Abstract
Background
Translocation of gastrointestinal bacteria in HIV-infected individuals is associated with systemic inflammation, HIV progression, mortality, and co-morbidities. HIV-infected individuals are also susceptible to fungal infection and colonization, but whether fungal translocation occurs and influences HIV progression or co-morbidities is unknown.
Methods
Serum (1→3)-β-D-glucan was measured by a Limulus Amebocyte Lysate assay (Fungitell®) in 132 HIV-infected outpatients. Selected plasma cytokines and markers of peripheral T-cell activation were measured. Pulmonary function testing and Doppler-echocardiography were performed. Relationship of high (≥40pg/ml) and low (<40pg/ml) levels of (1→3)-β-D-glucan with HIV-associated variables, inflammation markers, and pulmonary function and pulmonary hypertension measures were determined.
Results
Forty-eight percent had detectable (1→3)-β-D-glucan, and 16.7% had high levels. Individuals with high (1→3)-β-D-glucan were more likely to have CD4 counts below 200 cells/μl (31.8% vs. 8.4%, p=0.002), had higher log10 HIV viral levels (2.85 vs. 2.13 log copies/ml, p=0.004), and were less likely to use ART (68.2% vs. 90.0%, p=0.006). Plasma IL-8 (p=0.033), TNF-α (p=0.029), and CD8+CD38+ (p=0.046) andCD8+HLA-DR+ (p=0.029) were also increased with high levels. Abnormalities in diffusing capacity (p=0.041) and in pulmonary artery pressures (p=0.006 for pulmonary artery systolic pressure and 0.013 for tricuspid regurgitant velocity) were more common in those with high (1→3)-β-D-glucan.
Conclusions
We found evidence of peripheral fungal cell wall polysaccharides in an HIV-infected cohort. We also demonstrated an association between high serum (1→3)-β-D-glucan, HIV-associated immunosuppression, inflammation, and cardiopulmonary co-morbidity. These results implicate a new class of pathogen in HIV-associated microbial translocation and suggest a role in HIV progression and co-morbidities.
Keywords: HIV, COPD, (1→3)-β-D-glucan, pulmonary hypertension, emphysema, inflammation
INTRODUCTION
In HIV-infected individuals, microbial translocation is associated with systemic inflammation, HIV progression, mortality, and progression of co-morbidities [1-5]. Translocation of gastrointestinal bacteria secondary to CD4+ T-lymphocyte depletion occurs early in HIV, and bacterial products such as lipopolysaccharide (LPS) are detectable in peripheral blood [1, 4, 5]. Although HIV-infected individuals have increased susceptibility to fungal infections and chronic fungal colonization, whether fungal products are detectable in the blood and relate to HIV-associated outcomes has not been investigated. Translocation of fungal cell wall components and/or intact organisms could occur from sites such as the skin, the oral cavity and respiratory tract, the gastrointestinal tract, and the urogenital system. If such translocation occurs, it could act similarly to bacterial translocation in stimulating a systemic immune response that leads to HIV progression and co-morbidities.
(1→3)-β-D-glucan (BG) is a polysaccharide found in the cell wall of fungal organisms such as Aspergillus, Candida, and Pneumocystis. Because these cell wall polysaccharides are shed into the circulation during infection, elevated serum BG levels can be used to diagnose fungal pneumonia in both immunosuppressed and non-immunosuppressed populations [6-8]. Lower BG levels can also be detected in serum samples from healthy individuals, presumably from sloughing of commensal fungus into the bloodstream [9, 10]. BG is highly immunogenic. It activates macrophages, neutrophils, and T-cells and stimulates release of pro-inflammatory cytokines such as interleukin (IL)-8, tumor necrosis factor (TNF)-α, and IL-6 [6, 11, 12]. Whether BG is detectable in the serum of stable HIV-infected individuals and is associated with immune activation is currently unknown.
Both chronic obstructive pulmonary disease (COPD) and pulmonary hypertension have been reported to be increased in HIV-infected individuals [13-17]. We have recently demonstrated that airway obstruction, decreased diffusing capacity for carbon monoxide (DLco), and abnormal pulmonary artery systolic pressures as measured by Doppler-echocardiography commonly occur simultaneously in HIV and are associated with systemic and pulmonary inflammation, but triggers of this inflammation have not yet been identified [18]. We hypothesized that translocation of fungal components in HIV-infected individuals could drive these processes.
In order to assess if fungal cell wall products are detectable in serum of HIV-infected individuals and if they are related to HIV-associated outcomes, we measured serum levels of the fungal cell wall polysaccharide, BG and then related BG levels to measures of immunosuppression, immune activation, and COPD and pulmonary hypertension in HIV-infected individuals.
METHODS
Participants and data collection
Participants were 132 HIV-infected individuals recruited from the University of Pittsburgh HIV/AIDS clinic between January 2009 and July 2011. All were 18 years of age or older and were free from new or increasing respiratory symptoms and fevers within the past four weeks. They were recruited from the HIV clinic using advertisements and a research registry. Prior lung disease was not a criteria for either inclusion or exclusion. All participants signed informed consent, and the University of Pittsburgh IRB approved the protocol. A description of a subset of the cohort has been published previously [13]. Participants completed a standardized interview to obtain demographic and clinical data. Data obtained included age, gender, race/ethnicity, smoking history, alcohol and intravenous drug use (IDU), height and weight, time HIV-infected, use of antibiotics and antiretroviral medications, and history of previous pneumonia. Medical record review was performed to collect hemoglobin, creatinine, and the most recent CD4+ T-lymphocyte cell count and plasma HIV ribonucleic acid (RNA) level within three months. The lower limit of HIV RNA detection was 50 copies/ml. Whether a participant had started therapy with combination antiretrovirals in the past 18 months was also recorded. As albumin, innumoglobulin therapy, or amoxicillin-clavulinic acid can falsely elevate BG levels, we recorded use of these agents. No participants were receiving albumin or immunoglobulin therapy at the time of their visits. Two participants had received amoxicillin-clavulinic acid within a month of the study visit, but their BG levels were both categorized as low and they were continued in analyses. The glomerular filtration rate (GFR) was calculated using the Modification of Diet in Renal Disease equation [19].
(1→3)-β-D-glucan measurements
A BG-specific Limulus Amebocyte Lysate (LAL) assay for serum levels of (1→3)-β-D-glucan was performed using the Fungitell assay protocol (Associates of Cape Cod Incorporated, East Falmouth, MA)[9, 10]. Serum samples, in volumes of 5 μl, were repeated in duplicate on each plate and treated with the appropriate reagents, prior to microplate reader incubation and analysis, as per protocol. Average of duplicate samples was reported. The lower limit of detection of the assay was 7 pg/ml. Levels were also dichotomized as below or above 40 pg/ml based on the reported detectable levels in non-HIV-infected healthy individuals [9, 10]. For the overall cohort, BG was tested using blood samples obtained at the time of study visit. For the individuals who had started ART in the previous 18 months, a pre-ART sample was also analyzed that was obtained 18 months prior to the study visit. Samples were collected without the use of cotton gauze.
Measures of peripheral inflammation and lipopolysaccharide
Plasma interleukin (IL)-6, IL-8, IL-10, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and IFN-α were measured using Luminex (Luminex Corporation, Austin, TX). In individuals in whom fresh peripheral blood mononuclear cells (PBMCs) were available, PBMCs were isolated by density gradient centrifugation. Cell surface staining was performed using fluorochrome-conjugated monoclonal antibodies including isotype controls: fluorescein isothiocyanate (FITC) anti-CD8; phycoerythrin (PE) anti-CD38; PE-anti-HLA-DR; phycoerythrin-cyanine 7 (PE-Cy7) anti-CD3; and allophycocyanin (APC) CD4 (BD Pharmingen, San Diego, CA). Flow cytometry was performed on ≥10,000 live cells after lymphocyte gating using a BD FACS Calibur (BD Biosciences, San Jose, CA) and results analyzed using Cellquest Pro software (version 0.3.bfdb). Lipopolysaccharide (LPS) levels were measured in plasma samples from all participants using a limulus amebocyte assay (Cambrex) as previously described using endotoxin-free reagents and supplies [1, 2].
Pulmonary function testing
Post-bronchodilator spirometry was performed according to ATS standards [20, 21]. Hankinson prediction equations were used to calculate post-bronchodilator forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) [22]. Diffusing capacity for carbon monoxide (DLCO) percent predicted was adjusted for hemoglobin and carboxyhemoglobin, and Neas equations were used to calculate predicted values [23].
Sputum induction
Sputum induction was performed with nebulized 3% saline [24]. Sputum cell counts and percentages were determined as previously described, and samples with fewer than 30% squamous cells were considered acceptable [25]. Sputum supernatant IL-6, IL-8, IL-10, TNF-α, IFN-γ, and IFN-α were measured by Luminex (Luminex Corporation, Austin, TX). Pneumocystis colonization was determined in induced sputum samples by performing nested polymerase chain reaction (PCR) at the mitochondrial large subunit rRNA locus as previously described [26].
Doppler-echocardiography
Of the total cohort, 106 underwent echocardiography which was performed by a single operator on the same echocardiography machine (GE-Vingmed Vivid 7, GE Vingmed Ultrasound, Horten, Norway). Studies were read by one of three cardiologists blinded to participant status. Standard 2D views, pulsed and continuous wave Doppler measurements were obtained according to American Echocardiography Association recommendations [27, 28]. Peak pulmonary artery systolic pressures (PASP) and the maximum velocity of the tricuspid regurgitant jet (TRV) were estimated as previously described [29].
Statistical analyses
Statistical analyses were performed using Stata version 10 (StataCorp, College Station, Texas, USA). Demographic and clinical characteristics of the cohort were described. Variables were log10-transformed, dichotomized, or the square root was calculated as appropriate. BG levels were described as a continuous variable and dichotomized as low or high (below or above 40 pg/ml based on the reported levels in non-HIV-infected healthy individuals)[9, 10]. Analyses were also repeated using a BG cut-off of the 75th percentile of the cohort and were similar. Relationship of demographic and clinical variables such as age, gender, and race to low or high BG levels were determined using Wilcoxon ranksum and t-tests or Fisher's exact and chi-square as appropriate.
Differences in the square root of the CD4 cell count and log10-HIV viral RNA level according to low or high BG were determined using t-tests. Chi-square was performed to determine relationship of BG levels to use of ART in the previous three months. In individuals who recently started ART, the percentage with high BG pre- and post-ART were compared using McNemar's test.
Plasma and sputum cytokine levels were compared between individuals with high and low BG levels using t-tests or chi-square. Plasma cytokines were available for 122 participants, and sputum cytokines and cell counts were available in 111. These samples were not available in all participants secondary to lower blood volume initially collected (plasma) or because they either could not produce sufficient sputum or because sputum samples contained greater than 30% epithelial cells. In participants with flow cytometry data available (n=32 for CD38+, n=21 for HLA-DR+ based on availability of sufficient viable cells), Wilcoxon ranksum was used to compare percentages of T-cell subsets between those with high or low BG levels. Plasma LPS levels were compared between participants with high and low BG levels. Prevalence of Pneumocystis colonization defined as detection of human Pneumocystis in induced sputum was compared between individuals with high and low BG levels using chi-square.
In order to determine the relationship of BG levels to measures of pulmonary function and echocardiographic measures of pulmonary hypertension, post-bronchodilator FEV1 percent predicted, FEV1/FVC, DLco percent predicted adjusted for hemoglobin and carboxyhemoglobin and PASP and TRV were analyzed by t-tests by high or low BG levels. Pulmonary and cardiac outcomes were also dichotomized according to various clinically relevant cut-offs. Airway obstruction was defined as a post-bronchodilator FEV1/FVC below 0.70, and an abnormal DLco was defined as less than 60% predicted [18]. The DLco cut-off was chosen based on previous data demonstrating that this level of DLco impairment in HIV is associated with increased respiratory symptoms, inflammation, and pulmonary vascular abnormalities [18]. An abnormal PASP was defined as a pressure of at least 35mm Hg and an abnormal TRV as at least 3.0 m/sec. Individuals without a measurable tricuspid regurgitant jet and normal right ventricular function were assumed to have normal PASP and TRV [16, 30]. Chi-square or Fisher's exact were used to determine associations of these outcomes to BG levels, and odds ratios and 95% confidence intervals calculated. Because this study was an exploratory analysis, adjustments for multiple comparisons were not performed.
RESULTS
Detection of (1→3)-β-D-glucan
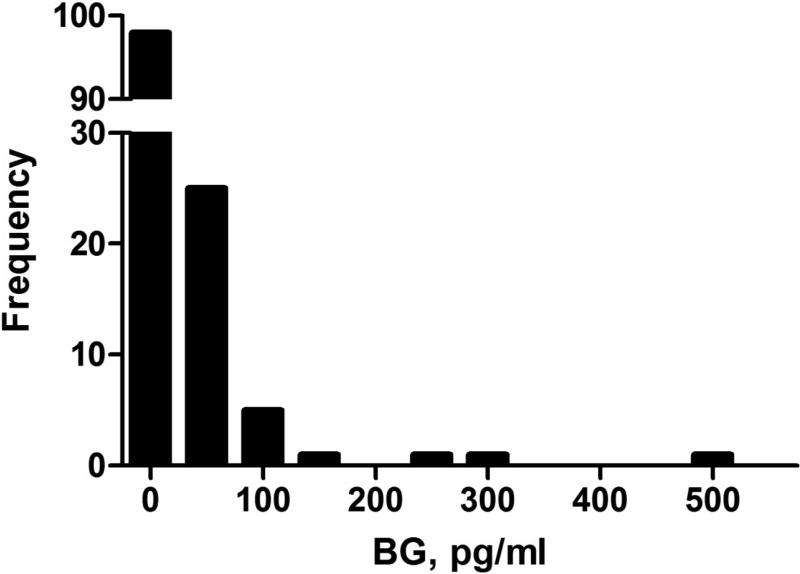
Serum BG was measured in 132 HIV-infected individuals. Average age of the cohort was 45.7 years, and 67.4% were male (Table 1). The median BG level was 7 pg/ml (the lower limit of the assay) with an upper level of 532.4 pg/ml (Figure 1). Forty-eight percent had a serum BG level above the threshold of detection, and 16.7% (n=22) had a BG level above 40 pg/ml. Individuals with high BG levels were more likely to have previously had pneumonia, but other characteristics including history of PCP and detection of Pneumocystis colonization were similar (Table 1).
Table 1.
Characteristics of HIV-infected participants by high (>40pg/ml) or low (≤40 pg/ml) serum (1→3)-β-D-glucan.
| Characteristic | Overall, n=132 | High BG n=22 | Low BG n=110 |
|---|---|---|---|
| Age, mean years (SD) | 45.7 (9.5) | 43.8 (9.5) | 46.1 (9.6) |
| Male, n (%) | 89 (67.4) | 12 (54.6) | 77 (70.0) |
| Race, n (%)* | |||
| White | 55 (41.7) | 6 (27.3) | 49 (44.5) |
| African-American/other | 77 (58.3) | 16 (72.7) | 61 (55.5) |
| HIV risk factor, n (%) | |||
| MSM | 56 (42.1) | 5 (22.7) | 51 (46.4) |
| Heterosexual | 50 (37.6) | 11 (50.0) | 37 (33.6) |
| Other or unknown | 26 (20.3) | 6 (27.3) | 22 (20.0) |
| Hepatitis ever, n (%) | 12 (9.1) | 2 (9.1) | 10 (9.1) |
| Diabetes, n (%) | 11 (8.3) | 1 (4.6) | 10 (9.1) |
| Previous pneumonia*, n (%) | 29 (22.0) | 9 (40.9) | 20 (18.2) |
| Previous PJP, n (%) | 3 (2.3) | 1 (4.6) | 2 (1.8) |
| Pneumocystis-colonized, n (% of 111) | 30 (27.0) | 5 (26.3) | 25 (27.2) |
| CD4 cell count below 200 cells/μl*, n (%) | 16 (12.1) | 7 (31.8) | 9 (8.2) |
| CD4 cell count from 200-500 cells/μl, n (%) | 40 (30.3) | 1 (4.5) | 39 (35.5) |
| CD4 cell count above 500 cells/μl, n (%) | 76 (57.6) | 14 (63.6) | 62 (56.4) |
| ART use >18 months, n (% of 114) | 101 (88.6) | 13 (86.7) | 88 (88.9) |
| IDU in past 6 months, n (%) | 1 (0.78) | 0 | 1 (0.91) |
| Ever smoker, n (%) | 110 (83.3) | 19 (86.4) | 91 (82.7) |
| Current smoker, n (%) | 75 (56.8) | 13 (59.1) | 62 (56.4) |
| Pack-year history, median (range) | 10.0 (0-75) | 15.0 (0-70) | 10 (0-75) |
| Body mass index, mean (SD) | 26.8 (6.0) | 26.9 (6.1) | 26.6 (4.7) |
| Hemoglobin, mean g/dl, (SD) | 13.6 (1.5) | 13.0 (1.2) | 13.7 (1.5) |
| GFR, median (range) | 93 (3-143) | 90 (3-143) | 95 (13-140) |
Abbreviations: ART, antiretroviral therapy; BG, serum (1→3)-β-D-glucan; GFR, glomerular filtration rate in ml/min per 1.73 m2; IDU, intravenous drug use; MSM, men who have sex with men; PJP, Pneumocystis jirovecii pneumonia; SD, standard deviation.
p=0.04 for race; p=0.019 for previous pneumonia;p=0.001 for CD4 cell count.
Figure 1.
Distribution of serum (1→3)-β-D-glucan in 132 HIV-infected individuals.
Abbreviations: BG, serum (1→3)-β-D-glucan
Correlation of (1→3)-β-D-glucan with HIV-associated variables
HIV-associated variables in individuals with high BG levels differed significantly from those with low levels (Table 2). Mean square root of the CD4 cell count tended to be lower, and more high BG individuals had CD4 cell counts below 200 cells/μl. Log10 HIV viral levels were also higher and percentage of those with a detectable viral level tended to be higher in individuals with high BG as well. Current ART use was less common among those with high BG levels. In the 13 individuals who had recently initiated ART, detectable BG (above 7 pg/ml) was present in pre-ART samples in 87.5% compared to 60.0% in the post-ART samples, although this comparison did not reach statistical significance (p=0.096). Those individuals who had used Pneumocystis prophylaxis in the past three months were more likely to have high BG levels.
Table 2.
HIV-associated variables differ significantly in individuals with high (>40pg/ml) or low (≤40 pg/ml) serum (1→3)-β-D-glucan.
| Characteristic | Overall, n=132 | High BG n=22 | Low BG n=110 | p-value |
|---|---|---|---|---|
| CD4 cell count, mean square root cells/μl, (SD) | 23.3 (7.4) | 21.4 (7.9) | 23.7 (7.2) | 0.185 |
| CD4 cell count below 200 cells/μl, n (%) | 16 (12.1) | 7 (31.8) | 9 (8.2) | 0.002 |
| Mean log10 HIV viral level, (SD) | 2.24 (1.08) | 2.85 (1.50) | 2.13 (0.95) | 0.004 |
| Detectable HIV viral level, n (%) | 39 (29.7) | 10 (45.5) | 29 (26.6) | 0.078 |
| Current ART use, n (%) | 114 (86.4) | 15 (68.2) | 99 (90.0) | 0.006 |
| PJP prophylaxis in past year, n (%) | 31 (23.5) | 9 (40.9) | 22 (20.0) | 0.035 |
| Time HIV-infected, median years (range) | 14.3 (1.6-30.6) | 11.8 (2.2-26.9) | 14.6 (1.6-30.6) | 0.456 |
Abbreviations: ART, antiretroviral therapy; BG, serum (1→3)-β-D-glucan; PJP; Pneumocystis jirovecii pneumonia; SD, standard deviation. Detectable HIV viral level defined as less than 50 copies/ml.
Immune activation
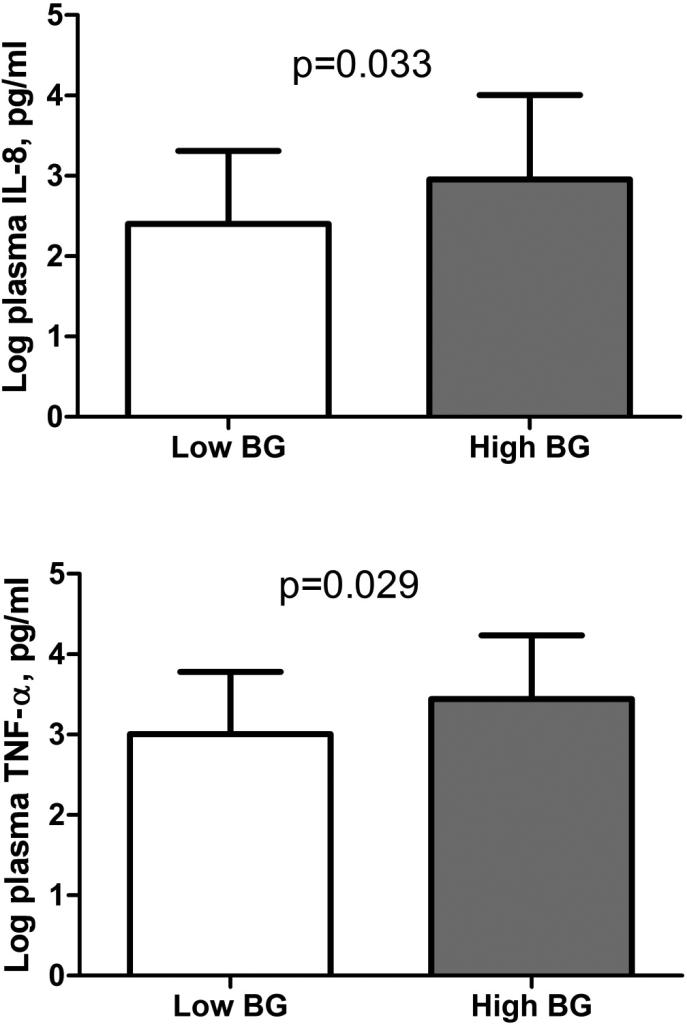
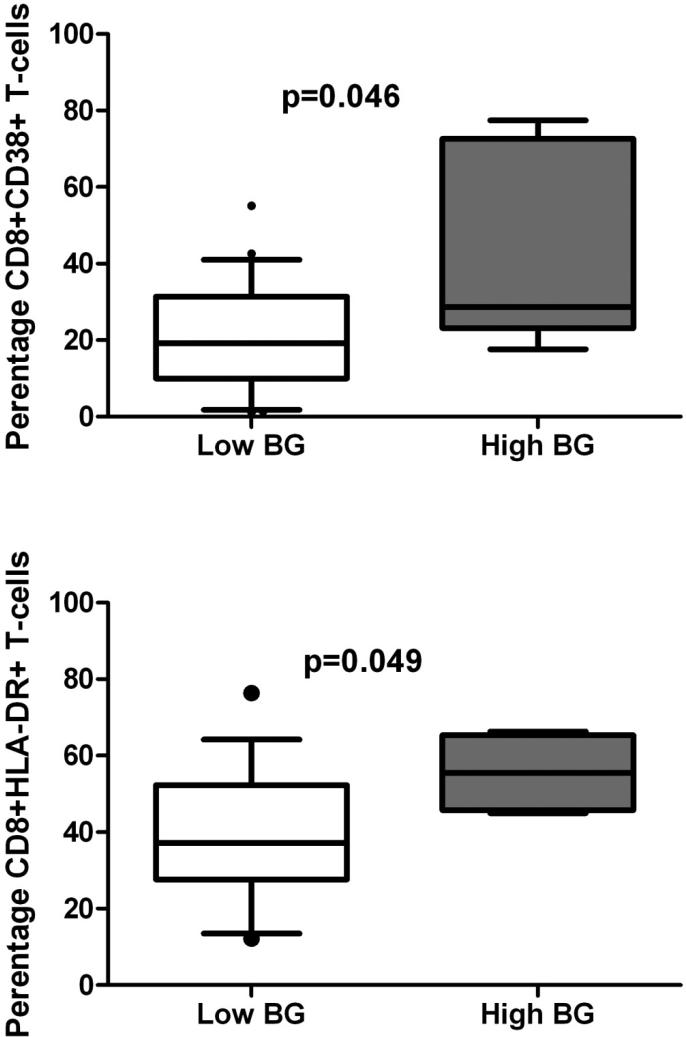
Of the plasma cytokines tested, IL-8 and TNF-α levels were higher in those with high BG levels (Figure 2). Those with high BG were also more likely to have high levels of IL-10 (90.0% of high BG vs. 62.4% of low BG, p=0.018). Levels of IFN-γ and IFN-α did not differ significantly between high and low BG groups (2.64 log pg/ml vs. 2.47 log pg/ml for low BG, p=0.544 for IFN-γ; 3.87 log pg/ml vs. 3.73 log pg/ml for low BG, p=0.630 for IFN- α). IL-6 was also not significantly different (1.40 log pg/ml vs. 1.10 log pg/ml for low BG, p=0.433). The percentages of CD8+ T-cells expressing HLA-DR and CD38 were significantly higher in those with high BG levels (Figure 3). Percentages of CD4+ HLA-DR+ (24.8% for high BG vs. 20.7% for low BG, p=0.706) and CD4+CD38+ T-cells (59.6% for high BG vs. 41.8% for low BG, p=0.102) did not differ by BG status. LPS levels were not significantly different between those with low and high BG levels (median LPS 16.3 pg/ml for low vs. 14.8 for high, p=0.756).
Figure 2.
Plasma cytokines are increased in HIV-infected individuals with high (>40pg/ml) or low (≤40 pg/ml) serum (1→3)-β-D-glucan.
Abbreviations: BG, serum (1→3)-β-D-glucan; IL, interleukin; TNF, tumor necrosis factor.
Figure 3.
T-cell markers of immune activation are increased in HIV-infected individuals with high (>40pg/ml) or low (≤40 pg/ml) serum (1→3)-β-D-glucan. n=71 for CD38+, n=21 for HLA-DR+
Abbreviations: BG, serum (1→3)-β-D-glucan. Bars=median, whiskers=10-90th percentile. P=0.046 for CD8+CD38+, p=0.049 for CD8+HLA-DR+
(1→3)-β-D-glucan and cardiopulmonary function
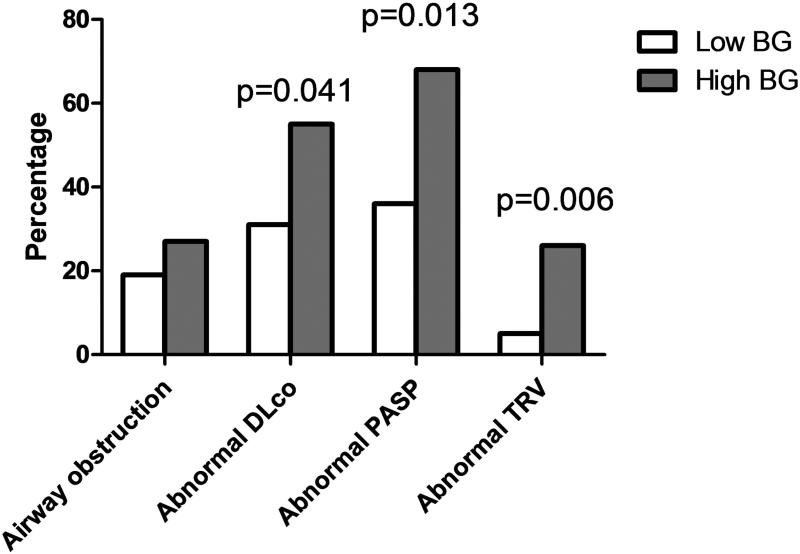
In analyses of pulmonary function, FEV1 percent predicted and DLco percent predicted tended to be lower in those with high BG levels (89.6% vs. 96.9%, p=0.078 for FEV1 percent predicted; 60.4% vs. 67.0%, p=0.051 for DLco percent predicted). FEV1/FVC did not differ by BG level (75.6% vs. 77.8%, p=0.35). A high BG level was significantly associated with having a DLco below 60% of predicted, but not with having airway obstruction (OR=2.65, 95% CI=1.04-6.72, p=0.041 for DLco; OR=1.59, 95%CI=0.56-4.56, p=0.388 for airway obstruction; Figure 4). Echocardiographic measurements of pulmonary hypertension were significantly increased in those with high BG levels (mean PASP 39.9 mmHg for high BG vs. 32.7 mmHg for low BG, p=0.0002; mean TRV=2.7 m/sec for high BG vs. 2.4 m/sec, p=0.0005). A high BG level was also associated with a greater likelihood of having an abnormal PASP (OR=3.84, 95% CI=1.33-11.13, p=0.013) or TRV (OR=7.32, 95% CI=1.75-30.65, p=0.006)(Figure 4).
Figure 4.
Prevalence of cardiopulmonary abnormalities by high (>40pg/ml) or low (≤40 pg/ml) serum (1→3)-β-D-glucan.
Abbreviations: Airway obstruction=forced expiratory volume in one second/forced vital capacity <0.70; abnormal DLco, diffusing capacity for carbon monoxide ≤60% predicted adjusted for hemoglobin and carboxyhemoglobin; abnormal PASP, pulmonary artery systolic pressure ≥35 mm Hg; abnormal TRV, tricuspid regurgitant jet velocity ≥3m/sec; BG, serum (1→3)-β-D-glucan.
CONCLUSIONS
This study demonstrates that fungal BG is detectable in the peripheral blood in HIV-infected outpatients without known active fungal infection. We found evidence of the fungal cell wall polysaccharide (1→3)-β-D-glucan in the serum of almost half of an HIV-infected outpatient cohort. This detection could represent translocation of the entire organism from another body site or shedding of the cell wall into the bloodstream. In addition, we demonstrated an association between high levels of serum (1→3)-β-D-glucan and degree of HIV-associated immunosuppression, systemic inflammation, and cardiopulmonary co-morbidity.
The findings of the current study parallel previous findings in bacterial translocation in several ways. For example, prior studies of bacterial translocation report detection of LPS, a component of the gram-negative bacterial cell wall, in peripheral blood of HIV-infected individuals without acute bacterial infection [1, 3, 31]. Here, we found a ubiquitous fungal cell-wall component in the peripheral blood of HIV-infected individuals not known to have active fungal infection. Higher levels of this cell-wall component were seen in those with lower CD4 cell counts, higher HIV viral levels, and in those not receiving ART, similar to findings in bacterial translocation [1, 4, 5]. We also saw a tendency for BG to decrease with ART, although numbers were small. LPS levels were not associated with BG levels, suggesting that BG is not a marker of general microbial translocation. As is the case with bacterial translocation, however, [1, 4, 5, 32, 33], fungal translocation was associated with immune activation, demonstrating increases in inflammatory cytokines and in T-cell expression of CD38 and HLA-DR. The bacterial translocation seen in HIV is presumed to occur from the gastrointestinal tract, but we do not know the origin or origins of fungal translocation. Given that HIV-infected individuals are susceptible to oral and esophageal candidiasis, Pneumocystis colonization of the respiratory tract, as well as other fungal infections, fungal translocation could occur from colonizing organisms of the mucosal surfaces of the gastrointestinal, respiratory, and genitourinary tracts or from diseased skin. Although it might be hypothesized that Pneumocystis prophylaxis would decrease BG levels, the association of higher BG with prophylactic antibiotics in combination with a lack of association of BG levels with Pneumocystis colonization suggests that other fungal species are important. Given that the prophylactic agents may also have anti-bacterial effects, this finding could implicate fungal overgrowth in the setting of decreased bacterial load as contributing to BG levels [34].
An important aspect of bacterial translocation is its ability to induce immune activation. BG is highly immunogenic, stimulating macrophages, neutrophils, and T-cells and leading to release of pro-inflammatory cytokines such as IL-8 and TNF-α [12, 35, 36]. We found that BG levels were associated with higher plasma IL-8, IL-10, TNF-α levels and with increases in activated CD8+ T-cells. We did not find an association with IL-6, IFN-α, or IFN-γ as has been reported in bacterial translocation.
Another important similarity with previous studies of bacterial translocation is the association with co-morbidities. Although bacterial translocation has not been associated with the specific cardiopulmonary co-morbidities studied here, it has been linked to other co-morbid processes including progression of hepatitis C, AIDS-associated dementia, and cardiovascular disease [3, 31]. We examined pulmonary function and pulmonary hypertension in our cohort. COPD, particularly abnormalities in DLco, and echocardiographic findings of pulmonary hypertension are common in HIV-infected individuals [13-17]. In addition, COPD and pulmonary hypertension often occur concurrently in HIV-infected individuals in association with systemic and pulmonary inflammation [18], but triggers of this immune activation are unknown.
The mechanism by which fungal translocation might result in cardiopulmonary dysfunction is unknown, but based on our findings, we hypothesize that chronic fungal translocation may have detrimental effects both by stimulating systemic immune activation and by direct local effects. For example, the dysregulated T-cells induced by peripheral BG may contribute to lung damage when they are recruited to the lungs in response to stimuli such as smoking, HIV, or other infections. T-cells may also stimulate pulmonary inflammation via direct interactions with alveolar macrophages as they pass through the pulmonary circulation. It is also possible that the fungal antigens act locally to cause end-organ damage. Alveolar macrophages, lung epithelial, and endothelial cells have fungal receptors that respond to circulating fungal antigens by secreting pro-inflammatory cytokines and proteases, resulting in local damage and recruitment of additional inflammatory cells to the lung [12, 35]. Exposure to fungal beta-glucans and other fungal cell wall components stimulates production of chitinases which have been linked to asthma and COPD in the non-HIV-infected population, providing another potential mechanism linking fungal translocation and pulmonary disease [37-40].
The study has several limitations. First, it is a single-site cohort study, and therefore results may not be generalizable to other HIV-infected populations. Second, we are unable to determine the origin of the fungal cell wall BG detected in peripheral blood. Although Pneumocystis colonization of induced sputum did not correlate with BG, studies of bronchoscopic samples might be more sensitive. Additional epidemiologic studies examining specific clinical risk factors for fungal infections as well as in-depth sampling of various body sites coupled with more specific fungal diagnostics could be informative. We also could not entirely rule-out the presence of occult fungal infections influencing the results, but participants were not known to have active disease, and we excluded symptomatic individuals. Further, the majority were receiving ART and had high CD4 cell counts, making fungal infection less likely; however, it remains possible that the BG levels detected may be an early indicator of future fungal infection. Additional limitations are that we did not adjust for multiple comparisons and that the analysis is cross-sectional. We do not know the trajectory of BG detection over time or its influence on long-term outcomes, nor can we determine causality or the nature of the relationship of BG, inflammation, advanced HIV, and end-organ damage. We also do not have a healthy HIV-uninfected control group for comparison. Finally, BG does not detect fungal species such as Cryptococcus or Mucormycoses and thus may have underestimated degree of fungal translocation.
In summary, our data support the novel theory that fungal translocation occurs in HIV-infected individuals and relates to important outcomes such as HIV-associated immunosuppression, inflammation, and cardiopulmonary co-morbidity. These results implicate a fungal species in HIV-associated microbial translocation and suggest a role in HIV progression and development of co-morbidities. Finally, the findings suggest that novel therapies such as anti-fungal agents or immune-modifying drugs could impact disease outcomes in HIV infection.
Acknowledgments
Funding: This work was supported by the National Institutes of Health [R01 HL083461, HL083461S, and R01 HL090339 to AM; T32 HL007563 and K23 HL108697 to MRG], Gilead Sciences to AM, and the University of Pittsburgh Clinical and Translational Science Institute [UL1 RR024153]. Associates of Cape Cod Incorporated donated (1→3)-β-D-glucan kits.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 2.Jiang W, Lederman MM, Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1175–1180. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchetti G, Bellistri GM, Borghi E, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008;22:2035–2038. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]
- 5.Marchetti G, Cozzi-Lepri A, Merlini E, et al. Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4+ cell count. AIDS. 2011;25:1385–1394. doi: 10.1097/QAD.0b013e3283471d10. [DOI] [PubMed] [Google Scholar]
- 6.Acosta J, Catalan M, Del Palacio-Perez-Medel A, et al. Prospective study in critically ill non-neutropenic patients: diagnostic potential of (1,3)-beta-D-glucan assay and circulating galactomannan for the diagnosis of invasive fungal disease. Eur J Clin Microbiol Infect Dis. 2011 doi: 10.1007/s10096-011-1365-0. [DOI] [PubMed] [Google Scholar]
- 7.Sax PE, Komarow L, Finkelman MA, et al. Blood (1->3)-beta-D-glucan as a diagnostic test for HIV-related Pneumocystis jirovecii pneumonia. Clin Infect Dis. 2011;53:197–202. doi: 10.1093/cid/cir335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desmet S, Van Wijngaerden E, Maertens J, et al. Serum (1-3)-beta-D-glucan as a tool for diagnosis of Pneumocystis jirovecii pneumonia in patients with human immunodeficiency virus infection or hematological malignancy. J Clin Microbiol. 2009;47:3871–3874. doi: 10.1128/JCM.01756-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Odabasi Z, Mattiuzzi G, Estey E, et al. Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin Infect Dis. 2004;39:199–205. doi: 10.1086/421944. [DOI] [PubMed] [Google Scholar]
- 10.Ostrosky-Zeichner L, Alexander BD, Kett DH, et al. Multicenter clinical evaluation of the (1-->3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis. 2005;41:654–659. doi: 10.1086/432470. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman OA, Standing JE, Limper AH. Pneumocystis carinii stimulates tumor necrosis factor-alpha release from alveolar macrophages through a beta-glucan-mediated mechanism. J Immunol. 1993;150:3932–3940. [PubMed] [Google Scholar]
- 12.Brown GD, Gordon S. Immune recognition of fungal beta-glucans. Cell Microbiol. 2005;7:471–479. doi: 10.1111/j.1462-5822.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 13.Gingo MR, George MP, Kessinger CJ, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med. 2010;182:790–796. doi: 10.1164/rccm.200912-1858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crothers K, Butt AA, Gibert CL, et al. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006;130:1326–1333. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- 15.Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183:388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsue PY, Deeks SG, Farah HH, et al. Role of HIV and human herpesvirus-8 infection in pulmonary arterial hypertension. AIDS. 2008;22:825–833. doi: 10.1097/QAD.0b013e3282f7cd42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mondy KE, Gottdiener J, Overton ET, et al. High prevalence of echocardiographic abnormalities among HIV-infected persons in the era of highly active antiretroviral therapy. Clin Infect Dis. 2011;52:378–386. doi: 10.1093/cid/ciq066. [DOI] [PubMed] [Google Scholar]
- 18.Morris A, Gingo MR, George MP, et al. Cardiopulmonary function in individuals with HIV infection in the antiretroviral therapy era. AIDS. 2012;26:731–740. doi: 10.1097/QAD.0b013e32835099ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 20.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 21.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 22.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 23.Neas LM, Schwartz J. The determinants of pulmonary diffusing capacity in a national sample of U.S. adults. Am J Respir Crit Care Med. 1996;153:656–664. doi: 10.1164/ajrccm.153.2.8564114. [DOI] [PubMed] [Google Scholar]
- 24.Gershman NH, Wong HH, Liu JT, et al. Comparison of two methods of collecting induced sputum in asthmatic subjects. Eur Respir J. 1996;9:2448–2453. doi: 10.1183/09031936.96.09122448. [DOI] [PubMed] [Google Scholar]
- 25.Fahy JV, Boushey HA, Lazarus SC, et al. Safety and reproducibility of sputum induction in asthmatic subjects in a multicenter study. Am J Respir Crit Care Med. 2001;163:1470–1475. doi: 10.1164/ajrccm.163.6.9901105. [DOI] [PubMed] [Google Scholar]
- 26.Morris A, Kingsley LA, Groner G, et al. Prevalence and clinical predictors of Pneumocystis colonization among HIV-infected men. AIDS. 2004;18:793–798. doi: 10.1097/00002030-200403260-00011. [DOI] [PubMed] [Google Scholar]
- 27.Henry WL, DeMaria A, Gramiak R, et al. Report of the American Society of Echocardiography Committee on Nomenclature and Standards in Two-dimensional Echocardiography. Circulation. 1980;62:212–217. doi: 10.1161/01.cir.62.2.212. [DOI] [PubMed] [Google Scholar]
- 28.Quinones MA, Otto CM, Stoddard M, et al. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 29.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657–662. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 30.Borgeson DD, Seward JB, Miller FA, Jr., et al. Frequency of Doppler measurable pulmonary artery pressures. J Am Soc Echocardiogr. 1996;9:832–837. doi: 10.1016/s0894-7317(96)90475-7. [DOI] [PubMed] [Google Scholar]
- 31.Balagopal A, Philp FH, Astemborski J, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226–233. doi: 10.1053/j.gastro.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anselmi A, Vendrame D, Rampon O, et al. Immune reconstitution in human immunodeficiency virus type 1-infected children with different virological responses to anti-retroviral therapy. Clin Exp Immunol. 2007;150:442–450. doi: 10.1111/j.1365-2249.2007.03526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallet MA, Rodriguez CA, Yin L, et al. Microbial translocation induces persistent macrophage activation unrelated to HIV-1 levels or T-cell activation following therapy. AIDS. 2010;24:1281–1290. doi: 10.1097/QAD.0b013e328339e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samonis G, Gikas A, Toloudis P, et al. Prospective study of the impact of broad-spectrum antibiotics on the yeast flora of the human gut. Eur J Clin Microbiol Infect Dis. 1994;13:665–667. doi: 10.1007/BF01973996. [DOI] [PubMed] [Google Scholar]
- 35.Brown GD, Gordon S. Fungal beta-glucans and mammalian immunity. Immunity. 2003;19:311–315. doi: 10.1016/s1074-7613(03)00233-4. [DOI] [PubMed] [Google Scholar]
- 36.Lee JN, Lee DY, Ji IH, et al. Purification of soluble beta-glucan with immune-enhancing activity from the cell wall of yeast. Biosci Biotechnol Biochem. 2001;65:837–841. doi: 10.1271/bbb.65.837. [DOI] [PubMed] [Google Scholar]
- 37.Seibold MA, Donnelly S, Solon M, et al. Chitotriosidase is the primary active chitinase in the human lung and is modulated by genotype and smoking habit. J Allergy Clin Immunol. 2008;122:944–950. e943. doi: 10.1016/j.jaci.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chupp GL, Lee CG, Jarjour N, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357:2016–2027. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 39.Letuve S, Kozhich A, Humbles A, et al. Lung chitinolytic activity and chitotriosidase are elevated in chronic obstructive pulmonary disease and contribute to lung inflammation. Am J Pathol. 2010;176:638–649. doi: 10.2353/ajpath.2010.090455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otsuka K, Matsumoto H, Niimi A, et al. Sputum YKL-40 levels and pathophysiology of asthma and chronic obstructive pulmonary disease. Respiration. 2012;83:507–519. doi: 10.1159/000330840. [DOI] [PubMed] [Google Scholar]