Abstract
Background
Prostate-specific antigen (PSA) testing for prostate cancer (PCa) is controversial, with concerning rates of both over- and under-screening. The reasons for the observed rates of screening are unknown, and few studies have examined the relationship of psychological health to PSA screening rates. Understanding this relationship can help guide interventions to improve informed-decision making (IDM) for screening.
Methods
A nationally-representative sample of men 57–85 years old without PCa (N=1,169) from the National Social life, Health and Aging Project (NSHAP) was analyzed. The independent relationship of validated psychological health scales measuring stress, anxiety, and depression to PSA testing rates was assessed using multvariable logistic regression analyses.
Results
PSA screening rates were significantly lower for men with higher perceived stress (OR=0.76, p=0.006), but not for higher depressive symptoms (OR=0.89, p=0.22) when accounting for stress. Anxiety influences PSA screening through an interaction with number of doctor visits (p=0.02). Among the men who visited the doctor 1 time, those with higher anxiety were less likely to be screened (OR=0.65, p=0.04). Conversely, among those who visited the doctor 10+ times with higher anxiety were more likely to be screened (OR=1.71, p=0.04).
Conclusions
Perceived stress significantly lowers PSA screening likelihood, and it appears to partly mediate the negative relationship of depression with screening likelihood. Anxiety affects PSA screening rates differently for men with different numbers of doctor visits. Interventions to influence PSA screening rates should recognize the role of the patients’ psychological state to improve their likelihood of making informed decisions and improve screening appropriateness.
Keywords: prostate cancer, screening, stress, anxiety, depression
Introduction
Prostate-specific antigen (PSA) testing has been used to screen men for prostate cancer (PCa) since the early 1990s.1 Despite its regular use, PSA screening remains controversial due to its uncertain mortality benefits and the possible exposure of men to unnecessary treatments and related toxicities.2 Recent large, randomized controlled trials (RCT) on the mortality benefits of PSA screening have failed to resolve the controversy,3,4 but data suggest that men with at least a 10-year remaining life expectancy (RLE) would have a disease-specific mortality benefit from PSA screening.3,5 As a consequence, guidelines emphasize the necessity for informed decision making (IDM). For example, both the American Cancer Society (ACS) and the American Urological Association (AUA) recommend that men with a RLE of at least 10 years have an opportunity to make an informed decision about being screened for PCa.6,7 In contrast, preliminary U.S. Preventive Services Task Force (USPSTF) guidelines recommend against routine PSA-based screening for PCa.8 Whatever the differences in recommendations, all emphasize the importance of a shared, informed decision-making process.
Despite this emphasis on informed decision making, multiple studies show that well over 30% of men with limited RLE and/or multiple comorbidities continue to be screened.2 Conversely, a similarly large percentage of men with long RLE and few comorbidities, for whom informed decisions resulting in PSA testing are potentially “appropriate,” are not receiving PSA tests.9 Proposed associations with inappropriate screening include difficulty for physicians in predicting RLE,10 and exposure to the “do-something” health care system.9,11 Nevertheless, it remains unclear why there is such variability in non-RLE-based PSA screening rates.
It has been suggested that a patient’s psychological state may influence cancer screening rates since emotions are known to effect health behavior, self-regulation, and uptake of health-related messages.12 For example, heightened anxiety has consistently predicted breast cancer screening rates.12 In the more limited studies on PCa screening in men, an inconsistent relationship has been found between anxiety and screening, likely due to small sample sizes, selected populations, or different measures of anxiety.13 One hospital-based study found that higher anxiety increased PSA screening rates in men, particularly for those with a family history of PCa seeking reassurance from a normal test result.14 Conversely, another study found a marginally significant, “inverted U”-shaped relationship between PSA screening and trait anxiety; men with both very low (i.e. no concern) or very high (i.e. fear) anxiety levels had lower screening rates, while individuals with moderate anxiety levels had the highest screening rates.15 Others show anxiety associated with decreased screening rates, possibly due to fear and avoidance of threatening situations.16,17 Still other studies have shown no relationship between trait anxiety and PSA screening.16
Depressive symptoms may also play a role in cancer screening behavior. Individuals with elevated depressive symptoms have lower overall rates of preventive care utilization and adherence to treatments,18,19 despite using more health care services overall.20 In regards to cancer screening, women with depressive symptoms have been found to have lower screening mammogram rates.21,22 In men, the relationship between depression and cancer screening remains largely unknown. One study on colorectal cancer screening found a decreased screening rate for men with depressive symptoms.21
Finally, perceived stress may play a role in cancer screening since increased stress has been associated with other preventive health behaviors such as increased smoking rates, increased dietary fat intake, and decreased exercise likelihood.23 One study examining the relationship between the likelihood of mammograms in those with psychological distress (a combined depressive and perceived stress symptoms measure) found decreased screening rates with increasing stress symptoms.24 However, no similar study has been conducted for PSA screening in men.
Previous studies have much more frequently focused on women, making an investigation of the relationship of psychological health to cancer screening behavior in men needed since each gender may be different. Reasons include differences in cancer incidence and detection patterns, perceptions of cancer risk,25 likelihoods of engaging in risky behaviors,26 and psychological distress influencing self-perception of health.27 The limited research on psychological health and PSA screening rates has been limited to small, non-representative samples of men. Additionally, previous research has focused almost entirely on anxiety, and very few studies have examined the role of other psychological states, such as depression and stress, on PSA screening rates. Understanding the relationship of different psychological states to PSA screening rates could help to direct interventions aimed at improving IDM. Identification of psychological factors that influence screening can also help guide physicians toward more appropriately screening men with longer RLE or less frequent potentially harmful screenings for men with limited RLE. In summary, patients’ psychological states currently have unclear relationships with cancer screening patterns in men, little empirical research has examined the role of psychological health -- including anxiety, depression, or stress -- on PSA screening, and none have been done in a nationally-representative population.
In response, this study uses a nationally-representative dataset, the National Social Life Health and Aging Project (NSHAP), to examine the role of patient anxiety, depression, and stress using validated measures on PSA screening rates. We hypothesize that: 1) anxiety has a curvilinear relationship with screening in which individuals with both low and high anxiety symptoms will have decreased screening rates, while those with moderate anxiety levels will have increased screening rates; 2) those with increased depressive symptoms will have decreased screening rates; and 3) those with increased perceived stress symptoms will have decreased screening rates.
Methods
Study sample
This study uses data from the first wave (2005) of the National Social life, Health, and Aging Project (NSHAP), a nationally-representative, community-based survey of 3,005 individuals aged 57–85 years old with interviews conducted in 2004–2005. An overall sample of 4,400 individuals was approached for potential enrollment in NSHAP and there was a 75.5% weighted response rate representing 3,005 total respondents. Full details of the sample design are reported elsewhere.28 For this sample, the women were removed, yielding 1,455 men available from the NSHAP dataset. Men with missing information on primary variables (n=125) or with a reported prior history of PCa or prostatectomy (n=161) were excluded from our analysis, yielding a final sample size of 1,169 men.
Dependent Variable - PSA screening
The primary outcome measure is the self-reported receipt of a PSA screening test in the last year, indicating appropriate screening for otherwise healthy men according to multiple guidelines at the time of data collection.7,29 This was assessed using the question, “About how long has it been since you last had a Prostate Specific Antigen test, also called a PSA test?”
Independent Variables - Psychological assessments
Three separate, validated psychological scales were included in NSHAP to assess symptoms of anxiety, depression, and stress.30 Anxiety was assessed using a 7-item modified version of the Hospital Anxiety and Depression Scale, Anxiety subscale (HADS-A). The measure assessed anxiety using a 0–3 Likert scale, giving a range of 0–21. The HADS-A has been shown to successfully assess symptom severity and identify cases of clinical anxiety,31 as well as have acceptable reliability and validity on a population level.30 The standardized Cronbach’s alpha coefficient for the modified version, indicating internal consistency and reliability, was 0.76, and a full account of the validity and acceptability of the modified HADS-A used for NSHAP is available elsewhere.30 Anxiety was assessed as a categorical variable for descriptive analysis, and included the previously-validated categories 0–7 (normal anxiety), 8–10 (mild anxiety), and 11+ (moderate/severe anxiety),32 with the mild anxiety score (≥8) being a reliable cutoff for identifying cases of significant anxiety.31
Depression was measured using the Center for Epidemiological Studies-Depression (CES-D) scale, a commonly-used tool for assessing depressive symptoms on a population level. Specifically, NSHAP used the 11-item “Iowa form” of the CES-D scale, which has been validated in population-based studies, including in older populations.33 Responses to questions were on a 0–3 Likert scale, giving a possible range of 0–33. The standardized Cronbach’s alpha was 0.80 in the NSHAP sample.30 In the full 20-item CES-D scale, which has a range of 0–60, a 16+ point cutoff is most commonly used to identify clinical cases of depression, which corresponds to 9+ points in the 11-item scale.33 Other studies have shown that for elderly populations, a cutoff point of 21+ for the full CES-D scale is more appropriate,34 which corresponds to approximately 12 points on the 11-item scale. Therefore, based on previous literature,35 categorical variable cutoffs were defined as 0–8 (normal), 9–11 (mild depression), and 12+ (moderate/severe depression) for descriptive analysis.
Stress was assessed using a 4-item modified version of Cohen’s Perceived Stress Scale (PSS-4). The PSS scale is a widely used measure of a global perception of stress, and questions focus on control over one’s life, self-confidence, and perception of life events.36 The modified PSS-4 scale focuses more on the perception of external stressors and ability to cope, rather than on general distress resulting from stress,37 distinguishing itself from the CES-D and HADS-A for which negative affective states are more relevant. The modified NSHAP version of the PSS-4 had a Cronbach alpha of 0.63, and relevant validity and acceptability data is summarized elsewhere.30 Questions were answered on a 0–3 Likert scale, giving a possible range of 0–12. There are no specific cut-off points for the modified PSS-4, however, previous studies have used dichotomous categories of 0–5 (low stress) and 6+ (high stress).38 Stress was therefore measured as a dichotomous categorical variable in descriptive analysis. To facilitate interpretation, each psychological scale was standardized to have mean 0 and SD equal to 1 prior to inclusion in the logistic regression models.
Health Status
Health status was assessed using a modified Charlson comorbidity index. The Charlson index was chosen because it is commonly used to assess worse health on the population level, and its survey form is well-validated, relatively simple to use, and has been tied to mortality.39,40 In addition, comorbidity indices have been recommended to physicians as a means to estimate RLE for guideline-based PSA screening,41 making it a clinically-relevant estimate physicians may utilize in assessing which patients are offered PSA screening.
Covariates
Several variables are associated with PSA screening and were included in the logistic regression models. Age was incorporated as both a linear and quadratic term in the regression models due to its curvilinear relationship to PSA screening. Ethnicity, marital status, education, and income have all been previously associated with PSA screening and psychological health status.1 Ethnicity was categorized as Caucasian, African American (AA), Hispanic, or Other. Marital status was classified as married or unmarried, where unmarried includes never married, widowed, and divorced men. Education was divided into four categories: “less than high school,” “high school diploma/GED,” “some college/vocational degree,” or “Bachelors or more.” Income was categorized based on the percentage of the federal poverty level (FPL) for single-adult and 2-adult households in 2005,42 with the categories “poor” (0–100% FPL), “near-poor” (101–200% FPL), and “not poor” (201% or more of the FPL). Health service utilization has also been described as an important potential confounder in the relationship between psychological distress and screening.11,20,43 Health care utilization was measured using the reported number of visits to a doctor’s office in the last 12 months.
Statistical Analysis
The rate of PSA screening was computed separately for subgroups based on each of the covariates. Separate logistic regressions were then used to evaluate the relationship between each psychological measure and the likelihood of screening, controlling for age (both linear and quadratic terms), race/ethnicity, marital status, education, visits to the doctor and comorbidity index. Interaction terms between each psychological measure and the number of doctor visits were tested, as were quadratic effects for each measure (to capture potential non-linearities in the relationships between these measures and the likelihood of screening). A combined model was then fit including all three measures. Marginal probabilities of screening based on the combined model were computed separately by age and selected values of the psychological measures. Finally, poverty status was added to the combined model, and the model was refit using multiple imputations to account for the missing data in household income and each of the psychological measures. Imputation using Chained Equations (ICE) was used, imputing each psychological measure via linear regression and household income via interval regression using information from the unfolding bracket questions that were administered when a respondent did not provide a specific dollar amount. Because the final model included an interaction between anxiety and visits to the doctor, imputation was performed separately for each category of doctor visits.44 A burn-in period of 20 iterations was used for each chain, and 20 sets of imputations were generated. Sampling weights and design-based standard errors using the linearization method were used throughout, based on the weight, strata and PSU variables distributed with the dataset All statistical analysis was performed using Stata 12.1.45
Results
Sample Characteristics and PSA Screening Rates (Table 1)
Table 1.
NSHAP national prevalence estimates and sample characteristics
| Total (%)
|
PSA Screening
|
|||
|---|---|---|---|---|
| (N=1,169) | 95% CI | (%) | 95% CI | |
| Overall | - | 53.4 | (49.3–57.5) | |
| Age1 | ||||
| <60 | 17.0 | (13.5–20.4) | 44.0 | (35.2–53.3) |
| 60–64 | 28.8 | (25.0–32.5) | 49.7 | (42.9–56.6) |
| 65–69 | 19.3 | (17.0–21.7) | 58.6 | (51.2–65.7) |
| 70–74 | 15.1 | (13.0–17.2) | 61.7 | (50.9–71.4) |
| 75–79 | 12.1 | (9.8–14.4) | 63.3 | (54.8–71.0) |
| 80–85 | 7.7 | (6.1–9.3) | 43.1 | (33.4–57.5) |
| Race/Ethnicity | ||||
| White | 81.6 | (77.8–85.4) | 55.9 | (51.7–60.0) |
| African American | 8.2 | (5.9–10.5) | 52.1 | (42.7–61.2) |
| Hispanic | 7.1 | (3.8–10.3) | 38.4 | (27.5–50.6) |
| Other | 3.2 | (1.8–4.6) | 26.4 | (16.7–39.1) |
| Education | ||||
| <High school | 16.1 | (12.4–19.8) | 40.3 | (31.6–49.6) |
| High school/GED | 23.2 | (20.0–26.3) | 54.9 | (48.5–61.1) |
| Some college | 28.3 | (24.3–32.3) | 56.4 | (49.5–62.9) |
| Bachelors+ | 32.4 | (27.4–37.5) | 56.3 | (48.7–63.6) |
| Marital Status | ||||
| Married | 78.0 | (74.8–81.3) | 55.9 | (51.2–60.5) |
| Unmarried | 22.0 | (18.7–25.2) | 44.6 | (38.2–51.2) |
| Income Level2 | ||||
| 0–100% FPL | 6.0 | (3.6–8.4) | 18.4 | (12.8–25.6) |
| 101–200% FPL | 13.2 | (10.7–15.7) | 47.8 | (38.2–57.7) |
| >200% FPL | 80.8 | (77.0–84.6) | 58.0 | (53.6–62.3) |
| Anxiety3 | ||||
| Mean Score | 3.2 (SD=3.6) | (3.0–3.5) | ||
| Normal (0–7) | 89.5 | (87.2–91.9) | 55.6 | (51.1–60.0) |
| Mild (8–10) | 7.4 | (5.7–9.1) | 52.4 | (40.4–64.1) |
| Moderate/Severe (11+) | 3.1 | (1.7–4.4) | 42.0 | (24.1–62.2) |
| Depression4 | ||||
| Mean Score | 4.9 (SD=5.2) | (4.5–5.3) | ||
| Normal (0–8) | 81.0 | (783–83.6) | 54.4 | (50.0–58.7) |
| Mild (9–11) | 8.7 | (6.5–10.9) | 57.2 | (48.8–65.2) |
| Moderate/Severe (12+) | 10.3 | (8.2–12.5) | 42.2 | (31.4–53.8) |
| Stress5 | ||||
| Mean Score | 1.5 (SD=2.3) | (1.4–1.7) | ||
| Low (0–5) | 93.1 | (91.3–94.9) | 56.1 | (51.6–60.5) |
| High (6+) | 6.9 | (5.1–8.7) | 39.1 | (26.7–53.1) |
| Doctor visits in last 12 months | ||||
| 0 | 8.9 | (6.7–11.1) | 2.9 | (1.0–8.0) |
| 1 | 13.4 | (11.1–15.7) | 41.3 | (32.2–51.1) |
| 2 or 3 | 31.7 | (28.9–34.4) | 58.5 | (51.5–65.1) |
| 4 to 9 | 31.4 | (27.5–35.3) | 60.0 | (52.9–66.7) |
| 10+ | 14.6 | (12.1–17.2) | 70.2 | (63.4–76.2) |
| Comorbidity Index6 | ||||
| Mean Score | 1.5 (SD=1.7) | (1.5–1.6) | ||
| 0 | 29.4 | (26.3–32.5) | 45.9 | (39.2–52.7) |
| 1 | 28.9 | (26.2–31.6) | 58.5 | (51.4–65.2) |
| 2 | 21.3 | (18.3–24.3) | 54.8 | (46.3–63.1) |
| 3 | 8.9 | (6.8–11.1) | 50.7 | (39.0–62.3) |
| 4 | 6.0 | (4.5–7.5) | 61.0 | (49.5–71.4) |
| 5+ | 5.4 | (4.0–6.9) | 57.9 | (44.5–70.3) |
<60 group is 57–59 years old
Income is measured as % of 2006 Federal Poverty Level (FPL), in 2006 the FPL for a one-adult household was $9,669, and $12,201 for a 2-adult household [46], N=900 individuals had reported income levels
Anxiety measured by the Hospital Anxiety and Depression Scale - Anxiety Subscale (HADS-A), Range: 0–21, N=1061
Depression measured by the Center for Epidemiological Studies-Depression Scale (CES-D), Range: 0–33, N=1162
Stress measured by the 4-item Perceived-Stress Scale (PSS-4), Range: 0–12, N=1062
Comorbidity index measured through a modified Charlson index
Abbreviations: CI - confidence interval, PSA - Prostate-specific antigen, SD - Standard Deviation
Age had both a linear and a curvilinear relationship with PSA screening, in which men less than 60 years old and 80–85 years old had low PSA screening rates compared to those in age categories in between. Clinical variables had marked trends with screening. Those who had more physician visits were screened more often, with 41.3% being screened if visiting the doctor 1 time, and 70.2% being screened if visiting the doctor 10 or more times. Individuals with higher comorbidity scores also received more screening, where those with a score of 0 had a rate of 45.9%, and individuals with a score of 5+ had a screening rate of 57.9%.
Considering the established cutoff level on the HADS for having clinically-significant anxiety, 10.5% of men were above the mild and greater categories. Similarly for depression on the CES-D, 10.3% of men had moderate/severe depression at a level above the established clinical cutoff for older adults. For perceived stress on the PSS, 6.9% of men had an elevated score. There was substantial variation in screening by each psychological variable. Individuals with normal levels of anxiety symptoms had a screening rate of 55.6% while those with moderate/severe anxiety had a rate of 42.0%. There was a negative trend with increased depressive symptoms, with a rate of 54.4% in the normal category compared to 42.2% in moderate/severe category. Finally, men with low stress had screening rates of 56.1%, while those with high stress levels had much lower PSA screening at 39.1%.
Logistic Regression Results (Tables 2 and 3)
Table 2.
Separate and combined multivariate logistic regressions for receiving a PSA screening test in last year
| Variable | OR | 95% CI | p-value | |
|---|---|---|---|---|
| Separate Models for each Psychological Measure (N = 1,166, 1,062 and 1,061, respectively)1 | ||||
| Psychological Health | ||||
| Depression (standardized) | 0.81 | (0.71–0.93) | 0.003 | |
| Stress (standardized) | 0.76 | (0.66–0.87) | <0.001 | |
| Anxiety (standardized) | ||||
| Visits to doctor | ||||
| 0 | 0.25 | (0.09–0.68) | 0.008 | |
| 1 | 0.53 | (0.36–0.77) | 0.001 | |
| 2–3 | 0.87 | (0.71–1.07) | 0.184 | |
| 4–9 | 0.94 | (0.67–1.31) | 0.707 | |
| 10+ | 1.44 | (0.97–2.12) | 0.067 | |
|
| ||||
| Combined Model (N = 1,059) | ||||
| Psychological Health | ||||
| Depression (standardized) | 0.88 | (0.72–1.06) | 0.165 | |
| Stress (standardized) | 0.76 | (0.63–0.91) | 0.003 | |
| Anxiety (standardized) | ||||
| Visits to doctor | ||||
| 0 | 0.31 | (0.12–0.82) | 0.019 | |
| 1 | 0.61 | (0.41–0.92) | 0.019 | |
| 2–3 | 1.08 | (0.84–1.39) | 0.529 | |
| 4–9 | 1.15 | (0.78–1.68) | 0.483 | |
| 10+ | 1.78 | (1.09–2.90) | 0.021 | |
|
| ||||
| Sociodemographics | ||||
| Age (decades) | Linear | 78.76 | (1.29–4,815.69) | 0.038 |
| Quadratic | 0.74 | (0.55–0.99) | 0.045 | |
|
| ||||
| Race/Ethnicity Group | White | Ref | - | - |
| African American (AA) | 1.03 | (0.64–1.67) | 0.892 | |
| Hispanic (non-AA) | 0.79 | (0.42–1.46) | 0.436 | |
| Other | 0.26 | (0.14–0.49) | <0.001 | |
|
| ||||
| Marital Status | Married | Ref | - | - |
| Not married2 | 0.75 | (0.54–1.06) | 0.100 | |
|
| ||||
| Education | <HS | Ref | - | - |
| HS/GED | 1.69 | (1.10–2.62) | 0.018 | |
| Some college | 2.17 | (1.25–3.76) | 0.007 | |
| Bachelors+ | 1.95 | (1.00–3.80) | 0.049 | |
|
| ||||
| Clinical | ||||
| Visits to Doctor (at mean anxiety) | 0 | 0.01 | (0.00–0.04) | <0.001 |
| 1 | 0.37 | (0.23–0.57) | <0.001 | |
| 2 or 3 | Ref | - | - | |
| 4 to 9 | 0.98 | (0.63–1.55) | 0.943 | |
| 10+ | 1.64 | (0.94–2.87) | 0.080 | |
|
| ||||
| Comorbidities | 1.01 | (0.90–1.13) | 0.921 | |
Each psychological variable was run in separate regression models on PSA screening while including all other covariates
“Not married” includes never married, widowed, and divorced men.
Table 3.
Logistic regression for receiving a PSA screening test in last year, including poverty status and estimated with multiple imputation (N = 1,169)
| Variable | OR | 95% CI | p-value | |
|---|---|---|---|---|
| Psychological Health | ||||
| Depression (standardized) | 0.89 | (0.73–1.08) | 0.219 | |
| Stress (standardized) | 0.76 | (0.62–0.92) | 0.006 | |
| Anxiety (standardized) | ||||
| Visits to doctor | ||||
| 0 | 0.45 | (0.19–1.05) | 0.065 | |
| 1 | 0.65 | (0.43–0.98) | 0.038 | |
| 2–3 | 1.11 | (0.86–1.43) | 0.435 | |
| 4–9 | 1.19 | (0.83–1.71) | 0.338 | |
| 10+ | 1.71 | (1.02–2.85) | 0.043 | |
|
| ||||
| Sociodemographics | ||||
| Age (decades) | Linear | 114.42 | (2.33–5,625.36) | 0.018 |
| Quadratic | 0.72 | (0.55–0.95) | 0.021 | |
|
| ||||
| Race/Ethnicity Group | White | Ref | - | - |
| African American (AA) | 1.01 | (0.64–1.59) | 0.957 | |
| Hispanic (non-AA) | 0.78 | (0.43–1.40) | 0.394 | |
| Other | 0.28 | (0.16–0.51) | <0.001 | |
|
| ||||
| Marital Status | Married | Ref | - | - |
| Not married1 | 0.79 | (0.57–1.11) | 0.166 | |
|
| ||||
| Education | <HS | Ref | - | - |
| HS/GED | 1.44 | (0.94–2.19) | 0.089 | |
| Some college | 1.63 | (0.97–2.76) | 0.066 | |
| Bachelors+ | 1.44 | (0.74–2.77) | 0.273 | |
|
| ||||
| Poverty Status | < Federal Poverty Level (FPL) | Ref | - | - |
| 101–200% FPL | 2.97 | (1.51–5.85) | 0.003 | |
| >200% FPL | 3.79 | (1.94–7.43) | <0.001 | |
|
| ||||
| Clinical | ||||
| Visits to Doctor (at mean anxiety) | 0 | 0.02 | (0.01–0.05) | <0.001 |
| 1 | 0.41 | (0.26–0.64) | <0.001 | |
| 2 or 3 | Ref | - | - | |
| 4 to 9 | 1.06 | (0.70–1.62) | 0.771 | |
| 10+ | 1.90 | (1.19–3.05) | 0.008 | |
|
| ||||
| Comorbidities | 0.98 | (0.87–1.09) | 0.675 | |
“Not married” includes never married, widowed, and divorced men.
In separate models for each psychological measure, quadratic terms were non-significant for anxiety (p = 0.90), depressive symptoms (p = 0.85), and stress (p = 0.71). A significant interaction between anxiety and number of doctor visits was found (p = 0.003), however the corresponding interactions for stress and depressive symptoms were not significant (p = 0.34 and p = 0.58, respectively). A quadratic term for age was narrowly significant in the combined model (p = 0.05), and was included throughout (Figure 1A).
Figure 1.
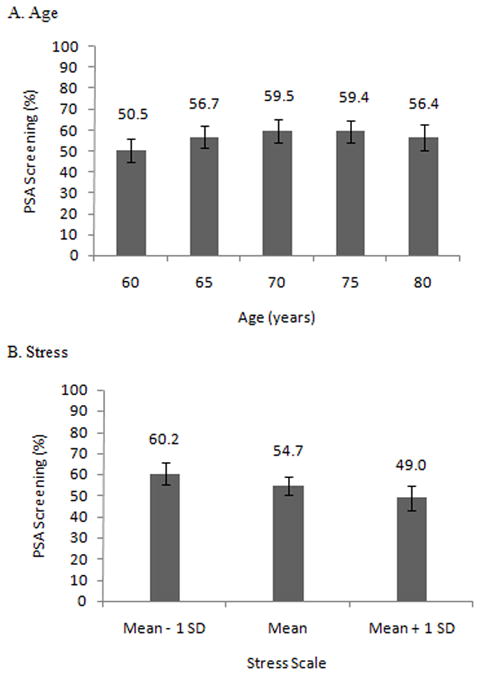
Marginal Probabilities of PSA Screening by selected covariates Model includes age, depression, stress, anxiety, ethnicity, marital status, education, visits to the doctor, and comorbidities
Abbreviations: Prostate Specific Antigen – PSA, Standard Deviation - SD
By itself, depressive symptoms was negatively associated with the likelihood of screening (OR = 0.81, p = 0.003), however this effect was diminished when adding stress to the model (OR = 0.88, p = 0.17) (Table 2). Stress was also negatively associated with screening, and the estimated effect was identical in both the separate and combined models (OR = 0.76, p = 0.003). Anxiety was negatively associated with screening for those who visited the doctor once or less in the last year (OR = 0.61, p = 0.02 for those who visited the doctor once), and positively associated with screening for those who visited the doctor 10 times or more (OR = 1.78, p = 0.02).
For those with an anxiety score near the mean, the likelihood of screening increased steadily with the number of doctor visits. Those without a high school degree were less likely to have been screened. Race/ethnicity, marital status and the comorbidity index were not associated with screening. Adding poverty status to the model and re-estimating using multiple imputation yielded very similar results (Table 3). Those below the Federal Poverty Level were less likely to be screened, and adding poverty status to the model diminished the estimated effect of education.
Discussion
As hypothesized, we found a substantial, independent, linear relationship between increasing levels of perceived stress and lower rates of PSA screening in this nationally-representative sample of older men. We also found that increased depressive symptoms decreased the likelihood of being screened, but this relationship was no longer significant after accounting for perceived stress. Finally, we found a strong, significant relationship between anxiety and PSA screening which differed depending on the number of doctor visits. To our knowledge, the relationship between perceived stress and screening and the impact of anxiety on screening depending on health care utilization have not been previously shown.
Men with increased perceived stress scores had a 17 percentage point difference in PSA screening rates compared to men with low perceived stress. There are some potential mechanisms that may explain the relationship. Stress has been linked to a reduced ability to appreciate long-term health goals, and stressed individuals therefore focus on short-term risk-averse behaviors, such as (avoiding) screening.23 In addition, older individuals perceive greater barriers to healthcare services, even with good access,46 and when combined with increased perception of external stressors, may have difficulty navigating the healthcare system. Further research examining the relationship of perceived stress to health care utilization is needed.
There was a significant interaction between anxiety levels and visits to the doctor, leading to different impacts on screening rates. Men with less doctor visits and high anxiety had lower screening rates, while men with more doctor visits and high anxiety had higher screening rates. It is important to consider both the source of anxiety, as well as the time course of symptom progression, when interpreting this relationship.13 For example, in men who have anxiety about the screening process, anxiety is associated with avoidance and less screening, at least in community studies.16,17 In contrast, men with anxiety about PCa utilize more screening services due to seeking peace of mind, regardless of the appropriateness of screening, at least in hospital-based studies.14 Therefore, despite the importance of physicians in the communication process regarding PSA screening,43 patient anxiety may importantly influence the decision making process. Our findings support both results, thereby drawing together seemingly disparate previous findings.
We found that men with depressive symptoms have more than a 10 percentage point lower rate of PSA screening, consistent with prior research showing a decreased likelihood of obtaining preventive health services, mammograms, and colorectal cancer screenings in women.19,22 However, this relationship was no longer statistically significant when the regression included perceived stress, suggesting it as a mediator between depressive symptoms and screening behavior. This is consistent with prior work showing that individuals with depression utilize less preventive care services, primarily due to a higher perception of care barriers.47 The relationship between stress, depression and PSA screening is interesting in light of previous research showing that men with greater psychological distress generally utilize more health services, not less.20
These findings have important policy implications for improving IDM regarding cancer screening in men. It is important to identify perceived stress and account for its impact on screening, especially for those also having depression. Organizational structures, such as collaborative care models which accommodate the needs of depressed or stressed patients, may importantly reduce perceived barriers.48 In addition, physicians must be aware that patient psychological characteristics may drive screening decisions, regardless of the appropriateness of screening in an individual situation. Indeed, physicians may need to recognize and actively engage men with psychological morbidity in communication.49 Strategies like motivational interviewing,47 can overcome psychological barriers to treatment adherence, encourage appropriate IDM, and improve the likelihood of appropriate screening. In summary, interventions must take into account patients’ psychological state to adequately empower patients to make informed decisions.
Our study has several limitations. First, it utilizes self-reported PSA screening rates, which may be subject to inaccuracies like recall bias.50 Nevertheless, our estimated rates of PSA screening are consistent with other studies.1,2 Second, the NSHAP (2005) dataset is cross-sectional, which limits causal inferences. This limitation may be partially addressed using future waves of NSHAP (2010) which would add longitudinal data. However, self-selection bias, in which those with more psychological morbidity have decreased participation in the study, may not be fully addressed, even with longitudinal data. Third, there are shifting opinions about the appropriateness of PSA screening, so it is unclear what screening regimen is appropriate. Nevertheless, our findings are likely to be general for other, more obviously appropriate screening services, so the importance of psychological morbidity to screening is important.
In conclusion, we found that high perceived stress levels have a strong, independent negative association with PSA screening rates in a large, nationally-representative sample. While we replicated the relationship between depression and decreased screening shown in previous studies, this relationship was no longer significant after controlling for perceived stress, suggesting stress as the more important influence. Finally, we found a strong interaction effect between anxiety, health care utilization, and PSA screening rates. These findings have important implications for IDM, suggesting the recognition and treatment of psychological morbidity may improve screening decisions. Future research should focus on interventions which can best address psychological influences on appropriate screening.
Figure 2.
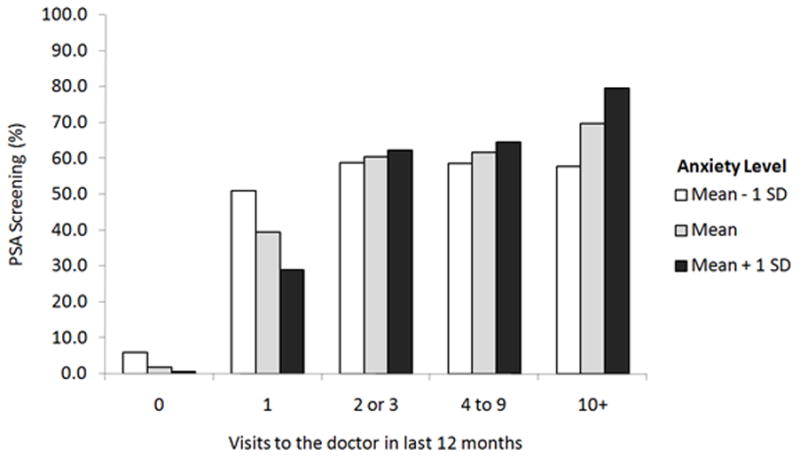
Marginal PSA screening rate by anxiety symptoms and visits to the doctor Model includes age, depression, stress, anxiety, ethnicity, marital status, education, visits to the doctor, and comorbidities
Acknowledgments
Funding: National Social Life Health and Aging Project (NSHAP) (NIH - 5R01 AG021487).
References
- 1.Ross L, Taylor Y, Richardson L, Howard D. Patterns in Prostate-Specific Antigen Test Use and Digital Rectal Examinations in the Behavioral Risk Factor Surveillance System, 2002–2006. Journal of the National Medical Association. 2009;101(4):317. doi: 10.1016/s0027-9684(15)30878-6. [DOI] [PubMed] [Google Scholar]
- 2.Walter L, Bertenthal D, Lindquist K, Konety B. PSA screening among elderly men with limited life expectancies. JAMA. 2006;296(19):2336. doi: 10.1001/jama.296.19.2336. [DOI] [PubMed] [Google Scholar]
- 3.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. New England Journal of Medicine. 2009;360(13):1320. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 4.Andriole GL, Crawford ED, Grubb RL, III, et al. Mortality results from a randomized prostate-cancer screening trial. The New England journal of medicine. 2009;360(13):1310. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford D, Grubb R, III, Black A, et al. Comorbidity and Mortality Results From a Randomized Prostate Cancer Screening Trial. Journal of Clinical Oncology. 2011;29(4):355–361. doi: 10.1200/JCO.2010.30.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf A, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA: A Cancer Journal for Clinicians. 2010;60(2):70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 7.AUA. Prostate-specific antigen (PSA) best practice policy. American Urological Association (AUA) Oncology (Williston Park) 2000;14(2):267–272. [PubMed] [Google Scholar]
- 8.USPSTF. Draft recommendation statement. 2011 http://www.uspreventiveservicestaskforce.org/draftrec3.htm.
- 9.Kotwal A, Mohile S, Dale W. Remaining life expectancy measurement and PSA screening of older men. Journal of Geriatric Oncology. 2012 doi: 10.1016/j.jgo.2012.02.003. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis C, Moore C, Golin C, Griffith J, Tytell-Brenner A, Pignone M. Resident Physicians? Life Expectancy Estimates and Colon Cancer Screening Recommendations in Elderly Patients. Medical Decision Making. 2008;28(2):254–261. doi: 10.1177/0272989X07311756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bynum J, Song Y, Fisher E. Variation in Prostate-Specific Antigen Screening in Men Aged 80 and Older in Fee-for-Service Medicare. Journal of the American Geriatrics Society. 2010;58(4):674–680. doi: 10.1111/j.1532-5415.2010.02761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Consedine NS, Magai C, Krivoshekova YS, Ryzewicz L, Neugut AI. Fear, anxiety, worry, and breast cancer screening behavior: a critical review. Cancer Epidemiology Biomarkers & Prevention. 2004;13(4):501. [PubMed] [Google Scholar]
- 13.Dale W, Bilir P, Han M, Meltzer D. The role of anxiety in prostate carcinoma. Cancer. 2005;104(3):467–478. doi: 10.1002/cncr.21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor K, Shelby R, Kerner J, Redd W, Lynch J. Impact of undergoing prostate carcinoma screening on prostate carcinoma-related knowledge and distress. Cancer. 2002;95(5):1037–1044. doi: 10.1002/cncr.10781. [DOI] [PubMed] [Google Scholar]
- 15.Consedine N, Morgenstern A, Kudadjie-Gyamfi E, Magai C, Neugut A. Prostate cancer screening behavior in men from seven ethnic groups: the fear factor. Cancer Epidemiology Biomarkers & Prevention. 2006;15(2):228. doi: 10.1158/1055-9965.EPI-05-0019. [DOI] [PubMed] [Google Scholar]
- 16.Consedine N, Adjei B, Ramirez P, McKiernan J. An Object Lesson: Source Determines the Relations That Trait Anxiety, Prostate Cancer Worry, and Screening Fear Hold with Prostate Screening Frequency. Cancer Epidemiology Biomarkers & Prevention. 2008;17(7):1631. doi: 10.1158/1055-9965.EPI-07-2538. [DOI] [PubMed] [Google Scholar]
- 17.Roumier X, Azzouzi R, Valéri A, et al. Adherence to an annual PSA screening program over 3 years for brothers and sons of men with prostate cancer. European urology. 2004;45(3):280–286. doi: 10.1016/j.eururo.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 18.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment. Arch Intern Med. 2000;160(14):2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 19.Thorpe JM, Kalinowski CT, Patterson ME, Sleath BL. Psychological distress as a barrier to preventive care in community-dwelling elderly in the United States. Medical Care. 2006;44(2):187. doi: 10.1097/01.mlr.0000196965.54871.d5. [DOI] [PubMed] [Google Scholar]
- 20.Callahan CM, Hui SL, Nienaber NA, Musick BS. Longitudinal study of depression and health services use among elderly primary care patients. Journal of the American Geriatrics Society. 1994 doi: 10.1111/j.1532-5415.1994.tb06554.x. [DOI] [PubMed] [Google Scholar]
- 21.Kodl MM, Powell AA, Noorbaloochi S, Grill JP, Bangerter AK, Partin MR. Mental Health, Frequency of Healthcare Visits, and Colorectal Cancer Screening. Medical Care. 2010;48(10):934. doi: 10.1097/MLR.0b013e3181e57901. [DOI] [PubMed] [Google Scholar]
- 22.Ludman EJ, Ichikawa LE, Simon GE, et al. Breast and Cervical Cancer Screening:: Specific Effects of Depression and Obesity. American journal of preventive medicine. 2010;38(3):303–310. doi: 10.1016/j.amepre.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng DM, Jeffery RW. Relationships between perceived stress and health behaviors in a sample of working adults. Health Psychology. 2003;22(6):638. doi: 10.1037/0278-6133.22.6.638. [DOI] [PubMed] [Google Scholar]
- 24.O’Donnell S, Goldstein B, DiMatteo MR, Fox SA, John CR, Obrzut JE. Adherence to Mammography and Colorectal Cancer Screening in Women 50–80 Years of Age:: The Role of Psychological Distress. Women’s Health Issues. 2010;20(5):343–349. doi: 10.1016/j.whi.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiology Biomarkers & Prevention. 2006;15(2):389–394. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 26.Courtenay WH, Mccreary DR, Merighi JR. Gender and ethnic differences in health beliefs and behaviors. Journal of health psychology. 2002;7(3):219. doi: 10.1177/1359105302007003216. [DOI] [PubMed] [Google Scholar]
- 27.Benyamini Y, Leventhal EA, Leventhal H. Gender differences in processing information for making self-assessments of health. Psychosomatic Medicine. 2000;62(3):354–364. doi: 10.1097/00006842-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 28.O’Muircheartaigh C, Eckman S, Smith S. Statistical Design and Estimation for the National Social Life, Health, and Aging Project. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2009 Nov 1;64B(suppl 1):i12–i19. doi: 10.1093/geronb/gbp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2009: a review of current American Cancer Society guidelines and issues in cancer screening. CA: A Cancer Journal for Clinicians. 2009;59(1):27. doi: 10.3322/caac.20008. [DOI] [PubMed] [Google Scholar]
- 30.Shiovitz-Ezra S, Leitsch S, Graber J, Karraker A. Quality of Life and Psychological Health Indicators in the National Social Life, Health, and Aging Project. The Journals of Gerontology: Series B. 2009;64(Supplement 1):i30. doi: 10.1093/geronb/gbn020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjelland I, Dahl A, Haug T, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale:: An updated literature review. Journal of Psychosomatic Research. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 32.Zigmond AS, Snaith R. The hospital anxiety and depression scale. Acta psychiatrica scandinavica. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 33.Kohout F, Berkman L, Evans D, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. Journal of Aging and Health. 1993;5(2):179. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 34.Lyness JM, Noel TK, Cox C, King DA, Conwell Y, Caine ED. Screening for depression in elderly primary care patients. A comparison of the Center for Epidemiologic Studies-Depression Scale and the Geriatric Depression Scale. Archives of Internal Medicine. 1997;157(4):449. [PubMed] [Google Scholar]
- 35.Unützer J, Patrick DL, Marmon T, Simon GE, Katon WJ. Depressive symptoms and mortality in a prospective study of 2,558 older adults. American Journal of Geriatric Psych. 2002;10(5):521. doi: 10.1097/00019442-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Cohen S. Perceived stress in a probability sample of the United States. 1988. [Google Scholar]
- 37.Hewitt PL, Flett GL, Mosher SW. The Perceived Stress Scale: Factor structure and relation to depression symptoms in a psychiatric sample. Journal of Psychopathology and Behavioral Assessment. 1992;14(3):247–257. [Google Scholar]
- 38.McClellan WM, Abramson J, Newsome B, et al. Physical and psychological burden of chronic kidney disease among older adults. American Journal of Nephrology. 2010;31(4):309–317. doi: 10.1159/000285113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katz J, Chang L, Sangha O, Fossel A, Bates D. Can comorbidity be measured by questionnaire rather than medical record review? Medical Care. 1996;34(1):73. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation* 1. Journal of chronic diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 41.Walter L, Covinsky K. Cancer screening in elderly patients: a framework for individualized decision making. Jama. 2001;285(21):2750. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 42.Qato D, Schumm P, Johnson M, Mihai A, Lindau S. Medication Data Collection and Coding in a Home-Based Survey of Older Adults. Journal of Gerontology. 2009;64B(S1):i86–i93. doi: 10.1093/geronb/gbp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han P, Coates R, Uhler R, Breen N. Decision Making in Prostate-Specific Antigen Screening:: National Health Interview Survey, 2000. American journal of preventive medicine. 2006;30(5):394–404. doi: 10.1016/j.amepre.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 44.White R, Royston P, Wood A. Multiple imputation using chained equations: Issues and guidance for practice. Statistics in Medicine. 2010;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 45.Stata Statistical Software: Release 12 [computer program] College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 46.Fitzpatrick AL, Powe NR, Cooper LS, Ives DG, Robbins JA. Barriers to health care access among the elderly and who perceives them. American journal of public health. 2004;94(10):1788. doi: 10.2105/ajph.94.10.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thorpe JM, Thorpe CT, Kennelty KA, Chewning BA. Medical Care. 2011. Depressive symptoms and reduced preventive care use in older adults: The mediating role of perceived access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Archives of Internal Medicine. 2006;166(21):2314. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- 49.Zolnierek KBH, DiMatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Medical Care. 2009;47(8):826. doi: 10.1097/MLR.0b013e31819a5acc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan E, Vernon S, Ahn C, Greisinger A. Do men know that they have had a prostate-specific antigen test? Accuracy of self-reports of testing at 2 sites. American journal of public health. 2004;94(8):1336. doi: 10.2105/ajph.94.8.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]


