Abstract
Background
Adult survivors of childhood cancer are at risk for long-term morbidities, which may be managed pharmacologically. Psychoactive medication treatment has been associated with adverse effects on specific neurocognitive processes in non-cancer populations, yet these associations have not been examined in adult survivors of childhood cancer.
Procedure
Outcomes were evaluated in 7,080 adult survivors from the Childhood Cancer Survivor Study using a validated self-report Neurocognitive Questionnaire. Multivariable logistic regression models were used to calculate odds ratios (OR) and 95% confidence intervals (CI) for neurocognitive impairment using demographic and treatment factors and survivors’ report of prescription medication use.
Results
Controlling for cranial radiation, pain, psychological distress, and stroke/seizure, use of antidepressant medications was associated with impaired task efficiency (OR=1.80, 95% CI=1.47–2.21), organization (OR=1.83, 95% CI=1.48–2.25), memory (OR=1.53, 95% CI=1.27–1.84) and emotional regulation (OR=2.06, 95% CI=1.70–2.51). Neuroleptics and stimulants were associated with impaired task efficiency (OR=2.46, 95% CI=1.29–4.69; OR=2.82, 95% CI=1.61–4.93, respectively) and memory (OR=2.08, 95% CI=1.13–3.82; OR=2.69, 95% CI=1.59–4.54, respectively). Anticonvulsants were associated with impaired task efficiency, memory and emotional regulation, although survivors who use these medications may be at risk for neurocognitive impairment on the basis of seizure disorder and/or underlying tumor location (CNS).
Conclusions
These findings suggest that specific psychoactive medications and/or mental health conditions may be associated with neurocognitive function in adult survivors of childhood cancer. The extent to which these associations are causal or indicative of underlying neurological impairment for which the medications are prescribed remains to be ascertained.
Keywords: psychoactive medication, neurocognition, survivorship
INTRODUCTION
Advances in medical treatments have contributed to a growing population of adult survivors of childhood cancer, prompting increased focus on the identification, characterization, and management of the long-term consequences of cancer and cancer-directed therapies. Survivors of childhood cancer are at risk for medical, functional and psychosocial late effects,1,2 for which pharmacotherapy may be considered as primary or adjunctive treatment. A forthcoming report from the Childhood Cancer Survivor Study (CCSS) indicates that 42% of adult survivors of childhood cancer reported treatment with one or more psychoactive medications over a 16-year period, a significantly larger proportion compared to sibling controls (33%)3
Neurocognitive dysfunction is one of the most common late effects experienced by long-term survivors of childhood acute lymphoblastic leukemia (ALL) and central nervous system tumors (CNS). Prevalence rates range from 20 to over 80%, varying due to the sample and cognitive process studied, measures employed, and definition of impairment utilized.4–6 Treatment with cranial radiation therapy (CRT) is a well-established risk factor for neurocognitive late effects,7 though antimetabolite chemotherapy and corticosteroids also have been implicated.8–10 Neurocognitive impairment includes deficits in attention, memory, processing speed, and executive function.7;11–13 These deficits often increase with time after treatment exposure7 and have the potential to impact multiple areas of adult functioning including educational attainment, employment, health behaviors, and quality of life.4,14,15 Given the potential pervasive impact of neurocognitive impairment on daily life, additional research is needed to better understand factors that have the potential to exacerbate or mitigate neurocognitive dysfunction.
Some psychoactive medications have been reported to impact neurocognitive functioning in non-cancer populations. Both tricyclic antidepressants16,17 and selective serotonin reuptake inhibitors (SSRIs)18,19 have been demonstrated to impair sustained attention and memory in non-depressed adults. Anticonvulsants have well documented negative effects on cognition in patients with epilepsy,20 and may also produce untoward cognitive effects on attention, memory, and psychomotor speed in healthy adults.21 Of note, these effects have been demonstrated to emerge in a dose-dependent fashion,21 providing further evidence of a direct effect of these medications on specific neurocognitive processes.
A report from CCSS indicates that 19% of adult survivors initiated treatment with antidepressants over a 10-year period, while nearly 7% initiated use of anticonvulsants.3 These medications are likely used for the management of symptoms of psychological distress and seizures, conditions often associated with neurocognitive dysfunction.22 Psychoactive medications may further exacerbate neurocognitive deficits in those already at risk for difficulties due to underlying medical and/or mental health conditions. Moreover, in some survivors neurocognitive problems may be related to an adverse response to the medication itself (see Figure 1 for a schematic representation of potential medication effects on neurocognitive outcomes). Importantly, the impact of psychoactive medication on neurocognitive processes of survivors may be more salient, given the heightened risk for brain injury following neurotoxic cancer treatment. Currently, little is known of the association between psychoactive medication treatment and neurocognitive outcomes in adult survivors of childhood cancer. The primary objective of this study is to investigate this association while accounting for the known effects of CRT, psychological distress, and stroke or seizure history on neurocognitive outcomes.
Figure 1.
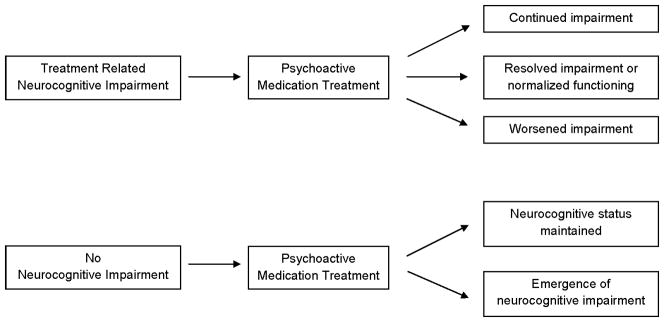
Model depicting potential impact of psychoactive mediation on neurocognitive functioning.
METHODS
Childhood Cancer Survivor Study
Detailed descriptions of the CCSS methodology and participants have been previously published.23–25 Briefly, the cohort consists of survivors of one of eight childhood cancers diagnosed younger than 21 years of age, treated at one of 26 institutions between 1970 and 1986, and who survived at least 5 years from their original diagnosis. Sibling controls were recruited from a randomly selected subset of survivors. The study was approved by the institutional review board at each of the collaborating institutions and informed consent was obtained from each study participant. Study participants completed a Baseline questionnaire between 1994 and 1998 and subsequent follow-up questionnaires in 2000, 2003 and 2007. The current study population included all cancer survivors and siblings who completed the Baseline questionnaire and the 2003 follow-up (Figure 2).
Figure 2.
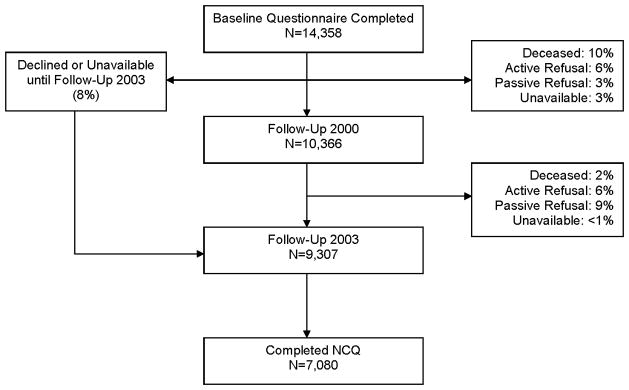
Flow diagram of study participation of survivors from the Childhood Cancer Survivor Study. NCQ = Neurocognitive Questionnaire.
Measures
Childhood Cancer Survivor Study Neurocognitive Questionnaire (CCSS-NCQ)
Self-report of neurocognitive function was assessed using the CCSS-NCQ, which was previously validated in adult survivors of childhood cancer. 26 The CCSS-NCQ is a 25-item questionnaire that provides a 3-point Likert scale (0=never a problem to 2=often a problem) for ratings of neurocognitive problems, and is comprised of 4 primary factors: Task Efficiency, Emotional Regulation, Organization and Memory. These factors provide measures of executive functioning (i.e., Emotional Regulation and Organization), attention and processing speed (i.e., Task Efficiency), and short and long-term memory (i.e., Memory). Consistent with previous CCSS studies, impaired performance was defined as a score falling ≥90th percentile based on values obtained in the sibling cohort.
Primary Predictors
Psychoactive medication use was the primary predictor in this study. Participants reported prescription drugs taken consistently for more than one month or >30 days in one year during the 2 year period prior to survey completion. Participants were instructed to report only medications prescribed by a physician and dispensed by a pharmacist and not report over-the-counter medications. Medications were classified using the American Hospital Formulary Service Drug Information database (AHFS).27 We identified 8 medication categories believed to include psychoactive properties: (1) antidepressants, (2) anxiolytics/sedatives/hypnotics, (3) anticonvulsants, (4) non-opioid analgesics, (5) opioids, (6) muscle relaxants, (7) central nervous system stimulants, and (8) neuroleptics. Supplemental Appendix I provides a list of the AHFS drug classes comprising each medication category. Polypharmacy was defined as the reported use of >1 psychoactive medication and was restricted to medications in the 8 classes identified above.
Covariates
Demographic and socioeconomic variables included sex, age at the time of follow-up, race/ethnicity, health insurance status, and household income, which was categorized as <$20,000 or ≥$20,000. Neurologic variables included history of headache, bodily pain and stroke or seizure. Cancer variables included age at diagnosis and treatment with cranial irradiation, which was categorized by dose intensity resulting in 3 groups: none, low (0 to <20Gy), or high (≥20Gy). The low-dose exposure group included scatter exposure from radiation treatment of adjacent body areas. Psychological distress was measured by the Brief-Symptom Inventory-18 (BSI-18)28 and subscales for anxiety, depression and somatization were used as covariates. Sex-specific scores were calculated based on standardized normative values and scores falling ≥90th percentile were classified as demonstrating a clinical level of acute emotional distress.
Statistical Analysis
Descriptive statistics were calculated for all outcomes, predictors and covariates used in the analyses. Logistic regression modeling was employed for each outcome variable from the CCSS-NCQ (Task Efficiency, Emotional Regulation, Organization and Memory). Performance was classified into yes/no impairment based on comparison to sibling norms and univariate models were constructed to identify each variable contributing to the four neurocognitive outcomes at p<0.10. All variables meeting this significance threshold were included in multivariable logistic regression models. Backward selection was performed for each of the neurocognitive models using SAS version 9.2 PROC Logistic (SAS Institute, Cary, NC). The least significant variables (largest p-value) were excluded one at a time until all variables remaining in the model were significant (p<0.05). All medication categories were forced into the multivariable models regardless of statistical significance. Odds ratios (OR) and 95% confidence intervals (CI) were calculated for predictors and covariates retained in the final models. Given the multicollinearity between cancer diagnosis and treatment variables, and in accord with previous research on cognitive late effects associated with treatment exposure, the models presented in this report include only treatment variables. Previous CCSS studies have failed to demonstrate an association between chemotherapy exposure and self-reported neurocognitive function independent of cranial radiation therapy.29 Thus, chemotherapy variables were not included in our multivariable models.
RESULTS
Table I shows descriptive statistics for survivor and sibling demographics as well as survivor treatment characteristics. Mean age at time of follow-up was 31.6 years (SD=7.5, range 17 to 54). The most frequent childhood cancer diagnoses were leukemia (33.3%), lymphoma (21.0%), and central nervous system tumors (11.9%). Compared with siblings, survivors were younger and less likely to be white/non-Hispanic, less likely to have health insurance and more likely to have a household income less than $20,000. Survivors were also more likely to report a history of pain and neurological event as well as acute psychological distress. Overall, 23% of survivors reported taking at least one psychoactive medication during the two-year period prior to survey completion compared to 16% of sibling controls (p=0.003). The proportion of survivors and siblings reporting psychoactive medication use by medication class is presented in Supplemental Appendix II. Notably, the difference in medication use appears to be largely driven by the practice of polypharmacy among survivors (7.2% vs. 2.3%, p<0.001). Supplemental Appendix III provides data on medication use across cancer diagnoses.
Table I.
Clinical Characteristics of Survivors and Siblings
| Survivors (N=7,080) | Siblings (N=384) | ||||
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | Range | Mean (SD) | Range | p-value | |
| Age at diagnosis, years | 7.9(5.9) | 0–20 | --- | --- | |
| Time since diagnosis, years | 23.6(4.6) | 15–34 | --- | --- | |
| Current age, years | 31.6(7.5) | 17–54 | 33.6(8.4) | 17–58 | <0.001 |
|
|
|||||
| Frequency | % | Frequency | % | ||
|
|
|||||
| Gender | 0.68 | ||||
| Male | 3468 | 49.0 | 184 | 47.9 | |
| Female | 3612 | 51.0 | 200 | 52.1 | |
| Race | <0.001 | ||||
| White, non-Hispanic | 6168 | 87.1 | 344 | 89.6 | |
| Other | 879 | 12.4 | 22 | 5.7 | |
| Not specified | 33 | 0.5 | 18 | 4.7 | |
| Current Insurance | 0.02 | ||||
| Yes* | 6263 | 88.5 | 354 | 92.2 | |
| No | 768 | 11.0 | 29 | 7.6 | |
| Household Income | 0.04 | ||||
| <$ 20,000 | 768 | 10.9 | 24 | 6.3 | |
| ≥$ 20,000 | 5488 | 77.5 | 334 | 87.0 | |
| Not Specified | 824 | 11.6 | 26 | 6.7 | |
| Pain | <0.001 | ||||
| Headache | 1171 | 25.0 | 77 | 20.1 | |
| Other bodily pain | 371 | 5.2 | 8 | 2.1 | |
| No pain | 4926 | 69.6 | 298 | 77.6 | |
| Stroke or Seizure | <0.001 | ||||
| Yes | 484 | 6.8 | 8 | 2.1 | |
| No | 6596 | 93.2 | 376 | 97.9 | |
| Psychological Distress | |||||
| Somatization | 981 | 13.9 | 26 | 6.8 | <0.001 |
| Depression | 838 | 11.8 | 31 | 8.1 | 0.02 |
| Anxiety | 545 | 7.7 | 17 | 4.4 | 0.02 |
| Diagnosis | |||||
| Leukemia | 2355 | 33.3 | --- | --- | |
| CNS Tumor | 844 | 11.9 | --- | --- | |
| Hodgkin Lymphoma | 951 | 13.4 | --- | --- | |
| Non-Hodgkin Lymphoma | 538 | 7.6 | --- | --- | |
| Kidney (Wilms Tumor) | 668 | 9.4 | --- | --- | |
| Neuroblastoma | 454 | 6.4 | --- | --- | |
| Soft tissue sarcoma | 645 | 9.1 | --- | --- | |
| Osteosarcoma | 625 | 8.8 | --- | --- | |
| Cranial Radiation | |||||
| None | 2307 | 32.6 | --- | --- | |
| <20Gy | 2812 | 39.7 | --- | --- | |
| ≥20Gy | 1436 | 20.3 | --- | --- | |
Yes includes Canadian citizen.
Rates of impairment on the 4 factors from the CCSS-NCQ are presented in Table II, with an expected impairment rate of 10% in sibling controls. The highest rate of impairment was observed on the Memory factor with 25% of survivors reporting problems related to short and long-term memory. Descriptive statistics for the neurocognitive outcome measures stratified by medication class are also presented in Table II. Across all eight medication categories, rate of neurocognitive impairment was greater for survivors who reported taking psychoactive medication compared with survivors who did not report medication use.
Table II.
Neurocognitive Impairment in Survivors by Medication Use*
| Task Efficiency | Emotional Regulation | Organization | Memory | ||
|---|---|---|---|---|---|
|
| |||||
| N(%) Impaired in Cohort | 1392(19.7%) | 1541(21.8%) | 946(13.4%) | 1787(25.2%) | |
|
| |||||
| N | %impaired | %impaired | %impaired | %impaired | |
| Non-Opioid Analgesics | |||||
| Yes | 275 | 29.1 | 39.6 | 18.2 | 34.6 |
| No | 6805 | 19.3 | 21.0 | 13.2 | 24.9 |
| Opioids | |||||
| Yes | 249 | 31.3 | 38.2 | 22.1 | 41.8 |
| No | 6831 | 19.2 | 21.2 | 13.0 | 24.6 |
| Antidepressants | |||||
| Yes | 921 | 38.3 | 45.1 | 25.4 | 41.9 |
| No | 6159 | 16.9 | 18.3 | 11.6 | 22.8 |
| Anticonvulsants | |||||
| Yes | 414 | 54.6 | 39.4 | 23.9 | 51.9 |
| No | 6666 | 17.5 | 20.7 | 12.7 | 23.6 |
| Anxiolytics/Sedatives/Hypnotics | |||||
| Yes | 258 | 37.2 | 43.0 | 21.3 | 45.7 |
| No | 6822 | 19.0 | 21.0 | 13.1 | 24.5 |
| Muscle Relaxants | |||||
| Yes | 75 | 42.7 | 58.7 | 26.7 | 50.7 |
| No | 7005 | 19.4 | 21.4 | 13.2 | 25.0 |
| Stimulants | |||||
| Yes | 85 | 48.2 | 40.0 | 42.4 | 52.9 |
| No | 6995 | 19.3 | 21.5 | 13.0 | 24.9 |
| Neuroleptics | |||||
| Yes | 73 | 67.1 | 49.3 | 35.6 | 64.4 |
| No | 7007 | 19.2 | 21.5 | 13.1 | 24.8 |
Impairment defined as performance > highest 10% of sibling controls.
Multivariable Prediction of Neurocognitive Outcome
Table III presents ORs for impairment for each neurocognitive outcome. Multivariable logistic regression models adjusting for all other covariates, including CRT and stroke/seizure, revealed significant associations between psychoactive medication use and impaired neurocognitive functioning. Antidepressants were associated with impairment across all areas including, task efficiency (OR=1.80, 95% CI=1.47–2.21), memory (OR=1.53, 95% CI=1.27–1.84), organization (OR=1.83, 95% CI=1.48–2.25) and emotional regulation (OR=2.06, 95% CI=1.70–2.51). Similarly, anticonvulsants were associated with impaired task efficiency (OR=2.29, 95% CI=1.69–3.09), memory (OR=1.51, 95% CI=1.13–2.02), and emotional regulation (OR=1.71, 95% CI=1.27–2.30). CNS stimulant medications were associated with a nearly 5–fold increased likelihood of impaired organization (OR=4.95, 95% CI=2.98–8.24) as well as increased likelihood of impaired task efficiency (OR=2.82, 95% CI=1.61–4.93) and memory (OR=2.69, 95% CI=1.59–4.54). Survivors taking neuroleptic medications were 2.5 times more likely to report impaired task efficiency (OR=2.46, 95% CI=1.29–4.69) and twice as likely to report memory difficulties (OR=2.08, 95% CI=1.13–3.82) compared with survivors who were not taking these medications.
Table III.
Multivariable Model Predicting Neurocognitive Outcomes
| Impaired Task Efficiency | Impaired Emotional Regulation | Impaired Organization | Impaired Memory | |
|---|---|---|---|---|
|
| ||||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Psychoactive Medication Use (yes vs. no) | ||||
| Non-Opioid Analgesics | 1.21(0.82,1.78) | 1.72(1.20,2.44) | 1.05(0.71,1.56) | 0.96(0.68,1.36) |
| Opioids | 0.72(0.48,1.09) | 0.81(0.54,1.20) | 1.04(0.70,1.55) | 1.01(0.70,1.44) |
| Antidepressants | 1.80(1.47,2.21) | 2.06(1.70,2.51) | 1.83(1.48,2.25) | 1.53(1.27,1.84) |
| Anticonvulsants | 2.29(1.69–3.09) | 1.71(1.27,2.30) | 1.20(0.87,1.67) | 1.51(1.13,2.02) |
| Anxiolytics/Sedatives/Hypnotics | 0.92(0.64,1.34) | 1.10(0.76,1.58) | 0.81(0.54,1.20) | 1.06(0.76,1.48) |
| Muscle Relaxants | 1.29(0.67,2.45) | 2.35(1.24,4.45) | 1.11(0.58,2.13) | 1.39(0.76,2.53) |
| CNS Stimulants | 2.82(1.61,4.93) | 1.66(0.92,2.97) | 4.95(2.98,8.24) | 2.69(1.59,4.54) |
| Neuroleptics | 2.46(1.29,4.69) | 1.25(0.65,2.42) | 1.67(0.92,3.01) | 2.08(1.13,3.82) |
| Clinical Characteristics | ||||
| Female Sex | --- | 1.86(1.60,2.18) | --- | --- |
| Race, White/non-Hispanic vs. Other | --- | --- | --- | --- |
| Current Age (years) | 0.97(0.96,0.98) | 0.96(0.95,0.97) | --- | --- |
| Health Insurance vs. None | --- | 0.74(0.58,0.93) | 0.76(0.59,0.97) | --- |
| Household Income < 20,000 vs. ≥ 20,000 | 1.73(1.41,2.12) | --- | 1.33(1.06,1.66) | 1.45(1.20,1.75) |
| Age at diagnosis (years) | --- | --- | --- | --- |
| Pain | ||||
| Headache vs. No pain | 1.46(1.23,1.73) | 1.36(1.15,1.61) | 1.27(1.06,1.52) | 1.65(1.43,1.91) |
| Other Bodily Pain vs. No pain | 1.46(1.06,2.01) | 1.11(0.80,1.53) | 1.32(0.94,1.84) | 1.10(0.82,1.47) |
| Medical Variables | ||||
| Neurologic Event vs. None | 2.65(2.02,3.47) | --- | 1.55(1.15,2.08) | 1.93(1.49,2.50) |
| ≥ 20Gy CRT vs. No CRT | 2.73(2.24,3.33) | 1.54(1.26,1.88) | 1.21(0.97,1.49) | 2.13(1.78,2.54) |
| < 20Gy CRT vs. No CRT | 1.15(0.96,1.39) | 1.22(1.03,1.45) | 0.90(0.75,1.09) | 1.20(1.03,1.40) |
| Psychological Distress (yes vs. no) | ||||
| Somatization | 2.07(1.68,2.54) | 1.72(1.40,2.11) | 1.63(1.32,2.02) | 2.18(1.81,2.62) |
| Depression | 2.94(2.36,3.65) | 4.74(3.84,5.85) | 2.07(1.67,2.57) | 2.18(1.77,2.68) |
| Anxiety | 2.11(1.62,2.77) | 4.01(3.06,5.26) | --- | 1.66(1.28,2.14) |
Variables listed were included in the final model. --- indicates variables dropped from final model through backward selection process.
Among the medical variables retained in the final models, survivors treated with high-dose cranial radiation were significantly more likely to report impaired task efficiency (OR=2.73, 95% CI=2.24–3.33), memory (OR=2.13, 95% CI=1.78–2.54), and emotional regulation (OR=1.54, 95% CI=1.26–1.88) compared to survivors who were not treated with cranial radiation. History of a neurologic event predicted difficulties with memory (OR=1.93, 95% CI=1.49–2.50) and organization (OR=1.55, 95% CI=1.15–2.08), as well as a 2.6-fold increased likelihood of impaired task efficiency (OR=2.65, 95% CI=2.02–3.47). Acute psychological distress emerged as an independent predictor of neurocognitive impairment. Survivors with elevated symptoms of depression were two to four times more likely to report problems with task efficiency (OR=2.94, 95% CI=2.36–3.65), memory (OR=2.18, 95% CI=1.77–2.68), organization (OR=2.07, 95% CI=1.67–2.57), and emotional regulation (OR=4.74, 95% CI=3.84–5.85).
DISCUSSION
This study reports on the association between psychoactive medication use and neurocognitive functioning in adult survivors of childhood cancer. Antidepressants, anticonvulsants, CNS stimulants, and neuroleptic medications were associated with report of impaired functioning across multiple domains of neurocognition. Importantly, psychoactive medications contributed to neurocognitive outcomes above and beyond CRT, neurologic history, and acute psychological distress, which are established predictors of neurocognitive impairment. These findings are consistent with previous studies that report adverse cognitive effects of psychoactive agents in non-cancer populations18,19,30 and suggest a contribution from specific medication classes on neurocognitive function in long-term survivors of childhood cancer.
Antidepressants were associated with impairment across all areas of neurocognitive functioning, with 38% and 42% of survivors who used antidepressants reporting impairment in task efficiency and memory, respectively. Previous research identified the adverse effects of tricyclic antidepressants on reaction time as well as sustained and selective attention.16,17 More recent data suggest that SSRIs, including fluoxetine,31 venlafaxine,32 and citalopram,33 also impair vigilance performance following acute and subchronic treatment. Additionally, paroxetine, a SSRI with affinity for anticholinergic activity, has been demonstrated to impair vigilance18 and long-term memory19 in healthy adults. Although our results parallel previous findings, even after adjusting for acute depressive symptoms, we must acknowledge the potential and likely contribution of underlying mental health factors to neurocognitive impairment. Difficulties with effortful attention, short-term and working memory, and executive functioning are reported among patients with major depressive disorder.22,34 Symptoms of depression, including slowed thinking, distractibility, and difficulty concentrating may mirror symptoms of neurocognitive impairment and cannot be differentiated using a single self-report measure. Additionally, patients with depression may be more apt to report cognitive impairment that extends beyond that which would be observed on objective measures of neurocognitive processes.35 While we acknowledge the contribution of mental health conditions to self-report of neurocognitive dysfunction, the associations we found between antidepressants and specific neurocognitive outcomes are consistent with research in healthy adults documenting the adverse impact of specific antidepressant agents on multiple attention processes and memory16,19,31,32 and suggest that future research is needed to better understand these associations in cancer survivors.
Similar to the findings regarding antidepressants, survivors taking anticonvulsant medications were significantly more likely to report impaired task efficiency, memory and emotional regulation. Research has demonstrated that anitconvlusants are associated with impairment across several areas of neurocognition in patients with epilepsy as well as health adults.21 Specifically, topiramate has been associated with adverse effects on measures of attention, verbal fluency, verbal memory, and psychomotor speed.21 It is important to note that survivors who were prescribed anticonvulsants may be independently at increased risk for neurocognitive impairment on the basis of seizure disorder and/or underlying tumor location (CNS). In fact, 21% of CNS tumor survivors in our sample reported taking anticonvulsants and research has consistently demonstrated heightened risk of neurocognitive dysfunction among survivors of pediatric brain tumors.36 Additionally, neuropsychological impairment is well documented in patients with epilepsy and may be related to structural brain abnormalities and/or the cumulative neuorobiological consequences of recurrent seizures.37 We also found that history of a stroke or seizure was independently associated with impaired task efficiency, organization and memory among adult survivors childhood cancer. Taken together, evidence suggests that anticonvulsant medications may exacerbate neurocognitive impairments in a population already vulnerable to cognitive dysfunction.
Neuroleptic medications were significantly associated with impaired task efficiency and memory. Of note, only one percent of our sample reported using neuroleptic medications, despite the inclusion of both antipsychotic and antimanic agents in this category. While this is consistent with prevalence rates reported in the general population, caution should be used when interpreting the associations between these medications and specific neurocognitive outcomes due to the relatively small sample. Antipsychotic and antimanic medications are often used for the management of psychotic symptoms and mood stabilization and may be prescribed for the treatment of Schizophrenia and/or Bipolar Disorder, psychiatric conditions with known effects on cognition.38,39 There is increasing evidence that second generation antipsychotic medications may ameliorate some cognitive symptoms associated with these disorders,40 although the clinical significance of observed improvements has been questioned and it is unclear if such improvements result in normalization of cognitive processes or simply a reduced degree of impairment. Thus, it is not surprising that survivors endorsing antipsychotic medication use also reported impaired neurocognitive functioning.
Among all medications, central nervous system stimulants showed the largest associations with impaired task efficiency, organization, and memory. Impressively, these associations were larger than those observed among neurocognitive impairment and high-dose cranial radiation therapy. Stimulant medications are often used for the treatment of attention-deficit/hyperactivity disorder (ADHD), which is generally characterized by impairments related to attention, concentration, hyperactivity, organization, task completion and memory.41 Stimulant medications may also be used for the management of fatigue in cancer survivors.42 Chronic fatigue has been associated with impaired cognition in non-cancer populations43 and recent data suggest that adult survivors of childhood cancer may be especially vulnerable to the adverse impact of poor sleep and fatigue on neurocognitive function.44 While stimulants may have beneficial effects on sustained attention among adults with ADHD and chronic fatigue,45 stimulant medication treatment does not appear to consistently improve or normalize other areas of cognitive functioning.46,47 Similarly, our findings suggest that stimulant medications do not appear to normalize neuropsychological function in adult survivors of childhood cancer. Importantly, potential effects of stimulants on neurocognitive processes may be dependent upon the etiology of underlying deficits. Future prospective, randomized studies are needed to delineate direct effects on neurocognitive functions in this patient population.
In contrast to other medication classes, pain medications were largely unrelated to impaired neurocognitive function. However, both non-opioid analgesics and muscle relaxants were associated with poor emotion regulation. We also found associations between acute symptoms of anxiety, depression and somatization and emotional regulation, suggesting that difficulties with emotional regulation may involve negative feelings such as sadness, nervousness, and frustration. Additionally, that patient report of headache was a significant predictor of problems with emotional regulation parallels previous reports of negative affect in patients with pain.48 Given that the experience of pain may be intensified by the presence of negative emotions such as anger and sadness,49 it is likely that our current finding regarding the connection between pain medication and poor emotional regulation is due to the complex interplay of acute emotional distress, pain and the management of these symptoms.
Despite the many strengths of our study, including the use of a large, well-characterized sample of long-term adult survivors of childhood cancer, there are important limitations that warrant discussion. The study design was retrospective, thus we were unable to establish a temporal relationship between onset of medication use and neurocognitive outcome. This limits our ability to discuss potential neurocognitive changes as a direct result of psychoactive medication treatment. As illustrated by Figure 1, survivors may initiate psychoactive medication treatment for the management of treatment-related morbidities with known effects on cognition, including seizures, depression, and fatigue. Such treatment may be associated with continued (i.e., no direct effect on cognition), resolved (i.e., beneficial effect on cognition) or worsened (i.e., adverse effect on cognition) impairment. Our data preclude determination of such causal associations and we recognize the potential for divergent outcomes based on individual response to treatment and the underlying symptoms for which medication treatment was initiated.
Additionally, we did not have information regarding medication dose or indication for medication use. This will be important information for future studies to consider as past research has demonstrated a dose-response effect of specific medications on cognitive processes.21 Although survivors reported used of multiple medications, we were not able to confirm that multiple medications were taken concurrently over the two-year study period, thus limiting our ability to examine polypharmacy in association with neurocognition. Given that polypharmacy appears to account for much of the difference in medication use between survivors and siblings, future studies should examine the contribution of concomitant medication use on neurocognition. This practice may be more salient in survivors given the potential for late effects to affect many organ systems and areas of function. Moreover, underlying treatment-related toxicities may make survivors vulnerable to adverse drug interactions. Both medication use and neurocognitive functioning were assessed via self-report rather than by direct assessment. It will be necessary to determine the extent to which the observed associations are maintained on performance-based measures of neurocognitive function. Lastly, these survivors were treated >15 years ago and patients treated on more contemporary protocols may demonstrate less treatment-related morbidity and reduced rates of psychoactive medication use.
In summary, this study provides preliminary data to suggest an association between specific psychoactive medications and neurocognitive outcomes in adult survivors of childhood cancer. Although this conclusion must be tempered by the fact that survivors taking psychoactive medications may be predisposed for neurocognitive impairment due to established medical and/or mental health conditions, these findings underscore the need for future research. Specifically, it will be important to investigate the impact of psychoactive medications on functional outcomes in a population with heightened risk for neurocognitive dysfunction. Clinically, these findings suggest the need for increased awareness among prescribing clinicians regarding the potential for medication effects on cognitive processes with monitoring of such effects warranted. Clinical research trials are necessary to understand dose-response relationships and whether childhood cancer survivors are at-risk for heightened sensitivity to medication effects.
Supplementary Material
Acknowledgments
This work was supported by grant U24 CA 55727 (L.L.R.) from the National Cancer Institute, with additional support provided to St. Jude Children’s Research Hospital by the Cancer Center Support (CORE) grant CA21765 and by the American Lebanese-Syrian Associated Charities (ALSAC).
References
- 1.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 2.Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290:1583–1592. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 3.Brinkman TM, Ullrich NJ, Zhang N, et al. Prevalence and predictors of prescription psychoactive medication use in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. doi: 10.1007/s11764-012-0250-x. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology. 23:705–717. doi: 10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulhern RK, Palmer SL. Neurocognitive late effects in pediatric cancer. Curr Probl Cancer. 2003;27:177–197. doi: 10.1016/s0147-0272(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 6.Maddrey AM, Bergeron JA, Lombardo ER, et al. Neuropsychological performance and quality of life of 10 year survivors of childhood medulloblastoma. J Neuroonco. 2005;72:245–253. doi: 10.1007/s11060-004-3009-z. [DOI] [PubMed] [Google Scholar]
- 7.Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004;22:706–713. doi: 10.1200/JCO.2004.05.186. [DOI] [PubMed] [Google Scholar]
- 8.Waber DP, Carpentieri SC, Klar N, et al. Cognitive sequelae in children treated for acute lymphoblastic leukemia with dexamethasone or prednisone. J Pediatr Hematol Oncol. 2000;22:206–213. doi: 10.1097/00043426-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Kadan-Lottick NS, Brouwers P, Breiger D, et al. A comparison of neurocognitive functioning in children previously randomized to dexamethasone or prednisone in the treatment of childhood acute lymphoblastic leukemia. Blood. 2009;114:1746–1752. doi: 10.1182/blood-2008-12-186502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mrakotsky CM, Silverman LB, Dahlberg SE, et al. Neurobehavioral side effects of corticosteroids during active treatment for acute lymphoblastic leukemia in children are age-dependent: report from Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. Pediatr Blood Cancer. 2011;57:492–498. doi: 10.1002/pbc.23060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reeves CB, Palmer SL, Reddick WE, et al. Attention and memory functioning among pediatric patients with medulloblastoma. J Pediatr Psychol. 2006;31:272–280. doi: 10.1093/jpepsy/jsj019. [DOI] [PubMed] [Google Scholar]
- 12.Reddick WE, White HA, Glass JO, et al. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer. 2003;97:2512–2519. doi: 10.1002/cncr.11355. [DOI] [PubMed] [Google Scholar]
- 13.Mabbott DJ, Penkman L, Witol A, Strother D, Bouffet E. Core neurocognitive functions in children treated for posterior fossa tumors. Neuropsychology. 2008;22:159–168. doi: 10.1037/0894-4105.22.2.159. [DOI] [PubMed] [Google Scholar]
- 14.Krull KR, Annett RD, Pan Z, et al. Neurocognitive functioning and health-related behaviours in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Eur J Cancer. 2011;47:1380–1388. doi: 10.1016/j.ejca.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ness KK, Gurney JG, Zeltzer LK, et al. The impact of limitations in physical, executive, and emotional function on health-related quality of life among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Arch Phys Med Rehabil. 2008;89:128–136. doi: 10.1016/j.apmr.2007.08.123. [DOI] [PubMed] [Google Scholar]
- 16.van Laar MW, Volkerts ER, Verbaten MN, Trooster S, van Megen HJ, Kenemans JL. Differential effects of amitriptyline, nefazodone and paroxetine on performance and brain indices of visual selective attention and working memory. Psychopharmacology (Berlin) 2002;162:351–363. doi: 10.1007/s00213-002-1116-0. [DOI] [PubMed] [Google Scholar]
- 17.Amado-Boccara I, Gougoulis N, Poirier Littre MF, Galinowski A, Loo H. Effects of antidepressants on cognitive functions: a review. Neurosci Biobehav Rev. 1995;19:479–493. doi: 10.1016/0149-7634(94)00068-c. [DOI] [PubMed] [Google Scholar]
- 18.Schmitt JA, Ramaekers JG, Kruizinga MJ, van Boxtel MP, Vuurman EF, Riedel WJ. Additional dopamine reuptake inhibition attenuates vigilance impairment induced by serotonin reuptake inhibition in man. J Psychopharmacol. 2002;16:207–214. doi: 10.1177/026988110201600303. [DOI] [PubMed] [Google Scholar]
- 19.Schmitt JA, Kruizinga MJ, Riedel WJ. Non-serotonergic pharmacological profiles and associated cognitive effects of serotonin reuptake inhibitors. J Psychopharmacol. 2001;15:173–179. doi: 10.1177/026988110101500304. [DOI] [PubMed] [Google Scholar]
- 20.Meador KJ, Loring DW, Hulihan JF, Kamin M, Karim R. Differential cognitive and behavioral effects of topiramate and valproate. Neurology. 2003;60:1483–1488. doi: 10.1212/01.wnl.0000063308.22506.19. [DOI] [PubMed] [Google Scholar]
- 21.Loring DW, Williamson DJ, Meador KJ, Wiegand F, Hulihan J. Topiramate dose effects on cognition: a randomized double-blind study. Neurology. 2011;76:131–137. doi: 10.1212/WNL.0b013e318206ca02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gualtieri CT, Johnson LG, Benedict KB. Neurocognition in depression: patients on and off medication versus healthy comparison subjects. J Neuropsychiatry Clin Neurosci. 2006;18:217–225. doi: 10.1176/jnp.2006.18.2.217. [DOI] [PubMed] [Google Scholar]
- 23.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 25.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krull KR, Gioia G, Ness KK, et al. Reliability and validity of the Childhood Cancer Survivor Study Neurocognitive Questionnaire. Cancer. 2008;113:2188–2197. doi: 10.1002/cncr.23809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Society of Health-System Pharmacists. [Accessed June 10, 2011.];AHFS Drug Information. Available from: http://www.ahfsdruginformation.com/
- 28.Derogatis L. Brief Symptom Inventory (BSI): Administration, scoring, and procedures manual. Minneapolis, MN: NCS Pearson; 2000. [Google Scholar]
- 29.Kadan-Lottick NS, Zeltzer LK, Liu Q, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. J Natl Cancer Inst. 2010;102:881–893. doi: 10.1093/jnci/djq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meador KJ, Loring DW, Vahle VJ, et al. Cognitive and behavioral effects of lamotrigine and topiramate in healthy volunteers. Neurology. 2005;64:2108–2114. doi: 10.1212/01.WNL.0000165994.46777.BE. [DOI] [PubMed] [Google Scholar]
- 31.Ramaekers JG, Muntjewerff ND, O’Hanlon JF. A comparative study of acute and subchronic effects of dothiepin, fluoxetine and placebo on psychomotor and actual driving performance. Br J Clin Pharmacol. 1995;39:397–404. doi: 10.1111/j.1365-2125.1995.tb04468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Hanlon JF, Robbe HW, Vermeeren A, van Leeuwen C, Danjou PE. Venlafaxine’s effects on healthy volunteers’ driving, psychomotor, and vigilance performance during 15-day fixed and incremental dosing regimens. J Clin Psychopharmacol. 1998;18:212–221. doi: 10.1097/00004714-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Riedel WJ, Eikmans K, Heldens A, Schmitt JA. Specific serotonergic reuptake inhibition impairs vigilance performance acutely and after subchronic treatment. J Psychopharmacol. 2005;19:12–20. doi: 10.1177/0269881105048887. [DOI] [PubMed] [Google Scholar]
- 34.Cohen R, Lohr I, Paul R, Boland R. Impairments of attention and effort among patients with major affective disorders. J Neuropsychiatry Clin Neurosci. 2001;13:385–395. doi: 10.1176/jnp.13.3.385. [DOI] [PubMed] [Google Scholar]
- 35.Farrin L, Hull L, Unwin C, Wykes T, David A. Effects of depressed mood on objective and subjective measures of attention. J Neuropsychiatry Clin Neurosci. 2003;15:98–104. doi: 10.1176/jnp.15.1.98. [DOI] [PubMed] [Google Scholar]
- 36.Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 37.Elger CE, Helmstaedter C, Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. 2004;3:663–672. doi: 10.1016/S1474-4422(04)00906-8. [DOI] [PubMed] [Google Scholar]
- 38.Sharma T, Antonova L. Cognitive function in schizophrenia. Deficits, functional consequences, and future treatment. Psychiatr Clin North Am. 2003;26:25–40. doi: 10.1016/s0193-953x(02)00084-9. [DOI] [PubMed] [Google Scholar]
- 39.Martinez-Aran A, Vieta E, Colom F, et al. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord. 2004;6:224–232. doi: 10.1111/j.1399-5618.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- 40.Hill SK, Bishop JR, Palumbo D, Sweeney JA. Effect of second-generation antipsychotics on cognition: current issues and future challenges. Expert Rev Neurother. 2010;10:43–57. doi: 10.1586/ern.09.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Fourth edition, text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 42.Breitbart W, Alici-Evcimen Y. Update on psychotropic medications for cancer-related fatigue. J Natl Compr Canc Netw. 2007;5:1081–1091. doi: 10.6004/jnccn.2007.0089. [DOI] [PubMed] [Google Scholar]
- 43.Majer M, Welberg LA, Capuron L, Miller AH, Pagnoni G, Reeves WC. Neuropsychological performance in persons with chronic fatigue syndrome: results from a population-based study. Psychosom Med. 2008;70:829–836. doi: 10.1097/PSY.0b013e31817b9793. [DOI] [PubMed] [Google Scholar]
- 44.Clanton NR, Klosky JL, Li C, et al. Fatigue, vitality, sleep, and neurocognitive functioning in adult survivors of childhood Cancer: A report from the childhood cancer survivor study. Cancer. 2011;117:2559–2568. doi: 10.1002/cncr.25797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blockmans D, Persoons P, Van Houdenhove B, Bobbaers H. Does methylphenidate reduce the symptoms of chronic fatigue syndrome? Am J Med. 2006;119:167 e123–130. doi: 10.1016/j.amjmed.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 46.Advokat C. What are the cognitive effects of stimulant medications? Emphasis on adults with attention-deficit/hyperactivity disorder (ADHD) Neurosci Biobehav Rev. 2010;34:1256–1266. doi: 10.1016/j.neubiorev.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 47.Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Relative lack of cognitive effects of methylphenidate in elderly male volunteers. Psychopharmacology (Berl) 2003;168:455–464. doi: 10.1007/s00213-003-1457-3. [DOI] [PubMed] [Google Scholar]
- 48.Keefe FJ, Lumley M, Anderson T, Lynch T, Studts JL, Carson KL. Pain and emotion: new research directions. J Clin Psychol. 2001;57:587–607. doi: 10.1002/jclp.1030. [DOI] [PubMed] [Google Scholar]
- 49.van Middendorp H, Lumley MA, Jacobs JW, Bijlsma JW, Geenen R. The effects of anger and sadness on clinical pain reports and experimentally-induced pain thresholds in women with and without fibromyalgia. Arthritis Care Res (Hoboken) 2010;62:1370–1376. doi: 10.1002/acr.20230. [DOI] [PubMed] [Google Scholar]
- 50.Calley CS, Tillman GD, Womack K, Moore P, Hart J, Jr, Kraut MA. Subjective report of word-finding and memory deficits in normal aging and dementia. Cogn Behav Neurol. 2010;23:185–191. doi: 10.1097/WNN.0b013e3181c5e2d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


