Abstract
Purpose
This study had two objectives: (1) to quantify the metabolic response to physical cooling in febrile patients with Systemic Inflammatory Response Syndrome (SIRS); and (2) to provide proof for the hypothesis that the efficiency of external cooling and the subsequent shivering response are influenced by site and temperature of surface cooling pads.
Methods
To quantify shivering thermogenesis during surface cooling for fever, we monitored oxygen consumption (VO2) in six febrile patients with SIRS during conventional cooling with cooling blankets and ice packs. To begin to determine how location and temperature of surface cooling influences shivering, we compared 5 cooling protocols for inducing mild hypothermia in six healthy volunteers.
Results
In the SIRS patients, core temperature decreased 0.67°C per hour, all patients shivered, VO2 increased 57.6% and blood pressure increased 15% during cooling. In healthy subjects, cooling with the 10°C vest was most comfortable and removed heat most efficiently without shivering or VO2 increase. Cooling with combined vest and thigh pads stimulated the most shivering and highest VO2, and increased core temperature. Reducing vest temperature from 10°C to 5°C failed to increase heat removal secondary to cutaneous vasoconstriction. Capsaicin, an agonist for TRPV1 warm-sensing channels, partially reversed this effect in 5 subjects.
Conclusions
Our results identify the hazards of surface cooling in febrile critically ill patients and support the concept that optimization of cooling pad temperature and position may improve cooling efficiency and reduce shivering.
Keywords: Fever, Hypothermia, Surface Cooling, Shivering
Introduction
Fever is a complex physiologic and behavioral response to infection or injury, the key feature of which is a temporary resetting of the body’s thermostatic set point causing an increase in core temperature1. Clinical studies suggest that the effects of fever in sick humans depend, in part, on the severity of the underlying illness 2. These studies demonstrate that fever shortens and antipyretic drugs prolong non-life-threatening illnesses, including chicken pox3, rhinovirus4, 5, and Shigellosis6.
The influence of fever in severe sepsis is less clear. Up to 90% of patients with sepsis are febrile 7–9. Retrospective studies of patients with invasive bacterial infections generally show fever to be associated with improved survival, but less consistently so than in patients with lower acuity infections10–14. For example, Bryant and colleagues10 reported their analysis of 218 patients with Gram-negative bacteremia demonstrating 2.4-fold higher survival (71% vs. 29%) in patients who were febrile on the day of bacteremia (maximum daily temperature greater than 38.3°C) compared with those who remained a febrile. In a reanalysis of published retrospective studies that we ranked based on acuity of illness, we found that fever-associated improvement in survival was lost in higher acuity disease 2. These studies 11, 15 also showed that survival decreased when fever exceeded 39.4°C, suggesting there is an upper limit to the optimal febrile range. Fever in critically ill patients persisting for ≥5 days is associated with longer mechanical ventilation and ICU stay and higher mortality 16. Mortality was higher and neurologic outcome worse in patients with brain injury and fever than those who remain a febrile17, 18.
Collectively, these studies suggest that suppressing fever in critically ill patients will have profound but difficult to predict consequences that depend on the clinical context and argues for rigorous prospective studies of fever suppression in well-defined illnesses. However, standard methods for fever reduction and suppression are either ineffective or unsafe in the critically ill patient population. Acetaminophen is poorly effective in critically ill patients 19, 20. Nonsteroidal anti-inflammatory agents such as ibuprofen are more effective in reducing fever 8, but the associated toxicity profile (e.g. renal toxicity and platelet dysfunction) raise concerns about its use in many critically ill patients. Physical cooling methods can reduce core temperature with variable efficiency but all methods cause shivering 21–23, increase metabolic rate 24–26, and cause cutaneous vasoconstriction 27, which interferes with surface cooling methods. Pharmacologic methods are available to reduce the shivering response; however, these drugs have side effects that may limit their usefulness in critically ill patients19, 20, 26, 28–36.
This study had two objectives focused on the problem of fever management in critically ill patients. We first quantified the increase in VO2 associated with shivering during conventional surface cooling in patients with the Systemic Inflammatory Response Syndrome (SIRS) in whom fever persisted despite acetaminophen treatment. We then tested the hypothesis that the efficiency of external cooling and the subsequent shivering response are influenced by site and temperature of surface cooling using a precision surface cooling system to induce mild hypothermia in healthy subjects.
Materials and Methods
Clinical Protocols
All protocols were approved by the University of Maryland Institutional Review Board.
Standard surface cooling in critically ill patients with fever
We analyzed the core temperature, oxygen consumption (VO2), and hemodynamic parameters in six patients with SIRS37 during external cooling for a fever (core temperature >38.3°C). All patients were endotracheally intubated and mechanically ventilated with FiO2 ≤ 60%. Following a decision by the treating intensivist to initiate surface cooling for fever, consent was obtained and baseline measurements of hemodynamic factors and VO2 were measured over 15 min. Two Cincinnati Sub-zero Blanketrol II cooling blankets set to 4°C were placed, one above and one below the patient, and axillary and inguinal icepacks were applied. VO2 was measured using a ViasysVmax 229 metabolic cart connected to the exhalation port of the Siemens Maquet Servo-i ventilator. Baseline hemodynamic and VO2 values were established over the 15 min period prior to initiation of cooling, and were measured every 15 min during 90 min cooling period. The change in VO2 was analyzed by calculating the maximal VO2 increase during cooling compared with baseline levels and also by calculating the area under the VO2 vs. time curve using the trapezoidal rule38. Core temperature was measured from either a urinary bladder catheter or central venous catheter probe that was already present at the time of the study. Blood pressure was measured noninvasively every 15 min. Heart and respiratory rates were determined from the cardiac monitor and ventilator, respectively.
Optimization of cooling during induction of mild hypothermia in normal subjects
Following informed consent, six healthy male volunteers were subjected to the same five cooling protocols using the Arctic Sun 5000 cooling system (Medivance; Denver, CO) and large-size standard cooling pad sets. The cooling pads sets consisted of a 2-piece vest that covered the back and lower abdomen and two thigh pads. All subjects underwent the following five 30 min cooling protocols in the order listed: (1) vest at 10°C, (2) vest at 5°C, (3) vest and thigh pads at 10°C, (4) thigh pads at 5°C, and (5) vest at 5°C with capsaicin 0.15% liquid (Capzaicin™) applied to the skin beneath the vest pad 15 min prior to cooling. Subjects were allowed to recover between cooling periods for at least 30 min and until the core temperature returned to within 0.5°C of the start of the previous cooling period. VO2, shivering, core temperature and cardiovascular response were recorded every 5 min. Core temperature was continuously monitored from an esophageal probe connected to the Arctic Sun controller. Heart rate was continuously monitored from standard cardiac electrodes and recorded every 5 min. Blood pressure was measured noninvasively using an automatic device (Dinamap™). Respiratory rate and shivering were assessed by visual inspection. A shivering score was determined every 5 min using the 4-point Bedside Shivering Assessment Scale described by Badjatia et al.21. VO2 was measured using a Viasys Elite metabolic cart, mouthpiece and nose clips. Six consecutive 20 sec VO2 measurements were recorded every 5 min. Heat removed during each cooling protocol was calculated from the flow rate and the temperature of the water exiting and entering the Arctic Sun controller from the cooling pads automatically, which was recorded by the Arctic Sun controller every min and downloaded at the completion of the cooling study. The total heat removed during each 30 min cooling period was calculated using equation:
Statistics
Data are presented as mean ± SE. Differences among groups were analyzed by applying a Tukey Honestly Significant Difference Test applied to a one-way ANOVA. Effects of cooling on physiologic parameters were analyzed by repeated measures one-way ANOVA.
Results
Consequences of standard surface cooling in critically ill patients with fever
To quantify the metabolic and cardiopulmonary stress during external cooling in critically ill, febrile patients, we measured VO2, respiratory rate, hemodynamic parameters, and core temperature during a 15 min pre-cooling period and every 15 min during a 90 min cooling period with two cooling blankets and inguinal and axillary ice packs. The patients, 4 men and 2 women, were 49.7±9.8 (SE) years old, met criteria for SIRS 37, and were mechanically ventilated through an endotracheal tube or tracheostomy tube with FiO2 0.4 to 0.6 using pressure-regulated volume control ventilation in four patients, pressure control ventilation in one, and pressure support in one,. All patients had received acetaminophen without significant reduction in core temperature. One patient was receiving midazolam and another fentanyl and these were continued without change during the cooling period. The other four patients were not receiving sedation at the time of the study.
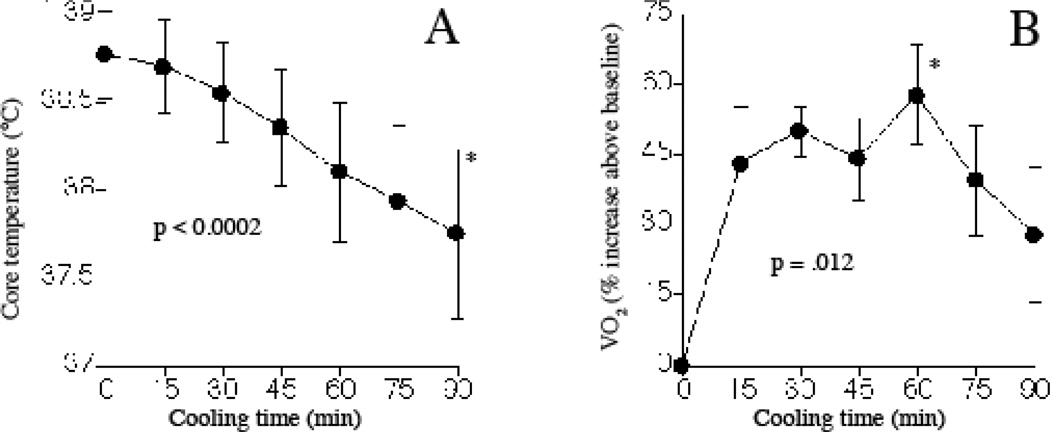
Pre-cooling core temperature was 38.8±0.25°C and decreased by 0.67°C per h during cooling (Fig. 1 A). VO2 was 273±54 ml/min in the febrile patients prior to cooling and increased to maximal levels 57.6±10.5% (mean ± SE) above pre-cooling baseline levels during cooling (Fig. 1B). An AUC analysis showed VO2 increased 34.8±7.5% over the 90-min cooling period compared with pre-cooling baseline VO2. All patients were noted to shiver during cooling. Mean arterial pressure increased 15.1±4.6% but there were no changes in heart rate or minute ventilation (Table 1).
Figure 1. Effect of external cooling on core temperature and VO2 in febrile patients with SIRS.
Six patients with SIRS and core temperature ≥38.8°C despite receiving acetaminophen were subjected to cooling using cooling blankets and ice packs for 90 min and core temperature (A) and VO2 (B) were measured every 15 min. Mean ± SE. Differences were tested by repeated measures ANOVA and p-values are indicated.
Table 1.
Physiologic response to cooling in patients with SIRS and fever1.
| Parameter | Baseline 2 | 15 min | 30 min | 45 min | 60 min | 75 min | 90 min |
|---|---|---|---|---|---|---|---|
| Heart rate | 108±29 | 108±31 | 107±24 | 105±24 | 106±24 | 103±20 | 102±213 |
| Systolic blood pressure (mm Hg) | 114±14.2 | 114±15.4 | 126±10.8 | 127±15.6 | 129±14.9 | 129±16.5 | 128±12.33 |
| Diastolic blood pressure (mm Hg) | 51±3.9 | 53±14 | 57±13 | 60±15 | 61±16 | 61±14 | 62±143 |
| Mean arterial pressure (mm Hg) | 68±4.9 | 71±14 | 77±16 | 80±17 | 83±17 | 82±16 | 82±164 |
| Respiratory rate (breaths per min) | 28±11 | 28±8.2 | 29±11 | 30±10 | 30±13 | 29±12 | 28±123 |
| VE (L/min) | 12.3±4.3 | 12.2±4 | 13.7±4.7 | 13.5±4.6 | 13.7±4.9 | 13±5 | 12.9±4.83 |
Measurements made every 15 min during cooling (mean±SD of 6 subjects)
Baseline measurements made every 5 min during 15 min pre-cooling baseline
not significant by repeated measures ANOVA.
p < 0.001 by repeated measures ANOVA.
Optimization of cooling during induction of mild hypothermia in normal subjects
To test the concept that the efficiency of surface cooling can be improved and the metabolic stress reduced by optimizing the location and temperature of surface cooling, we utilized a cooling system that allows precise placement and temperature regulation of cooling pads and quantification of heat removal. We studied six healthy men between 34 and 57 years old with BMI 24.4–35.9. We used the standard Arctic Sun™ cooling pad set, which includes a 2-piece vest with total surface area of 0.41 sq. m that covers most of the back and abdomen and a pair of circumferential thigh pads with combined surface area of 0.314 sq. m. We operated the device in the manual cooling mode with cooling pad temperature set to either 5°C or 10°C. Each cooling period lasted 30 min and was to have been terminated earlier if requested by the subject or if core temperature dropped by >1°C, neither of which occurred.
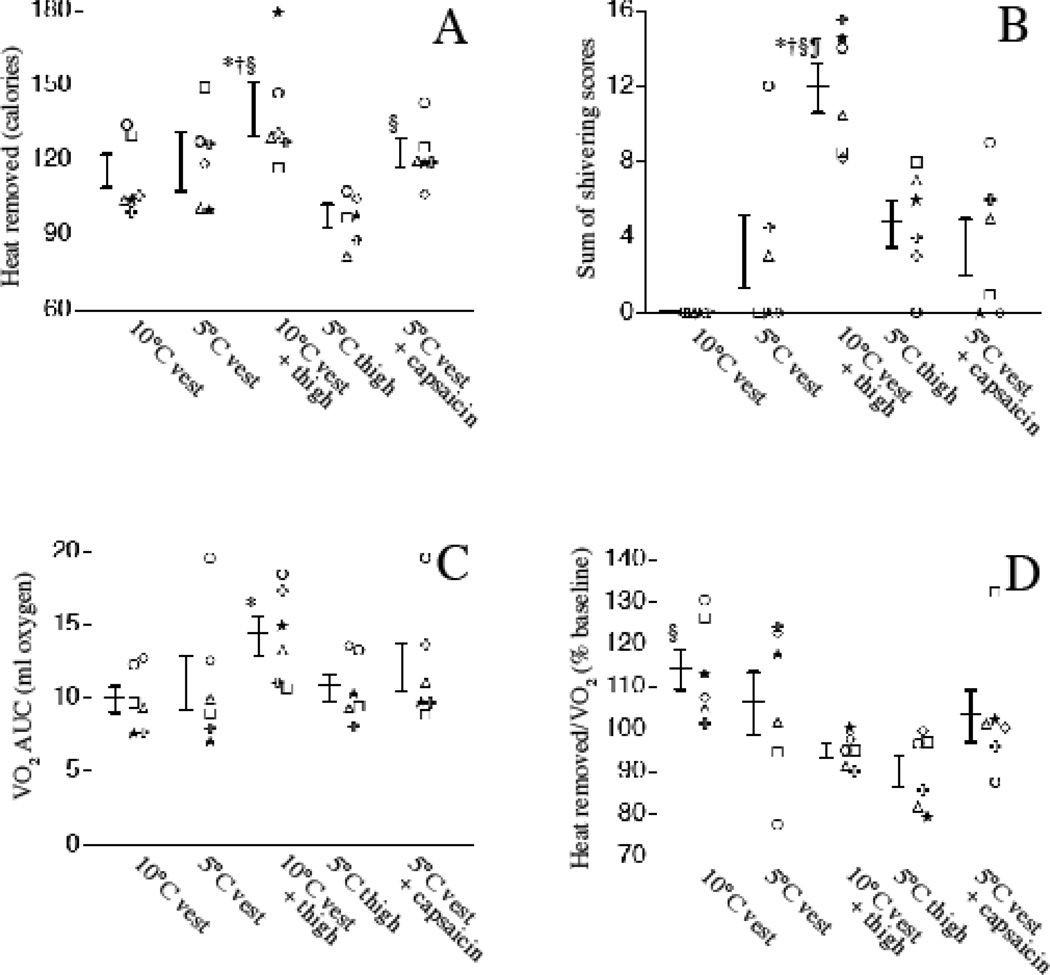
Reducing the temperature of the vest pads from 10°C to 5°C did not increase heat removal (Fig. 2A). Cooling with 10°C thigh pads and 10°C vest pads removed 26% more heat than 10°C vest pads alone, but represented 77% greater cooling pad surface area than vest pads alone. Cooling with the 5°C thigh pads alone removed 15% less heat than the 5°C vest, which was expected based on the 23% less surface area of the thigh pads vs. the vest.
Figure 2. Effect of different cooling protocols on heat removal, shivering, and oxygen consumption in normal subjects.
Six normal volunteers were subjected to 30 min cooling with the indicated pad positioning and temperature without or with 0.15% capsaicin applied beneath the pad. The same symbol represents each subject in all panels in figures 2 and 3. A. Total heat removed (calories) was estimated from the temperature of the circulating refrigerant entering and leaving the cooling device and the flow rate recorded every min during each 30 min cooling period is shown (see Methods). B. Shivering was measured objectively every 5 min during cooling using a 4-point bedside shivering assessment scale 21. The sum the shivering scores is shown. C. VO2 was measured every 5 min during cooling and AUC for the 30 min cooling period calculated using the trapezoidal rule 38. D. The ratio of heat removed to VO2 AUC for each 30 min cooling period was calculated. Individual subject data and mean ± SE are shown. *, †, §, and ¶ denote p < 0.05 vs. 10°C vest, 5°C vest, 5°C thigh pads, and 5°C vest with capsaicin, respectively.
All six subjects tolerated cooling with the 10°C vest pads without objective or subjective shivering (Fig. 2B) or change in VO2 (Fig. 2C), while cooling with the 10°C vest and thigh pad combination induced the highest shivering scores and increase in VO2. Shivering occurred almost immediately upon cooling in all six subjects during cooling with 10°C vest and thigh pads. Shivering occurred in three of six subjects during cooling with the 5°C vest pads, in four of six subjects when capsaicin was applied beneath the 5°C vest pads, and in five of six subjects during cooing with 5°C thigh pads. However, the mean shivering score in each cooling regimen except the combined vest and thigh pads was not significantly increased from baseline. The ratio of heat removed to VO2 during each cooling period was greatest for the 10°C vest and lowest for 5°C thighs (Fig. 2D).
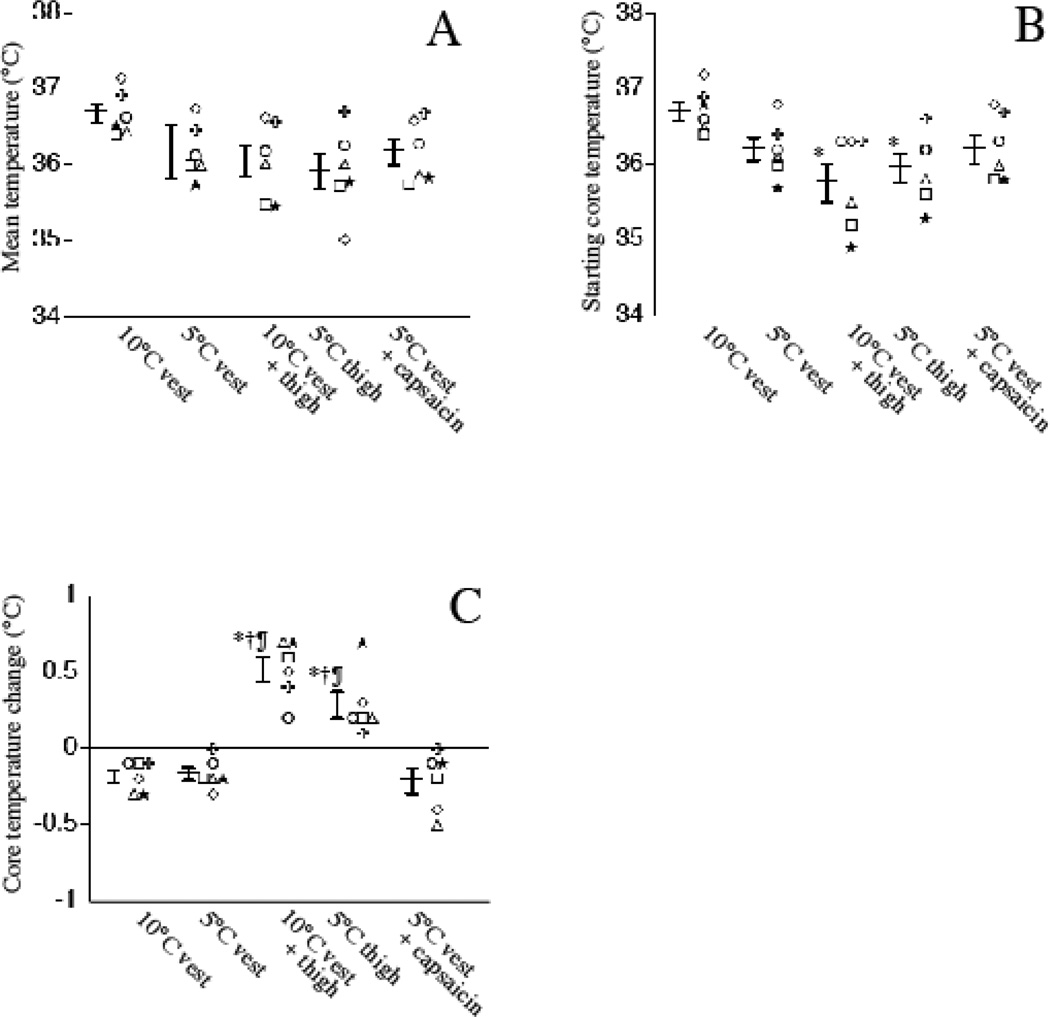
Each of the subjects were exposed to the five cooling protocols in the same order and were allowed to recover between cooling periods until core temperature was within 0.5°C of the previous starting period. There were no significant differences in mean core temperature among the five 30 min cooling periods (Fig. 3A). The starting temperature with the 5°C thigh pads and combination of 10°C vest and 10°C thigh pads was 0.7 and 0.9°C lower than with the 10°C vest but the starting core temperature among the 5°C vest, 10°C vest/thigh combination, 5°C thigh pad, and 5°C vest pads with capsaicin cooling periods were similar (Fig. 3B).Importantly, while core temperature decreased during cooling with the 10°C vest, 5°C vest, and combination of capsaicin and 5°C vest protocols, core temperature increased during cooling with the 5°C thigh pads and the combination of 10°C vest and 10°C thigh pads (Fig. 3C).
Figure 3. Effect of different cooling protocols on core temperature in normal subjects.
Six normal volunteers were subjected to 30 min cooling with the indicated pad positioning and temperature without or with 0.15% capsaicin applied beneath the pad. Core temperature was recorded from esophageal probes just before and every 5 min during each 30 min cooling period. A. The mean temperature for 30 min cooling period was calculated from the six individual measurements. B. The starting temperature for each cooling period is shown C. The change in core temperature during cooling was calculated as the difference between the core temperatures at the end and beginning of each 30 min cooling period. Individual subject data and mean ± SE are shown. *, †, and ¶ denote p < 0.05 vs. 10°C vest, 5°C vest, and 5°C vest with capsaicin, respectively.
All six subjects reported cooling with the 10°C vest to be most comfortable and the combination of 10°C vest and thigh pads to be least comfortable.
Discussion
The cutaneous vasoconstriction, shivering, and metabolic responses to physical cooling not only oppose core temperature reduction, but also increase metabolic stress, VO2, and cardiovascular demand 21, 39, 40. While the metabolic response to surface cooling has been quantified in normal subjects during cold exposure40, the metabolic and cardiopulmonary stress imposed by surface cooling in febrile, critically ill patients had not been previously quantified. In the present study, we measured the VO2 and cardiopulmonary response to conventional external cooling with cooling blankets and ice packs applied to the entire body surface in febrile patients with SIRS. All six febrile patients shivered during cooling and increased VO2 by as much as 57% despite having received acetaminophen, which is consistent with the previously described poor antipyretic activity of acetaminophen in this patient population 19, 20, 41. While the increase in metabolic rate is a substantial stress for critically ill patients, it is less than the 3-fold increase in VO2 reported by Horvath et al.40 to accompany shivering during external cooling in a febrile volunteers. The smaller fold-increase in VO2 during cooling in our SIRS patients compared with Horvath’s subjects may reflect the higher pre-cooling baseline VO2 that accompanied fever in our patients or the effect of the underlying critical illness. For example, all 6 patients had hypoxic respiratory failure and hypoxia impairs the shivering response to cooling in animal models42.
Since pharmacologic therapies to reduce the shivering and metabolic responses during physical cooling are limited by poor efficacy and potential toxicity28,29. 19, 20, 26, 30–36, we tested the hypothesis that the shivering response during external cooling might be mitigated by changing the temperature and/or site of cooling. The hypothesis is based on animal data demonstrating heterogeneous distribution and nonlinear, dynamic responsiveness of cutaneous cool sensing neurons 43–45. The Arctic Sun™ (Medivance) cooling system used in this study has high cooling efficiency 23, 49, allows accurate and precise cooling pad location and temperature, and provided readouts of heat removal and core temperature trends. We estimated the heat removed from each subject as the temperature difference between refrigerant returning to the Arctic Sun controller from the cooling pads and the refrigerant exiting the device. This estimate ignores environmental heat transferred to the refrigerant through the outside surface of the cooling pads and the conducting tubing, both of which were well-insulated. In using this assumption, we have overestimated the heat removed during each cooling period; however, the error should be small and similar among the five cooling protocols in each subject.
According to Fourier’s law of conductive heat transfer, the rate of conductive heat transfer is the product of thermal conductivity, interface surface area, and temperature gradient. Assuming that skin temperature of the subjects was ~33°C 50, the reduction in vest pad temperature from 10°C to 5°C would increase the driving force for heat transfer by ~22% yet heat removed at the two thermal pad temperatures was nearly identical (Fig. 2A). Similarly adding 10°C thigh pads to 10°C vest pads increased surface area for heat exchange by 77% but only increased heat removal by 26%. These results demonstrate that both the cooling pad temperature and position modify thermal conductance, most likely secondary to alterations in cutaneous blood flow.
We used two measures of shivering thermogenesis, a validated bedside shivering assessment scale based on direct observation of motor activity21 and direct measurement of VO2. We found good agreement between changes in shivering score and VO2 across the five treatment groups (compare figures 2B and C). The combination of 10°C vest and thigh pads stimulated the most shivering and the highest VO2. Even though this cooling protocol achieved the highest heat removal of the five protocols tested, core temperature increased by 0.52°C during the cooling period, demonstrating the capacity of shivering thermogenesis to oppose cooling. We used a ratio of heat removed-to-VO2 as a single measure of cooling efficiency and thermogenic response (Fig. 2D). As expected this ratio was highest for the three cooling protocols that reduced core temperature and lowest for the two protocols that raised core temperature. Cooling with the 10°C vest achieved the highest heat removed-to-VO2 ratio and was reported to be the most comfortable cooling protocol by all six subjects.
We also tested whether applying topical capsaicin to the skin beneath the 5°C vest pads reduced the vasoconstrictor and shivering responses to cooling. Capsaicin is a chemical agonist for the TRPV1 warm-sensing channels and causes a sensation of warmth and cutaneous vasodilatation 51, 52. We found that treating the skin underlying the 5°C cooling pad with capsaicin increased heat removal compared with 5°C vest cooling alone in 5 of 6 subjects, but the difference did not reach statistical significance and there was no detectable blunting of the shivering score. Nonetheless, we believe these limited data support the continued investigation of topical capsaicin and other agonists of cutaneous thermal sensing neurons to improve the efficiency, comfort, and safety of external cooling.
Thermal comfort and shivering thermogenesis are determined by input from both central and peripheral thermal sensors 53. The influence of peripheral thermal sensing on the shivering response was demonstrated by the classic study of Horvath et al.40 who showed that shivering in normal volunteers during cold exposure began prior to decrease in rectal temperature. The differences we found in heat removal efficiency and shivering response among the five cooling protocols support the importance of peripheral thermal sensing and suggest that cooling pad temperature and location may exert substantial influence on the vasoconstrictor and shivering thermogenesis responses. However, since the protocol only required subjects to return to within 0.5°C of the previous cooling period, the starting temperatures for the later cooling periods were lower than during the initial cooling period with the 10°C vest. The potential contribution of lower core temperature to initiation of the shivering response during cooling with 10°C vest and thigh pads cannot be excluded. However, we note that there were no differences in starting core temperature among the 10°C vest/thigh pad combination, 5°C thigh pads, 5°C vest and vest/capsaicin combination even though there were substantial differences in shivering between the 10°C vest/thigh combination and all other cooling regimens.
In summary, we have presented data that support the concept that the efficiency, comfort, and metabolic cost can be optimized by modifying the location and temperature of cooling pads and perhaps by applying chemical agonists for TRPV1 warm-sensing channels. These data support the need for additional studies to further optimize cooling parameters to develop effective, safe, and comfortable methods of cooling. Because of safety and feasibility concerns of subjecting febrile patients to multiple cooling protocols, we performed the initial proof of concept study in healthy volunteers subjected to mild hypothermia. Whether these results can be translated to febrile, critically ill patients awaits empiric testing.
Acknowledgements
Supported by NIH grants HL69057 and HL085256, by VA Merit Review Award (JDH), and by a gift of equipment and supplies by Medivance, Inc. We thank Dr. Phillip Mackowiak for his expert review and critique of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare. The study sponsors had no role in collection or analysis of the data presented.
References
- 1.Mackowiak PA. Concepts of fever. Arch Intern Med. 1998;158:1870–1881. doi: 10.1001/archinte.158.17.1870. [DOI] [PubMed] [Google Scholar]
- 2.Hasday JD, Fairchild KD, Shanholtz C. The role of fever in the infected host. Microbes and Infection. 2000;2:1–14. doi: 10.1016/s1286-4579(00)01337-x. [DOI] [PubMed] [Google Scholar]
- 3.Doran TF, DeAngelis C, Baumgardner RA, et al. Acetaminophen: more harm than good for chicken pox? J Pediatr. 1989;114:1045–1048. doi: 10.1016/s0022-3476(89)80461-5. [DOI] [PubMed] [Google Scholar]
- 4.Graham NMH, Burrell CJ, Douglas RM, et al. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus-infected volunteers. J Infect Dis. 1990;162:1277–1282. doi: 10.1093/infdis/162.6.1277. [DOI] [PubMed] [Google Scholar]
- 5.Stanley ED, Jackson GG, Panusarn C, et al. Increased viral shedding with aspirin treatment of rhinovirus infection. JAMA. 1975;231:1248–1251. [PubMed] [Google Scholar]
- 6.Mackowiak PA, Wasserman SS, Levine MM. An analysis of the quantitative relationship between oral temperature and severity of illness in experimental shigellosis. J Infect Dis. 1992;166:1181–1184. doi: 10.1093/infdis/166.5.1181. [DOI] [PubMed] [Google Scholar]
- 7.Effect of high-dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. The Veterans Administration Systemic Sepsis Cooperative Study Group. N Engl J Med. 1987;317:659–665. doi: 10.1056/NEJM198709103171102. [DOI] [PubMed] [Google Scholar]
- 8.Bernard GR, Wheeler AP, Russell JA, et al. The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group [see comments] N Engl J Med. 1997;336:912–918. doi: 10.1056/NEJM199703273361303. [DOI] [PubMed] [Google Scholar]
- 9.Clemmer TP, Fisher CJ, Jr, Bone RC, et al. Hypothermia in the sepsis syndrome and clinical outcome. The Methylprednisolone Severe Sepsis Study Group [see comments] Crit Care Med. 1992;20:1395–1401. doi: 10.1097/00003246-199210000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Bryant RE, Hood AF, Hood CE, et al. Factors affecting mortality of gram-negative rod bacteremia. Arch Intern Med. 1971;127:120–128. [PubMed] [Google Scholar]
- 11.Mackowiak PA, Browne RH, Southern PM, Jr, et al. Polymicrobial sepsis: an analysis of 184 cases using log linear models. Am J Med Sci. 1980;280:73–80. doi: 10.1097/00000441-198009000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Weinstein MP, Iannini PB, Stratton CW, et al. Spontaneous bacterial peritonitis. A review of 28 cases with emphasis on improved survival and factors influencing prognosis. Am J Med. 1978;64:592–598. doi: 10.1016/0002-9343(78)90578-8. [DOI] [PubMed] [Google Scholar]
- 13.Hoefs JC, Canawati HN, Sapico FL, et al. Spontaneous bacterial peritonitis. Hepatology. 1982;2:399–407. doi: 10.1002/hep.1840020402. [DOI] [PubMed] [Google Scholar]
- 14.DuPont HL, Spink WW. Infections due to gram-negative organisms: an analysis of 860 patients with bacteremia at the University of Minnesota Medical Center, 1958–1966. Medicine (Baltimore) 1969;48:307–332. doi: 10.1097/00005792-196907000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Hodgin UG, Sanford JP. Gram-negative rod bacteremia. An analysis of 100 patients. Am J Med. 1965;39:952–960. doi: 10.1016/0002-9343(65)90118-x. [DOI] [PubMed] [Google Scholar]
- 16.Peres Bota D, Lopes Ferreira F, Melot C, et al. Body temperature alterations in the critically ill. Intensive Care Med. 2004;30:811–816. doi: 10.1007/s00134-004-2166-z. [DOI] [PubMed] [Google Scholar]
- 17.Hajat C, Hajat S, Sharma P. Effects of poststroke pyrexia on stroke outcome : a meta-analysis of studies in patients. Stroke. 2000;31:410–414. doi: 10.1161/01.str.31.2.410. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz S, Hafner K, Aschoff A, et al. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology. 2000;54:354–361. doi: 10.1212/wnl.54.2.354. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg RS, Chen H, Hasday JD. Acetaminophen has limited antipyretic activity in critically ill patients. J Crit Care. 2010;25:363, e361–e367. doi: 10.1016/j.jcrc.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackenzie I, Forrest K, Thompson F, et al. Effects of acetaminophen administration to patients in intensive care. Intensive Care Med. 2000;26:1408. doi: 10.1007/s001340000614. [DOI] [PubMed] [Google Scholar]
- 21.Badjatia N, Strongilis E, Gordon E, et al. Metabolic impact of shivering during therapeutic temperature modulation: the Bedside Shivering Assessment Scale. Stroke. 2008;39:3242–3247. doi: 10.1161/STROKEAHA.108.523654. [DOI] [PubMed] [Google Scholar]
- 22.Carhuapoma JR, Gupta K, Coplin WM, et al. Treatment of refractory fever in the neurosciences critical care unit using a novel, water-circulating cooling device. A single-center pilot experience. J Neurosurg Anesthesiol. 2003;15:313–318. doi: 10.1097/00008506-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Mayer SA, Kowalski RG, Presciutti M, et al. Clinical trial of a novel surface cooling system for fever control in neurocritical care patients. Crit Care Med. 2004;32:2508–2515. doi: 10.1097/01.ccm.0000147441.39670.37. [DOI] [PubMed] [Google Scholar]
- 24.Eyolfson DA, Tikuisis P, Xu X, et al. Measurement and prediction of peak shivering intensity in humans. Eur J Appl Physiol. 2001;84:100–106. doi: 10.1007/s004210000329. [DOI] [PubMed] [Google Scholar]
- 25.Kimberger O, Ali SZ, Markstaller M, et al. Meperidine and skin surface warming additively reduce the shivering threshold: a volunteer study. Crit Care. 2007;11:R29. doi: 10.1186/cc5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mokhtarani M, Mahgoub AN, Morioka N, et al. Buspirone and meperidine synergistically reduce the shivering threshold. Anesth Analg. 2001;93:1233–1239. doi: 10.1097/00000539-200111000-00038. [DOI] [PubMed] [Google Scholar]
- 27.Kurz A, Sessler DI, Birnbauer F, et al. Thermoregulatory vasoconstriction impairs active core cooling. Anesthesiology. 1995;82:870–876. doi: 10.1097/00000542-199504000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Dupuis JY, Nathan HJ, DeLima L, et al. Pancuronium or vecuronium for treatment of shivering after cardiac surgery. Anesth Analg. 1994;79:472–481. doi: 10.1213/00000539-199409000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Gehr LC, Sessler CN. Neuromuscular blockade in the intensive care unit. Semin Respir Crit Care Med. 2001;22:175–188. doi: 10.1055/s-2001-13831. [DOI] [PubMed] [Google Scholar]
- 30.Kizilirmak S, Karakas SE, Akca O, et al. Magnesium sulfate stops postanesthetic shivering. Ann N Y Acad Sci. 1997;813:799–806. doi: 10.1111/j.1749-6632.1997.tb51784.x. [DOI] [PubMed] [Google Scholar]
- 31.Piper SN, Röhm KD, Suttner SW, et al. A comparison of nefopam and clonidine for the prevention of postanaesthetic shivering: a comparative, double-blind and placebo-controlled dose-ranging study. Anesthesia. 2004;59:559–564. doi: 10.1111/j.1365-2044.2004.03734.x. [DOI] [PubMed] [Google Scholar]
- 32.Wadhwa A, Sengupta P, Durrani J, et al. Magnesium sulphate only slightly reduces the shivering threshold in humans. Br J Anaesth. 2005;94:756–762. doi: 10.1093/bja/aei105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zweifler RM, Voorhees ME, Mahmood MA, et al. Induction and maintenance of mild hypothermia by surface cooling in non-intubated subjects. J Stroke Cerebrovasc Dis. 2003;12:237–243. doi: 10.1016/j.jstrokecerebrovasdis.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 34.McGettigan P, Henry D. Current problems with non-specific COX inhibitors. Curr Pharm Des. 2000;6:1693–1724. doi: 10.2174/1381612003398690. [DOI] [PubMed] [Google Scholar]
- 35.Doufas AG, Lin CM, Suleman MI, et al. Dexmedetomidine and meperidine additively reduce the shivering threshold in humans. Stroke. 2003;34:1218–1223. doi: 10.1161/01.STR.0000068787.76670.A4. [DOI] [PubMed] [Google Scholar]
- 36.Kurz A, Ikeda T, Sessler DI, et al. Meperidine decreases the shivering threshold twice as much as the vasoconstriction threshold. Anesthesiology. 1997;86:1046–1054. doi: 10.1097/00000542-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Bone RC, Fisher CJJ, Clemmer TP, et al. Sepsis syndrome: a valid clinical entity. Crit Care Med. 1989;17:389–393. [PubMed] [Google Scholar]
- 38.Givens GH, Hoeting JA. Computational Statistics (ed First) New York City, N.Y.: Wiley-Interscience; 2005. [Google Scholar]
- 39.Gozzoli V, Treggiari MM, Kleger GR, et al. Randomized trial of the effect of antipyresis by metamizol, propacetamol or external cooling on metabolism, hemodynamics and inflammatory respons. Intensive Care Med. 2004;30:401–407. doi: 10.1007/s00134-003-2087-2. [DOI] [PubMed] [Google Scholar]
- 40.Horvath S, Spurr G, Hutt B, et al. Metabolic cost of shivering. J Appl Physiol. 1956;8:595–602. doi: 10.1152/jappl.1956.8.6.595. [DOI] [PubMed] [Google Scholar]
- 41.Henker R, Rogers S, Kramer DJ, et al. Comparison of fever treatments in the critically ill: a pilot stud. Am J Crit Care. 2001;10:276–280. [PubMed] [Google Scholar]
- 42.Hemingway A, Birzis L. Effect of hypoxia on shivering. J Appl Physiol. 1956;8:577–579. doi: 10.1152/jappl.1956.8.6.577. [DOI] [PubMed] [Google Scholar]
- 43.Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R37–R46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- 44.Kenshalo DR, Duncan DG, Weymark C. Thresholds for thermal stimulation of the inner thigh, footpad, and face of cats. J Comp Physiol Psychol. 1967;63:133–138. doi: 10.1037/h0024177. [DOI] [PubMed] [Google Scholar]
- 45.Simon E, Pierau FK, Taylor DC. Central and peripheral thermal control of effectors in homeothermic temperature regulation. Physiol Rev. 1986;66:235–300. doi: 10.1152/physrev.1986.66.2.235. [DOI] [PubMed] [Google Scholar]
- 46.Badjatia N, O'Donnell J, Baker JR, et al. Achieving normothermia in patients with febrile subarachnoid hemorrhage: feasibility and safety of a novel intravascular cooling catheter. Neurocrit Care. 2004;1:145–156. doi: 10.1385/NCC:1:2:145. [DOI] [PubMed] [Google Scholar]
- 47.Pichon N, Amiel JB, Francois B, et al. Efficacy of and tolerance to mild induced hypothermia after out-of-hospital cardiac arrest using an endovascular cooling system. Crit Care. 2007;11:R71. doi: 10.1186/cc5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simosa HF, Petersen DJ, Agarwal SK, et al. Increased risk of deep venous thrombosis with endovascular cooling in patients with traumatic head injury. Am Surg. 2007;73:461–464. [PubMed] [Google Scholar]
- 49.English MJ, Hemmerling TM. Heat transfer coefficient: Medivance Arctic Sun Temperature Management System. vs. water immersion. Eur J Anaesthesiol. 2008;25:531–537. doi: 10.1017/S0265021508003931. [DOI] [PubMed] [Google Scholar]
- 50.Buguet AG, Livingstone SD, Reed LD, et al. Cold-induced shivering in men with thermoneutral skin temperatures. J Appl Physiol. 1976;41:142–145. doi: 10.1152/jappl.1976.41.2.142. [DOI] [PubMed] [Google Scholar]
- 51.Gavva NR. Body-temperature maintenance as the predominant function of the vanilloid receptor TRPV1. Trends Pharmacol Sci. 2008;29:550–557. doi: 10.1016/j.tips.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Stephens DP, Charkoudian N, Benevento JM, et al. The influence of topical capsaicin on the local thermal control of skin blood flow in humans. Am J Physiol Regul Integr Comp Physiol. 2001;281:R894–R901. doi: 10.1152/ajpregu.2001.281.3.R894. [DOI] [PubMed] [Google Scholar]
- 53.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci. 2008;11:62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]