Abstract
Study Objective
Knowledge of current areas of activity in emergency medicine research may improve collaboration among investigators and may help inform decisions about future research priorities. Randomized controlled trials are a key component of research activity and an essential tool for improving care. We investigated the characteristics of randomized trials recently published in emergency medicine journals.
Methods
This was a retrospective analysis of randomized trials published in the five highest-impact emergency medicine journals. Pubmed was searched for reports of randomized trials involving human subjects indexed to MEDLINE between January 1, 2008 and December 31, 2011. Included trials were classified with respect to study topic, funding source, presence of age-related inclusion criteria, and country of origin.
Results
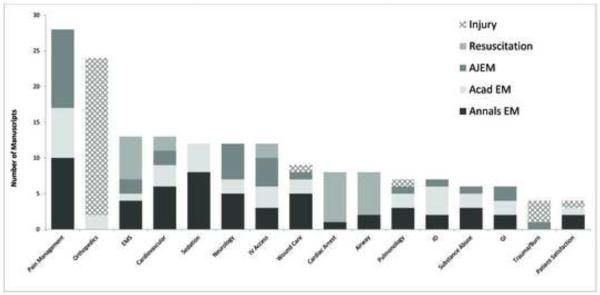
163 published studies were included for analysis. Pain management was the most commonly studied topic (N=28, 17%) followed by orthopedics (N = 24, 15%), cardiovascular disease (N=13, 8%), and pre-hospital medicine (N=13, 8%). Less than half of studies received extramural funding support. Children were specifically examined in 22 (13%) of trials; only 5 trials (3%) specifically examined patients age 60 or older.
Conclusions
Emergency medicine journals publish randomized trials addressing a wide range of clinical topics. Randomized trials focusing on geriatric patients are not commonly published in these journals.
Keywords: Emergency medicine, randomized controlled trials, geriatrics
INTRODUCTION
Background and Importance
Clinical research provides the evidence base for improvements in the quality of emergency medical care and helps to create the unique body of knowledge that defines the specialty of emergency medicine. Randomized trials provide the highest available level of evidence to guide such improvements. Because of the breadth of emergency medicine research and the many journals in which this work may be published, identifying emergency medicine research is a significant challenge.1 Further, although priorities in emergency medicine research have been defined,1, 2 there is little available information about the current areas of emphasis in emergency medicine research. Defining active areas of emergency medicine research may facilitate collaboration among researchers, help clinicians and medical administrators identify study results which can inform policies and standards to improve patient care, and help researchers and funders identify future research priorities. Emergency medicine journals are an obvious and important mechanism for disseminating the results of randomized trials relevant to emergency medical care.
Goals of this Investigation
We sought to describe the research topics, funding sources, study populations, and country of origin of randomized trials recently published in emergency medicine journals.
METHODS
Study Design and Trial Selection
We conducted a descriptive study of randomized controlled trials indexed to PubMed between January 1st 2008 and December 31st 2011 and published in the five emergency medicine journals with the highest impact factors: the American Journal of Emergency Medicine, Academic Emergency Medicine, Annals of Emergency Medicine, Injury, and Resuscitation.3 Randomized trials were identified by searching the selected journals for manuscripts classified by MEDLINE as ‘randomized controlled trials’ and by performing an unrestricted search for the term “random*.” This search mechanism examines titles, key words, and abstracts for words beginning with “random.” We excluded all non-randomized trials, all trials which did not involve human patients, simulation-based studies, and secondary analyses of previously published data. Abstracts and when necessary manuscripts were examined for all articles identified by the MEDLINE search to assess exclusion criteria.
Data Collection and Processing
A study author (CWJ) extracted information about each manuscript using a standardized template. Study topics were not mutually exclusive. For example, a study assessing pain control for migraine headache patients was classified under both ‘neurology’ and ‘pain control.’ Funding source was categorized as industry sponsored, government sponsored, foundation supported or unfunded. Trials were considered industry sponsored if a corporation provided any funding or study supplies free of charge. Other data collected included the number of patients enrolled, the presence of age-related inclusion criteria, and the country of origin of the corresponding author. Studies that restricted the sample to patients aged 18 years or younger were defined as pediatric studies. Studies that restricted the sample to patients aged 60 years or older were defined as geriatric studies.
Primary Data Analysis
Trials were categorized by publishing journal and by topic. Within these categories proportions were compared with respect to funding source, registration rate, and geographic origin. Median sample sizes were calculated. Statistical analysis was performed using SPSS version 17.0 (SPSS Inc, Chicago, IL).
RESULTS
After searching MEDLINE, 771 potentially relevant abstracts were assessed for study inclusion. Of these, 608 were excluded: 306 trials were animal, in-vitro, or simulation-based studies; 143 were non-randomized studies; 128 were review articles or meta-analyses; and 31 were secondary analyses of previously published data. This left 163 eligible manuscripts for analysis. Overall, fewer than half of studies reported receiving funding from external sources, including the government (N=35, 20%), industry (N=25, 15%), and foundations (N=16, 10%) (Table 1). Included studies are cited in a supplementary appendix (Appendix I).
Table 1.
Sources of funding, study characteristics, and age-related inclusion criteria of randomized trials published in emergency medicine journals (2008-2011).
| Journal | Manuscripts N (%) |
Median sample size |
Government Funded N (%) |
Industry Supported N (%) |
Foundation Supported N (%) |
Pediatric population N (%) |
Population over age 60 N (%) |
From the United States N (%) |
|---|---|---|---|---|---|---|---|---|
| AJEM a | 31 (19%) | 100 | 2 (6%) | 3 (10%) | 0 | 4 (13%) | 0 | 13 (42%) |
|
Annals of
EM |
52 (32%) | 181 | 16 (31%) | 7 (13%) | 9 (17%) | 7 (13%) | 0 | 40 (77%) |
|
Academic
EM |
35(21%) | 120 | 12 (34%) | 5 (14%) | 5 (14%) | 9 (26%) | 0 | 29 (83%) |
| Resuscitation | 19 (12%) | 101 | 4 (21%) | 5 (26%) | 1 (5%) | 2 (11%) | 0 | 2 (11%) |
| Injury | 26 (16%) | 57 | 1 (4%) | 5 (19%) | 1 (4%) | 0 | 5 (19%) | 2 (8%) |
|
| ||||||||
| Total | 163 | 110 | 35 (20%) | 25 (15%) | 16 (10%) | 22 (13%) | 5 (3%) | 86 (53%) |
American Journal of Emergency Medicine
Pediatric patients were the primary study population in 22 (13%) studies (Table 1). Injury was the only journal during the period analyzed to publish trials which focused specifically on older patients. Of the five studies which restricted enrollment to patients aged 60 or older, 4 dealt with intra-operative management of hip fractures, and 1 dealt with operative versus conservative management of complex humeral fractures. No other studies focused primarily on older patients.
The most common study topics among all journals were: pain management, orthopedics, prehospital care, cardiovascular disease, procedural sedation, neurology and intravenous access (Figure). Seventeen percent of all trials investigated pain management. Orthopedic trials accounted for 15% of the included studies; all but two were published in Injury (Figure). Government funding was highest for studies related to neurology (5/12, 42%) (Table 2).
Figure.
The topics most commonly studied in randomized trials published in five emergency medicine journals from January 2008 through December 2011.
Table 2.
Manuscript characteristics of the eight topics most frequently studied in randomized trials published in emergency medicine journals (2008-2011)
| Condition Studied: |
Manuscripts N (%) |
Median sample size |
Government Funded (%) |
Industry Supported (%) |
Foundation Supported (%) |
From the United States (%) |
|---|---|---|---|---|---|---|
| Pain Control | 28 (17) | 103 | 11% | 4% | 11% | 68% |
| Orthopedics | 24 (15) | 57 | 4% | 13% | 4% | 17% |
| Pre-hospital | 13 (8) | 149 | 15% | 15% | 15% | 15% |
| Cardiovascular | 13 (8) | 124 | 23% | 31% | 15% | 46% |
| Procedural | 12 (7) | 136 | 0% | 8% | 25% | 58% |
| Sedation | ||||||
| Neurology | 12 (7) | 89 | 42% | 0% | 0% | 83% |
| IV Access | 12 (7) | 80 | 0% | 25% | 0% | 75% |
| Wound Care | 9 (6) | 164 | 22% | 11% | 11% | 67% |
|
| ||||||
| Overall | 163 | 110 | 20% | 15% | 10% | 53% |
Manuscripts originated from 24 countries, including 86 (53%) from the United States. Every journal published trials from at least 3 different continents. Studies from North America made up large majorities of the trials published in Annals of Emergency Medicine (81%) and Academic Emergency Medicine (89%). The American Journal of Emergency Medicine published 31 trials, 14 (45%) from North America, 10 (32%) from Asia, 5 (16%) from Europe, and 2 (6%) from Africa. No other journal published African trials. Europe was the source of 69% of trials published in Injury. The majority of trials published in Resuscitation were from either Europe (42%) or Asia (26%). The total number of trials led by non-US investigators published in US-based journals (N=36) was similar to the number of trials by non-US investigators published in the two non-US based journals, Injury and Resuscitation (N=41).
LIMITATIONS
This study has several important limitations. We did not analyze emergency medicine research published in other specialty or general medical journals, which considerably constrains our ability to draw conclusions about the characteristics of all emergency medicine research. Certain types of trials, particularly studies related to trauma care and resuscitation, are often done with large numbers of patients and a large investment of research money and achieve results whose relevance extends beyond the field of emergency medicine. Studies of this type are probably more likely to be published in general medical journals than in emergency medicine journals. Consequently, the distribution of topics of trials published in emergency medicine journals is likely to differ from the distribution found among the broader groups of all studies by emergency medicine researchers or all studies which have implications for emergency medicine practice. This study is none-the-less informative about activity within emergency medicine journals and likely suggests areas of activity or lack of activity in the field. It is also possible that our search strategy failed to identify some randomized trials published in these five journals during the period analyzed. If trials were missed in a systematic fashion, this may have affected the trends reported here. Information about trial funding is based on sources of funding as reported in published manuscripts. If authors failed to report this information then our estimate of the percentage of trials receiving external funding will be low. Study data was abstracted by a single author, and it is possible that other abstractors would characterize studies differently. Finally, this investigation provides aggregate data from trials published over a four year period. The small number of studies in many of the categories included here limits our ability to comment on the strength of many of the described associations.
DISCUSSION
Our results indicate that randomized trials recently published in emergency medicine journals address a wide range of clinical topics. Investigations into pain management, orthopedic injuries, prehospital case and cardiovascular disease are particularly well represented. Most of the topics studied are published in multiple journals, and four of the five journals included in this study published on a broad range of clinical topics. Orthopedic studies were nearly exclusively published in Injury, comprising most of the randomized trials published in that journal. Many of the included studies were conducted without the support of external funding sources such as the National Institutes of Health (NIH) or other governmental agencies. Much current emergency medicine research is published in non-emergency medicine journals and much important research occurs through means other than randomized trials. However, the data presented here provides insight into current patterns of randomized trial funding as well as current areas of active emergency medicine research.
Research addressing the emergency department care of geriatric patients has been highlighted as a priority for future clinical trials.1, 4 The prioritization of research which is relevant to care for geriatric patients is particularly important given the rapidly aging United States population and the increasing rates of emergency department utilization by older adults.5, 6 Older adults are less likely to be enrolled in ED based research, with increased rates of exclusion due to cognitive impairment and family declining enrollment.7 Older adults are also less likely to be enrolled in randomized trials for cancer treatment and acute lung injury.8, 9 These limitations may be addressed in part by designing trials which specifically enroll and address the needs of geriatric research participants. Among the studies we examined, patients aged 60 or older were specifically examined in just 3% of trials. None of these received NIH or other governmental support.
Nearly 50% of the trials identified were published outside of the United States, demonstrating the important contribution of emergency medicine researchers from around the world. During the four year period analyzed, the three US-based journals published almost half of the studies originating outside the US. The large contribution of non-US researchers is particularly noteworthy because in many countries emergency medicine has only recently been recognized as an independent medical specialty and infrastructure for both emergency medicine-related research and clinical training remains under-developed in much of the world.10
In conclusion, emergency medicine journals publish randomized trials addressing a diverse range of clinical topics, many of which did not receive external funding. Trials focusing on geriatric patients are not commonly published in emergency medicine journals.
Supplementary Material
Acknowledgments
Funding: This study was supported by Award Number KL2 RR025746 and UL1 RR025747 from the National Center for Research Resources through the North Carolina Translational and Clinical Science Institute (Dr Platts-Mills).
Role of the Sponsors: The National Center for Research Resources had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review or approval of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the National Institutes of Health, or the North Carolina Translational and Clinical Science Institute.
Footnotes
Supplementary Appendix. This appendix includes the manuscript title, authorship, journal information, and PubMed identification number for included trials.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Address for reprints: Reprints not available from authors
Contributor Information
Katherine M. Hunold, University of North Carolina Chapel Hill School of Public Health
Cameron G. Isaacs, University of North Carolina Chapel Hill Department of Emergency Medicine.
Timothy F. Platts-Mills, University of North Carolina Chapel Hill Department of Emergency Medicine.
REFERENCES
- 1.Kaji AH, Lewis RJ, Beavers-May T, et al. Summary of NIH Medical-Surgical Emergency Research Roundtable held on April 30 to May 1, 2009. Ann Emerg Med. Nov;56(5):522–537. doi: 10.1016/j.annemergmed.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Miller SZ, Rincon H, Kuppermann N. Revisiting the emergency medicine services for children research agenda: priorities for multicenter research in pediatric emergency care. Acad Emerg Med. 2008 Apr;15(4):377–383. doi: 10.1111/j.1553-2712.2008.00072.x. [DOI] [PubMed] [Google Scholar]
- 3.2009 Journal Citation Reports. Science Edition Thompson Reuters; 2010. [Google Scholar]
- 4.Schumacher JG. Emergency medicine and older adults: continuing challenges and opportunities. Am J Emerg Med. 2005 Jul;23(4):556–560. doi: 10.1016/j.ajem.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 5.He W. 65+ in the United States : 2005. U.S. Dept. of Commerce, Economics and Statistics Administration For sale by the Supt. of Docs., U.S. G.P.O.; Washington, D.C.: 2005. [Google Scholar]
- 6.Xu KT, Nelson BK, Berk S. The changing profile of patients who used emergency department services in the United States: 1996 to 2005. Ann Emerg Med. 2009 Dec;54(6):805–810. e801–807. doi: 10.1016/j.annemergmed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Glickman SW, Anstrom KJ, Lin L, et al. Challenges in enrollment of minority, pediatric, and geriatric patients in emergency and acute care clinical research. Ann Emerg Med. 2008 Jun;51(6):775–780. e773. doi: 10.1016/j.annemergmed.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999 Dec 30;341(27):2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 9.Cooke CR, Erickson SE, Watkins TR, et al. Age-, sex-, and race-based differences among patients enrolled versus not enrolled in acute lung injury clinical trials. Crit Care Med. Jun;38(6):1450–1457. doi: 10.1097/CCM.0b013e3181de451b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alagappan K, Schafermeyer R, Holliman CJ, et al. International emergency medicine and the role for academic emergency medicine. Acad Emerg Med. 2007 May;14(5):451–456. doi: 10.1197/j.aem.2007.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.