIR treatment (Figs. 4C and 5). The potential cooperation of HK2 with MnSOD within mitochondria may be responsible for the enhanced apoptotic resistance and could contribute to the overall LDIR adaptive response.
Fig. 2.
UnitProt ID’s were provided for GORetriever to retrieve existing GO annotations for all proteins identified by MALDI-TOF/TOF. Subsequently, GO annotations were functionally categorized in CateGOrizer using a custom classification list (Supplemental Table 1). A pie chart is shown to display the percent of GO annotations that were listed for each GO category. A large number of putative binding partners of MnSOD were found to have functions relating to DNA repair/binding/replication and cell growth/death/apoptosis. These results indicate that MnSOD may act as a signaling mediator to activate the cellular adaptive response induced by LDIR.
Fig. 5.
A list of UniProt IDs for all putative binding partners, plus MnSOD, was submitted to STRING database. A functional protein association network was created using parameters: custom confidence level 0.24 and showing no more than 5 integrators. The type of protein-protein association (neighborhood, gene fusion, concurrence, experimental data, databases, text mining) corresponds to the connection color. Protein color clustering was performed using KMEANS. Protein-protein interactions verified by immunoblotting analysis for selected proteins were highlighted by red boxes. This map displays a potential overall protein connection of MnSOD and its putative signaling networks in LDIR-induced radioprotection in human skin keratinocytes.
MnSOD interaction partners in DNA repair network
The group of MnSOD interacting proteins, RAD50, RAD51, RFC1, OXR1, CBARA1, ACTB and SRSF1, in Fig. 2 were functionally categorized in stress response (GO: 0006950) and are also well known in genotoxic derived DNA repair. In fact, OXR1 (oxidation resistance protein) is recognized in the prevention and repair of oxidative damage caused by ROS [33] although its mechanisms are to be elucidated. OXR1 is shown to be induced by oxidative stress, which can cause it to localize to the mitochondria and allows for protection against further oxidative damage [33]. Due to electron transport activities during respiration, mitochondria have naturally high ROS levels. Our data shows that MnSOD interacts with components of the mitochondrial respiration chain, NDUFA10 by mass spectrometry data (Table 1) and NDUFA11 by Co-IP (Fig. 4C). MnSOD must be in close proximity to the respiration chain so that it can detoxify the released free radicals, created by oxidative phosphorylation and interact with chain proteins. Thus, our finding of MnSOD interaction with OXR1, NDUFA10 and NDUFA11 could imply that LDIR-induced oxidative stress could cause OXR1 to relocate to the mitochondria where they cooperate with MnSOD at the site of the respiration chain to prevent the accrual of toxic radicals and subsequent DNA damage. It warrants a further investigation on whether the antioxidant function of MnSOD and OXR1 could be mutually activated.
Proteins RAD50, RAD51 and RFC1 (GO:0006281 DNA repair), all actively involved in DNA repair, and NF2 (GO:0006260 DNA synthesis/replication), the DNA replication regulator, were also found to have the potential to interact with MnSOD. Activation of DNA repair pathways has been well established in adaptive radioprotection. It is believed that LDIR is able to activate DNA repair mechanism in healthy cells as a mean of preparing for later more severe genotoxic damage [34–36]. In human lymphocytes, the expression of RAD50 is increased 2.5 hr after LDIR treatment when compared to sham irradiated controls [37]. In addition, adenoviral proteins can impair DNA repair machinery MRN complex (composed of proteins MRE11, RAD50 and NBS1) enough to lead to buildup of DNA damage and cause cell death. In fact, another group has reported that MnSOD interacts with H2AFX, a well-known histone family member localized in the nucleus [38]. H2AFX can interact with RAD50 [39] and cause the recruitment of DNA repair factors, giving it an essential role in DNA repair, replication and overall chromosome stability necessary after damage induced by IR [40–42]. Similarly, Ganesan et al discovered that MnSOD could be a SUMO1 substrate in melanoma cells [43]. SUMO1 (also known as UBL1) regulates double-stranded DNA repair by forming a complex with RAD51 and RAD52 [43–45]. Together with our current data, these findings suggest that MnSOD is involved in the LDIR induced DNA repair pathways and apoptosis regulation, possibly by acting as a signaling molecule or as an integral component of the adaptive response cascade, potentially even from within mitochondria or by acting on DNA through a signal transduction mechanism.
The communication of MnSOD with these well-known DNA repair proteins supports the concept that mitochondrial DNA damages are repairable and MnSOD is involved. Mitochondrial DNA is believed to be more susceptible to oxidative damage by ROS than nuclear DNA due to its lack of shielding histones [46]. Although the exact mechanism regulating the mitochondrial DNA repair system has been controversial although some evidence has been provided [46–49], our current data support the concept that MnSOD-related ability to prevent and repair mitochondrial DNA damages may contribute to the overall mitochondrial functions. Previously, overexpression of MnSOD was shown to protect mitochondrial DNA from ROS-induced damage [50, 51], but the details of this cellular mechanism are unknown. Histone H1 is well known in providing nucleosome structure for nuclear DNA. Recent studies show that isoform Histone 1.2 can convey apoptotic signals to the mitochondria in response to a DNA double-stranded break [52], or even translocate to the mitochondria after induced apoptosis [52, 53]. In agreement with these reports, our data demonstrate that MnSOD interacts with Histone H1 at a higher level after being induced by LDIR (Fig. 3), confirmed with Co-IP in Fig. 4C. Therefore, it is plausible that MnSOD interacts with Histone H1 and H2AFX to mediate or initiate DNA structure to allow for repair. The protein interactions found in this report may shed additional light on this mechanism and suggest that LDIR triggers MnSOD protein interactions to protect the cell from mitochondrial DNA damage and to enable the repair by interacting and communicating with RAD50, RAD51 and RFC1.
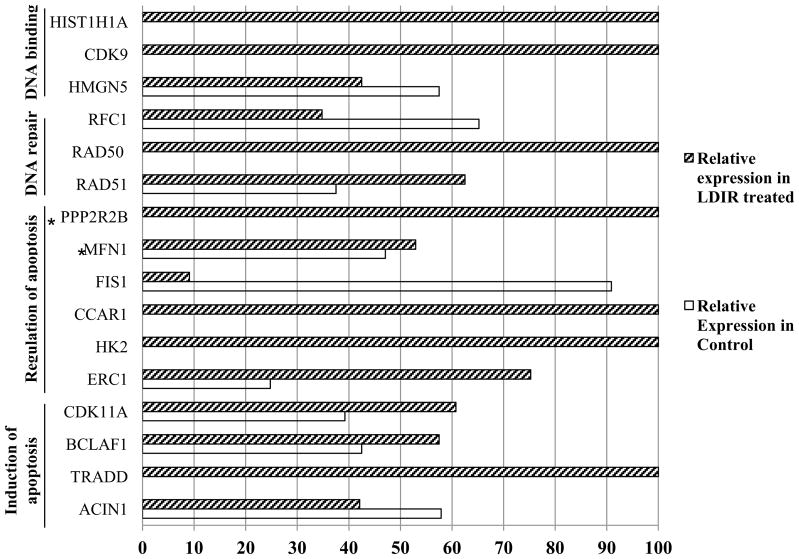
Fig. 3.
Proteins with GO annotations in the following categories (induction of apoptosis GO:0006917, regulation of apoptosis GO: 0042981, DNA repair GO:0006281, DNA binding GO:0003677) were graphed according to their relative abundance in control samples and after LDIR treatment. This shows the immense variation in MnSOD levels of interaction with these important survival related proteins. *Indicates mitochondrial protein.
MnSOD’s involvement in LDIR-induced apoptosis
Many of MnSOD’s identified interacting partners were functionally classified into apoptosis categories (GO:0008219, cell death; GO:0006915, apoptosis; GO:0042981 regulation of apoptosis; GO:0006916 anti-apoptosis; GO:0045767 regulation of anti-apoptosis; GO:0006917, induction of apoptosis), PP2R2B, MFN1, FIS1, CCAR1, HK2, ERC1, CDK11A, BCLAF1, TRADD and ACIN1. Fig. 3 shows the variance in their interaction levels with MnSOD after being treated with LDIR. Some of these proteins are actively involved in the mitochondrial regulation on apoptosis. For example, BCL2 can inhibit the release of cytochrome c [54, 55], which prevents triggering of apoptosis and MnSOD averts apoptosis by reducing ROS levels [56–58]. Interestingly, our co-IP analysis confirmed the interaction between BCL2 and MnSOD (Fig. 4C). MFN1 and FIS1 are known to cooperate to regulate mitochondrial morphology, which is in constant fluctuation during the process of apoptosis [59]. CDK11 is shown to relocate to the mitochondria and linked with mitochondrial apoptosis. Overexpression of CDK11 inhibits protein synthesis and induces apoptosis, while silencing CDK11 blocks mitochondrial cytochrome c release and inhibits apoptosis [60]. All of the evidence in conjunction with our findings suggests a unique new function of MnSOD that allows cells to cope with the stress under certain low level genotoxic condition via MnSOD-regulated anti-apoptotic pathways.
Increased MnSOD enzymatic activity and interaction with key effectors after LDIR
In agreement with reported results [5, 20, 61], MnSOD enzymatic activity was 3-fold increased in LDIR-treated cells compared to sham control cells (Fig. 4A) with enhanced clonogenic survival when exposed to the lethal high dose 5 Gy irradiation (Fig. 4B). Thus, LDIR is able to trigger MnSOD so as not only to become more active enzymatically, but also to induce prosurvival network. Five major effectors with different cellular functions including BCL2, RAD51, NFUFA10, HK2 and Histone H1, were further confirmed to interact with MnSOD by Co-IP analysis (Fig. 4C).
MnSOD’s protein-association map reveals a complex yet closely associated protein web
We submitted the putative MnSOD interacting partners (Tables 1–3) to STRING 9.0 database that is a search tool for the retrieval of interacting genes/proteins and utilizes a variety of different sources such as, experimental data, computational predictions, publication text mining, curated databases and pathway analysis, to associate proteins into a network. The close clustering of the majority of the identified proteins is displayed as a protein-association map shown in Fig. 5. Protein color clustering was performed using KMEANS statistical calculations, which resulted in proteins of the same color having the closest associations. The protein-protein interaction map created by the database provides a systematical interpretation of our proteomic data. The tight association of these proteins, many of them have not been reported in the mitochondria, in the MnSOD connection map clearly shows a signaling network accounting for an array of fundamental functions in LDIR-induced functional cooperation in adaptive radiation protection. To date, this association seems to be mostly through co-expression, text mining and database searching. Therefore for many of these proteins, this report is the first to experimentally prove the direct interaction with MnSOD.
Conclusions
Fully understanding the protein interactions of MnSOD is necessary to comprehend the whole picture of signaling networks related to the adaptive radioprotection in normal tissues after exposure to genotoxic stress conditions such as LDIR. This pathway is of importance to further elucidate the exact role of MnSOD in the stress-induced adaptive radioprotection. This study demonstrates the first list of MnSOD interaction partners, many of which are confirmed to function in the mitochondrial regulation of metabolism, DNA repair and apoptosis. These results emphasize the possibility that the role of MnSOD in the adaptive response is not only due to its enzymatic dismutase activity, but also relates to its interactions and communications with many cellular and mitochondrial proteins. It should be noticed that the MnSOD interactome had many fluctuations due to LDIR treatment. Such interactions could be induced or inhibited by LDIR and MnSOD may facilitate these cellular fate-determining pathways. Overall, our results suggest that MnSOD can act as a multi-functional factor in LDIR-induced adaptive radioprotection via interaction with array cellular and mitochondrial proteins involved in mitochondrial apoptosis and DNA repair in addition to its known function of detoxifying superoxide in mitochondria.
Supplementary Material
Highlights.
MnSOD interacts with an array of proteins under LDIR-mediated adaptive response.
MnSOD-interacting proteins were linked with mitochondrial function and DNA repair.
MnSOD may function as a signaling regulator in stress-induced adaptive response.
Acknowledgments
This work was supported by NIH/NCI grant RO1CA152313 (JJL) and the Department of Energy Office of Science Grant DE-SC0001271 (GW, JJL, DJG).
Footnotes
Author Contributions
A.E., B.C. and M. F. performed the experiments. M.F., B. C., J.L. designed the study. A.E., G.W., D.G. B.C., and JL designed the study, performed result analysis and wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morgan WF. Will radiation-induced bystander effects or adaptive responses impact on the shape of the dose response relationships at low doses of ionizing radiation? Dose Response. 2006;4:257–262. doi: 10.2203/dose-response.06-110.Morgan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hei TK, Zhou H, Chai Y, Ponnaiya B, Ivanov VN. Radiation induced non-targeted response: mechanism and potential clinical implications. Curr Mol Pharmacol. 2011;4:96–105. doi: 10.2174/1874467211104020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai L. Research of the adaptive response induced by low-dose radiation: where have we been and where should we go? Hum Exp Toxicol. 1999;18:419–425. doi: 10.1191/096032799678840291. [DOI] [PubMed] [Google Scholar]
- 4.Feinendegen LE, Bond VP, Sondhaus CA, Altman KI. Cellular signal adaptation with damage control at low doses versus the predominance of DNA damage at high doses. C R Acad Sci III. 1999;322:245–251. doi: 10.1016/s0764-4469(99)80051-1. [DOI] [PubMed] [Google Scholar]
- 5.Fan M, Ahmed KM, Coleman MC, Spitz DR, Li JJ. Nuclear factor-kappaB and manganese superoxide dismutase mediate adaptive radioresistance in low-dose irradiated mouse skin epithelial cells. Cancer Res. 2007;67:3220–3228. doi: 10.1158/0008-5472.CAN-06-2728. [DOI] [PubMed] [Google Scholar]
- 6.Jiang H, Li W, Li X, Cai L, Wang G. Low-dose radiation induces adaptive response in normal cells, but not in tumor cells: in vitro and in vivo studies. J Radiat Res (Tokyo) 2008;49:219–230. doi: 10.1269/jrr.07072. [DOI] [PubMed] [Google Scholar]
- 7.St Clair DK, Wan XS, Oberley TD, Muse KE, St Clair WH. Suppression of radiation-induced neoplastic transformation by overexpression of mitochondrial superoxide dismutase. Mol Carcinog. 1992;6:238–242. doi: 10.1002/mc.2940060404. [DOI] [PubMed] [Google Scholar]
- 8.Li JJ, Oberley LW, St Clair DK, Ridnour LA, Oberley TD. Phenotypic changes induced in human breast cancer cells by overexpression of manganese-containing superoxide dismutase. Oncogene. 1995;10:1989–2000. [PubMed] [Google Scholar]
- 9.Li JJ, Oberley LW, Fan M, Colburn NH. Inhibition of AP-1 and NF-kappaB by manganese-containing superoxide dismutase in human breast cancer cells. FASEB J. 1998;12:1713–1723. doi: 10.1096/fasebj.12.15.1713. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, Chaiswing L, Oberley TD, Batinic-Haberle I, St Clair W, Epstein CJ, St Clair D. A mechanism-based antioxidant approach for the reduction of skin carcinogenesis. Cancer Res. 2005;65:1401–1405. doi: 10.1158/0008-5472.CAN-04-3334. [DOI] [PubMed] [Google Scholar]
- 11.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 12.Murley JS, Kataoka Y, Baker KL, Diamond AM, Morgan WF, Grdina DJ. Manganese superoxide dismutase (SOD2)-mediated delayed radioprotection induced by the free thiol form of amifostine and tumor necrosis factor alpha. Radiat Res. 2007;167:465–474. doi: 10.1667/RR0758.1. [DOI] [PubMed] [Google Scholar]
- 13.Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–322. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- 14.Oberley LW, McCormick ML, Sierra-Rivera E, StClair DK. Manganese superoxide dismutase in normal and transformed human embryonic lung fibroblasts. Free Radical Biol Med. 1989;6:379–384. doi: 10.1016/0891-5849(89)90083-x. [DOI] [PubMed] [Google Scholar]
- 15.St Clair DK, Holland JC. Complementary DNA encoding human colon cancer manganese superoxide dismutase and the expression of its gene in human cells. Cancer Res. 1991;51:939–943. [PubMed] [Google Scholar]
- 16.Murley JS, Nantajit D, Baker KL, Kataoka Y, Li JJ, Grdina DJ. Maintenance of manganese superoxide dismutase (SOD2)-mediated delayed radioprotection induced by repeated administration of the free thiol form of amifostine. Radiat Res. 2008;169:495–505. doi: 10.1667/RR1194.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams MD, Van Remmen H, Conrad CC, Huang TT, Epstein CJ, Richardson A. Increased oxidative damage is correlated to altered mitochondrial function in heterozygous manganese superoxide dismutase knockout mice. J Biol Chem. 1998;273:28510–28505. doi: 10.1074/jbc.273.43.28510. [DOI] [PubMed] [Google Scholar]
- 18.Summers RW, Maves BV, Reeves RD, Arjes LJ, Oberley LW. Irradiation increases superoxide dismutase in rat intestinal smooth muscle. Free Radic Biol Med. 1989;6: 261–270. doi: 10.1016/0891-5849(89)90053-1. [DOI] [PubMed] [Google Scholar]
- 19.Epperly MW, Sikora C, Defilippi S, Bray J, Koe G, Liggitt D, Luketich JD, Greenberger JS. Plasmid/liposome transfer of the human manganese superoxide dismutase transgene prevents ionizing irradiation-induced apoptosis in human esophagus organ explant culture. Int J Cancer. 2000;90:128–137. doi: 10.1002/1097-0215(20000620)90:3<128::aid-ijc2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed KM, Li JJ. NF-kappa B-mediated adaptive resistance to ionizing radiation. Free Radic Biol Med. 2008;44:1–13. doi: 10.1016/j.freeradbiomed.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murley JS, Baker KL, Miller RC, Darga TE, Weichselbaum RR, Grdina DJ. SOD2-mediated adaptive responses induced by low-dose ionizing radiation via TNF signaling and amifostine. Free Radic Biol Med. 2011;51:1918–1925. doi: 10.1016/j.freeradbiomed.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong W, Oberley LW, Oberley TD, Yan T, Domann FE, St Clair DK. Inhibition of cell growth and sensitization to oxidative damage by overexpression of manganese superoxide dismutase in rat glioma cells. Cell Growth Differ. 1996;7:1175–1186. [PubMed] [Google Scholar]
- 23.Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–395. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- 24.Oberley LW. Anticancer therapy by overexpression of superoxide dismutase. Antioxid Redox Signal. 2001;3:461–472. doi: 10.1089/15230860152409095. [DOI] [PubMed] [Google Scholar]
- 25.Oberley LW. Mechanism of the tumor suppressive effect of MnSOD overexpression. Biomed Pharmacother. 2005;59:143–148. doi: 10.1016/j.biopha.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Pei XF, Meck JM, Greenhalgh D, Schlegel R. Cotransfection of HPV-18 and v-fos DNA induces tumorigenicity of primary human keratinocytes. Virology. 1993;196:855–860. doi: 10.1006/viro.1993.1546. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed KM, Fan M, Nantajit D, Cao N, Li JJ. Cyclin D1 in low-dose radiation-induced adaptive resistance. Oncogene. 2008;27:6738–6748. doi: 10.1038/onc.2008.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed KM, Nantajit D, Fan M, Murley JS, Grdina DJ, Li JJ. Coactivation of ATM/ERK/NF-kappaB in the low-dose radiation-induced radioadaptive response in human skin keratinocytes. Free Radic Biol Med. 2009;46:1543–1550. doi: 10.1016/j.freeradbiomed.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolf A, Agnihotri S, Micallef J, Mukherjee J, Sabha N, Cairns R, Hawkins C, Guha A. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208:313–326. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plas DR, Thompson CB. Cell metabolism in the regulation of programmed cell death. Trends Endocrinol Metab. 2002;13:75–78. doi: 10.1016/s1043-2760(01)00528-8. [DOI] [PubMed] [Google Scholar]
- 32.Vogt C, Yki-Jarvinen H, Iozzo P, Pipek R, Pendergrass M, Koval J, Ardehali H, Printz R, Granner D, Defronzo R, Mandarino L. Effects of insulin on subcellular localization of hexokinase II in human skeletal muscle in vivo. J Clin Endocrinol Metab. 1998;83:230–234. doi: 10.1210/jcem.83.1.4476. [DOI] [PubMed] [Google Scholar]
- 33.Elliott NA, Volkert MR. Stress induction and mitochondrial localization of Oxr1 proteins in yeast and humans. Mol Cell Biol. 2004;24:3180–3187. doi: 10.1128/MCB.24.8.3180-3187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esposito G, Campa A, Pinto M, Simone G, Tabocchini MA, Belli M. Adaptive response: modelling and experimental studies. Radiat Prot Dosimetry. 2011;143:320–324. doi: 10.1093/rpd/ncq474. [DOI] [PubMed] [Google Scholar]
- 35.Vares G, Wang B, Tanaka K, Kakimoto A, Eguchi-Kasai K, Nenoi M. Mutagenic adaptive response to high-LET radiation in human lymphoblastoid cells exposed to low doses of heavy-ion radiation. Mutat Res. 2011;712:49–54. doi: 10.1016/j.mrfmmm.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Hamada N, Maeda M, Otsuka K, Tomita M. Signaling pathways underpinning the manifestations of ionizing radiation-induced bystander effects. Curr Mol Pharmacol. 2011;4:79–95. doi: 10.2174/1874467211104020079. [DOI] [PubMed] [Google Scholar]
- 37.Singh S, Bala M, Kumar R, Kumar A, Dhiman SC. Modification in the expression of Mre11/Rad50/Nbs1 complex in low dose irradiated human lymphocytes. Dose Response. 2009;7: 193–207. doi: 10.2203/dose-response.09-001.Singh. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang X, Zou P, Yao J, Yun D, Bao H, Du R, Long J, Chen X. Proteomic dissection of cell type-specific H2AX-interacting protein complex associated with hepatocellular carcinoma. J Proteome Res. 2010;9:1402–1415. doi: 10.1021/pr900932y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobayashi J, Tauchi H, Sakamoto S, Nakamura A, Morishima K, Matsuura S, Kobayashi T, Tamai K, Tanimoto K, Komatsu K. NBS1 localizes to gamma-H2AX foci through interaction with the FHA/BRCT domain. Curr Biol. 2002;12:1846–1851. doi: 10.1016/s0960-9822(02)01259-9. [DOI] [PubMed] [Google Scholar]
- 40.Cook PJ, Ju BG, Telese F, Wang X, Glass CK, Rosenfeld MG. Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature. 2009;458:591–596. doi: 10.1038/nature07849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandez-Capetillo O, Celeste A, Nussenzweig A. Focusing on foci: H2AX and the recruitment of DNA-damage response factors. Cell Cycle. 2003;2:426–427. [PubMed] [Google Scholar]
- 42.Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 43.Ganesan AK, Kho Y, Kim SC, Chen Y, Zhao Y, White MA. Broad spectrum identification of SUMO substrates in melanoma cells. Proteomics. 2007;7:2216–2221. doi: 10.1002/pmic.200600971. [DOI] [PubMed] [Google Scholar]
- 44.Shen Z, Pardington-Purtymun PE, Comeaux JC, Moyzis RK, Chen DJ. Associations of UBE2I with RAD52, UBL1, p53, and RAD51 proteins in a yeast two-hybrid system. Genomics. 1996;37:183–186. doi: 10.1006/geno.1996.0540. [DOI] [PubMed] [Google Scholar]
- 45.Li W, Hesabi B, Babbo A, Pacione C, Liu J, Chen DJ, Nickoloff JA, Shen Z. Regulation of double-strand break-induced mammalian homologous recombination by UBL1, a RAD51-interacting protein. Nucleic Acids Res. 2000;28:1145–1153. doi: 10.1093/nar/28.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mandavilli BS, Santos JH, Van Houten B. Mitochondrial DNA repair and aging. Mutat Res. 2002;509:127–151. doi: 10.1016/s0027-5107(02)00220-8. [DOI] [PubMed] [Google Scholar]
- 47.Grishko VI, Rachek LI, Spitz DR, Wilson GL, LeDoux SP. Contribution of mitochondrial DNA repair to cell resistance from oxidative stress. J Biol Chem. 2005;280:8901–8905. doi: 10.1074/jbc.M413022200. [DOI] [PubMed] [Google Scholar]
- 48.LeDoux SP, Druzhyna NM, Hollensworth SB, Harrison JF, Wilson GL. Mitochondrial DNA repair: a critical player in the response of cells of the CNS to genotoxic insults. Neuroscience. 2007;145:1249–1259. doi: 10.1016/j.neuroscience.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tahbaz N, Subedi S, Weinfeld M. Role of polynucleotide kinase/phosphatase in mitochondrial DNA repair. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grishko V, Solomon M, Wilson GL, LeDoux SP, Gillespie MN. Oxygen radical-induced mitochondrial DNA damage and repair in pulmonary vascular endothelial cell phenotypes. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1300–1308. doi: 10.1152/ajplung.2001.280.6.L1300. [DOI] [PubMed] [Google Scholar]
- 51.Lenaz G, Bovina C, D’Aurelio M, Fato R, Formiggini G, Genova ML, Giuliano G, Merlo Pich M, Paolucci U, Parenti Castelli G, Ventura B. Role of mitochondria in oxidative stress and aging. Ann N Y Acad Sci. 2002;959:199–213. doi: 10.1111/j.1749-6632.2002.tb02094.x. [DOI] [PubMed] [Google Scholar]
- 52.Konishi A, Shimizu S, Hirota J, Takao T, Fan Y, Matsuoka Y, Zhang L, Yoneda Y, Fujii Y, Skoultchi AI, Tsujimoto Y. Involvement of histone H1.2 in apoptosis induced by DNA double-strand breaks. Cell. 2003;114:673–688. doi: 10.1016/s0092-8674(03)00719-0. [DOI] [PubMed] [Google Scholar]
- 53.Yan N, Shi Y. Histone H1.2 as a trigger for apoptosis. Nat Struct Biol. 2003;10:983–985. doi: 10.1038/nsb1203-983. [DOI] [PubMed] [Google Scholar]
- 54.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 55.Dlugosz PJ, Billen LP, Annis MG, Zhu W, Zhang Z, Lin J, Leber B, Andrews DW. Bcl-2 changes conformation to inhibit Bax oligomerization. Embo J. 2006;25:2287–2296. doi: 10.1038/sj.emboj.7601126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delhalle S, Deregowski V, Benoit V, Merville MP, Bours V. NF-kappaB-dependent MnSOD expression protects adenocarcinoma cells from TNF-alpha-induced apoptosis. Oncogene. 2002;21:3917–3924. doi: 10.1038/sj.onc.1205489. [DOI] [PubMed] [Google Scholar]
- 57.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. J Cell Sci. 2007;120:4155–4166. doi: 10.1242/jcs.011163. [DOI] [PubMed] [Google Scholar]
- 58.Liu JW, Chandra D, Rudd MD, Butler AP, Pallotta V, Brown D, Coffer PJ, Tang DG. Induction of prosurvival molecules by apoptotic stimuli: involvement of FOXO3a and ROS. Oncogene. 2005;24:2020–2031. doi: 10.1038/sj.onc.1208385. [DOI] [PubMed] [Google Scholar]
- 59.Choudhary V, Kaddour-Djebbar I, Lakshmikanthan V, Ghazaly T, Thangjam GS, Sreekumar A, Lewis RW, Mills IG, Bollag WB, Kumar MV. Novel role of androgens in mitochondrial fission and apoptosis. Mol Cancer Res. 2011;9:1067–1077. doi: 10.1158/1541-7786.MCR-10-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng Y, Ariza ME, Goulet AC, Shi J, Nelson MA. Death-signal-induced relocalization of cyclin-dependent kinase 11 to mitochondria. Biochem J. 2005;392:65–73. doi: 10.1042/BJ20050195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Veeraraghavan J, Natarajan M, Herman TS, Aravindan N. Low-dose gamma-radiation-induced oxidative stress response in mouse brain and gut: regulation by NFkappaB-MnSOD cross-signaling. Mutat Res. 2011;718:44–55. doi: 10.1016/j.mrgentox.2010.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.