Abstract
This study addresses a gap in the attachment literature by investigating maternal neural response to cry related to infant attachment classifications and behaviors. Twenty-two primiparous mothers and their 18-month old infants completed the Strange Situation Procedure (SS) to elicit attachment behaviors. During a separate functional MRI session, mothers were exposed to their own infant’s cry sound, as well as an unfamiliar infant’s cry and control sound. Maternal neural response to own infant cry related to both overall attachment security and specific infant behaviors. Mothers of less secure infants maintained greater activation to their cry in left parahippocampal and amygdala regions and the right posterior insula. consistent with a negative schematic response bias. Mothers of infants exhibiting more avoidant or contact maintaining behaviors during the SS showed diminished response across left prefrontal, parietal, and cerebellar areas involved in attentional processing and cognitive control. Mothers of infants exhibiting more disorganized behavior showed reduced response in bilateral temporal and subcallosal areas relevant to social cognition and emotion regulation. No differences by attachment classification were found. Implications for attachment transmission models are discussed.
Keywords: attachment, mother, infant, fMRI, cry
1. The missing link: Mothers’ neural response to infant cry related to infant attachment behaviors
The attachment relationship that develops through repeated caregiver-infant interactions has important implications not only for infants’ subsequent functioning (e.g., Kochanska et al., 2010) but also for their own children, given intergenerational transmission of insecurity (Shah, Fonagy, & Strathearn, 2011). As suggested by a recent overview of attachment research, such transmission is likely carried through distorted patterns of cognitive and affective responses to interpersonal distress, learned through inadequate caregiver responses to negative emotion and expressed in interactions with one’s own infant (Dykas & Cassidy, 2011). A next step in this research is to identify neural substrates for such differences in cognitive/affective response, bringing into focus which components underlie which aspects of attachment formation. The current study takes an important step in this direction by examining the relation between mothers’ neural response to their infant’s distress cues and their infants’ attachment behaviors with them.
An infant’s ability to form a secure attachment to the mother—to use her as a secure base for exploration and a safe haven in the face of threat (see Bowlby, 1969)—depends on the mother’s responsiveness to infant cues, particularly when these involve distress. To the extent that the mother’s response is sensitive, characterized by mutuality, synchrony, and consideration of the infant’s mental state (or “mind-mindedness”), she fosters a secure attachment (deWolff & van Ijzendoorn, 1997; Meins, Fernyhough, Fradley, & Tuckey, 2001). These response qualities depend, in turn, on open, flexible attention to and interpretation of a range of emotion in herself and her infant, which may be hindered by her own attachment experiences (Dykas & Cassidy, 2011). In particular, adults with insecure representations of their own attachment tend to suppress processing or use negative schemas to process attachment-relevant social information, which is proposed to contribute to insecurity in their children.
Depending on the types of emotion the mother preferentially responds to and the consistency of her responsiveness, different forms of insecurity may develop. Persistent nonresponse to infant negative emotion has more typically been associated with avoidant attachment, and inconsistent responses or nonresponse to positive emotion with anxious attachment (Goldberg, MacKay-Soroka, & Rochester, 1994; Grossmann, Grossmann, Kindler, & Zimmermann, 2008). This suggests that a mother who herself carries anxious or avoidant attachment representations reproduces her attentional bias and associated emotional dysregulation in her infant, though some research supports an inversion of attachment style (i.e., a mother with an anxious attachment representation producing an avoidant attachment relationship with her infant: Shah et al., 2010). Finally, disconnected and/or extremely insensitive maternal responses to infant emotion—perhaps based on PTSD-like dissociation—have been associated with disorganized attachment (Out, Bakermans-Kranenburg & van Ijzendoorn, 2009). Ongoing questions about the origins and specificity of these patterns observed at cognitive and behavioral levels may be resolved by a clearer picture of neural mechanisms involved.
The emerging field of parental neuroimaging has begun to delineate normative “maternal” response circuits activated by infant auditory/visual cues, as well as individual differences in response relevant to parenting quality (see Barrett & Fleming, 2010). A study of mothers responding to video of their own infants during both a distressing task (Strange Situation Procedure) and a non-distressing play session showed higher activity in the left orbitofrontal cortex (OFC) related to more happy/joyful feelings, and higher activity in the right OFC related to more anxious feelings toward their infants (Noriuchi, Kikuchi, & Senoo, 2008). This study also related mothers’ feelings of love and excitement for their infants to increased infant distress-specific responses in the superior temporal sulci. Such differences in affective response may, in turn, guide behavioral differences relevant to attachment formation.
Two studies have investigated maternal behavioral sensitivity in relation to neural response to their own infant’s distress (cry sound). The first demonstrated an association between observed sensitivity during mother-infant interaction at 3-4 months and activation of the right superior frontal gyrus and amygdala at 1 month postnatal (Kim et al., 2011), and the second (conducted within our lab) related mothers’ observed sensitivity with their 18-month-old infants to heightened right frontopolar and inferior frontal activation (Musser, Laurent, & Ablow, in press). Together with the above, these studies suggest prefrontal and temporal areas play a critical role in guiding more or less optimal maternal responses to infant expressions of negative emotion. Consistent with attachment theory, there is also evidence that mothers’ own early care experiences influence these circuits, with mothers reporting better care themselves showing enhanced structural development and infant-related activation in the OFC and temporal cortex, as well as occipital, parietal, and cerebellar circuits important for attending to and interpreting infant sensory cues (Kim et al., 2010).
In the absence of any previous research directly addressing infant attachment-related differences in maternal neural response, we turn to studies of adult attachment, which should inform infant attachment as discussed above. Neuroimaging research has demonstrated effects of adult attachment on brain structure and function relevant to social-cognitive processing and emotion regulation. Studies of specific attachment styles1 support the idea that adults with anxious attachment representations have difficulty downregulating negative emotion via left lateral OFC activity (Benetti et al., 2010; Gillath, Bunge, Wendelken, & Mikulinder, 2005). In addition, the amygdala hyperactivation thought to characterize insecurity more generally (Lemche et al., 2006) appears most pronounced among anxiously attached adults responding to negative emotion cues (Vrticka, Andersson, Grandjean, Sander, & Vuilleumier, 2008). The picture for avoidant attachment representations is more complex, with findings of both diminished activation to negative emotion cues in regions generating a sensory-emotional response—OFC, striatal, somatosensory—and increased activation or failure to deactivate in cognitive/affective control circuits—dorsolateral prefrontal and subcallosal cortices (Gillath et al., 2005; Strathearn, Fonagy, Amico, & Montague, 2009; Suslow et al., 2009). These findings have been interpreted to illustrate costs of an avoidant style, in that efforts to control felt responses to negative emotional information may lead to ineffective responding and/or recovery.
To our knowledge, only one pilot study has investigated effects of disorganized attachment representations on adults’ neural responses to negative emotion cues; the authors failed to detect significant overall differences between resolved and unresolved groups, but the latter tended to show greater medial temporal lobe activity with exposure to more negative emotional content (Buchheim et al., 2006). Related research on trauma survivors further suggests a breakdown in temporal and subcallosal (i.e., subgenual anterior cingulate) function underlying dissociation and severely dysregulated emotional responses (Buchheim et al., 2008; Hopper, Frewen, van der Kolk, & Lanius, 2007). Overall, these studies suggest a brain basis for insecure adults’ difficulties taking in and responding accurately to negative emotional information. As yet, no research offers insight into parental brain responses underlying differences in their infants’ attachment to them.
This study was designed to address suggestive gaps in attachment research. First, we aimed to identify maternal neural response to infant distress cues related to infant attachment security, and to compare this response with neural correlates of adult attachment based on the existing literature reviewed above. This would ground theorizing about mechanisms for the transmission of insecurity—i.e., suppression or negative interpretation of threatening emotional information—in a neurophysiological framework. Based on current risk transmission models, we hypothesized that infant insecurity would be associated with similar maternal neural patterns as those previously found in less emotionally/behaviorally responsive mothers and in adult insecurity—i.e., compromised OFC, temporal, and/or subcallosal function. Second, we wished to investigate maternal neural response differences related not only to infant attachment classifications, but also to a continuous security classifier (reflecting the likelihood of secure vs. insecure attachment), and to continuous measures of specific attachment behaviors. Most previous research has focused on categorical differences between secure and insecure groups, but continuous variability in behavioral strategies may better reflect attachment differences (Fraley & Spieker, 2003). Therefore, a secondary aim of this research was to examine both infant attachment classifications, and the behaviors giving rise to such classifications, in relation to maternal neural response.
2. Method
2.1 Participants
Twenty-two primiparous mothers (M age = 24.1 years, SD = 4.1) of 15-18-month old infants were recruited through the Women Infants Children (WIC) program to participate in this study. Mothers gave informed consent and were screened for MRI contraindications and for psychopathology using the Structured Clinical Interview for the DSM-IV (SCID); as described further in Laurent & Ablow (2011), half of participants were required to meet criteria for a major depressive episode during the perinatal period, and the other half to show no diagnosable psychopathology. By the time of the current study assessments, none of the mothers met criteria for a major depressive episode (according to the SCID), and both depression status and current symptoms were found to be unrelated to attachment variables. Thus, while the sample represents a relatively high-risk group of mother-infant dyads, the mood-related elements of risk appeared to operate independently of infant attachment-related risk. Three mothers reported taking psychotropic medication (SSRI’s); analyses excluding these participants yielded no differences from the full sample, so they were retained. These 22 mothers represent the subset of those originally screened into the study (n = 36) eligible to complete it; reasons for discontinuation included new pregnancy and failure to collect a cry sample. No systematic differences were found between those who completed and did not complete the study.
Reflective of the community from which they were drawn, mothers tended to be Caucasian (77%; 14% African American; 9% Latina) and low SES (32% reporting household income < $10,000 per year; 36% $20,000-40,000; 32% > $40,000). Most had experienced a vaginal delivery (18% caesarian section) and breastfed their infants at least 3 months (27% 0-3 mo; 36.5% 3-12 mo; 36.5% >12 mo). Just over half were married (36%) or in a stable cohabiting relationship (23%), and a minority (32%) were still partnered with the infant’s biological father. There were no demographic differences related to attachment variables.
2.2 Measures
2.2.1 Infant attachment
The Strange Situation Procedure (SS; Ainsworth, Blehar, Waters, & Wall, 1978) was conducted during a laboratory visit within one week of the scanning session. The standard series of separations and reunions was used to access differences in infants’ attachment security with their mothers, and videotapes were sent for expert classification and attachment behavior coding by Elizabeth Carlson, Ph.D. at the University of Minnesota. Infants were classified as Avoidant (A), Resistant (C), Disorganized (D), or Secure (B) based on their patterns of SS behavior as described by Ainsworth and colleagues (1978).
Infants were also coded on a 1-7 scale during each reunion for interactive behaviors that could be indicative of insecure attachment: avoidance referred to the persistence and promptness of the infant’s avoidance of interaction with the mother; contact maintenance referred to the intensity and persistence of the infant’s efforts to maintain contact with the mother once in contact with her; resistance referred to the frequency and duration of resistance to contact (e.g., fussiness, angry distress); and disorganization referred to the degree to which the infant displayed conflicting or incoherent behavioral strategies, which could include a mixture of organized strategies and/or uncommon behaviors such as freezing (see Main and Solomon, 1990). As described in previous work investigating attachment in terms of both categorical classifications and continuous behaviors (Fraley & Spieker, 2003), A infants should be distinguished by high avoidance and low contact maintenance, C infants by high contact maintenance and resistance and low avoidance, D infants by high disorganization, and B infants by low levels of avoidance, resistance, and disorganization.
Disorganized behavior was coded as a single score across both reunions, and mean scores across the two reunions for the remaining insecure behaviors were computed for use as continuous predictors. Substantial correlations (average r = .56) between reunion 1 and 2 behaviors supported this approach. At the same time, the differential contributions of reunion 1 vs. 2 behaviors to judgments of attachment security were taken into account with a weighted security composite score calculated according to a version of Richters and colleagues’ (1988) algorithm (see Van Ijzendoorn & Kroonenberg, 1990 for the scoring used in this paper). This provided a second, continuous measure of the likelihood that the infant was securely vs. insecurely attached.
2.2.2 Infant temperament control
Mothers reported on their infant’s temperament with the Early Childhood Behavior Questionnaire (Putnam, Gartstein, & Rothbart, 2006). Negative affect (alpha in this sample = .92), surgency (alpha = .79), and effortful control (alpha = .64) dimension scores were calculated and considered as possible confounds in attachment effects.
2.2.3 Maternal depressive symptom control
Within 1 week of scanning, mothers reported on current depressive symptoms using the Center for Epidemiologic Studies Depression Scale (Radloff, 1977). Total depressive symptom scores (alpha = .93) were also considered as a control variable to distinguish the influence of maternal mood in attachment-related responses.
2.3 Stimulus Collection and Presentation
A sample of each mother’s own infant’s cry following injections was recorded at their 18-month well baby visit. Twenty-one seconds from the beginning of the first cry expiration were selected for the “own cry” stimulus. In addition, cry sound from an unfamiliar infant was collected using the same procedures to be presented to all participants, and a non-cry control sound was developed by editing a rising and falling tone to have a fundamental frequency within the range of normal infant cry (400-600 Hz; Zeskind & Lester, 1978). All sounds were edited to have the same maximum amplitude but were otherwise unaltered to retain their natural frequency and temporal characteristics. No attachment-related differences in cry sounds were found.
The stimulus protocol consisted of two 9-minute runs of a block design presenting own cry, other cry, control sound, and rest periods. Ordering of blocks within runs was counterbalanced within and across participants, and each run contained 6 repetitions of each block (2s pause + 21s sound for cry/control sound; 21s pause for rest). Participants were instructed to simply listen to the sounds to allow the most natural range of response to cry. Sound was presented via earphones in the scanner, and a sound check carried out before each scan to ensure audibility.
2.4 Scanning
MR imaging was conducted with a 3T Siemens Allegra 3 magnet. A standard birdcage coil was used to acquire data from the whole brain. Sessions began with a shimming routine to optimize signal-to-noise ratio, followed by a fast localizer scan (FISP) and Siemens Autoalign routine, then the two functional runs and anatomical scan.
2.4.1 Functional
T2*-weighted gradient echo sequence, 64 × 64 voxel matrix, TE = 30ms, TR = 2000ms, flip angle = 80, 32 contiguous slices thickness = 4mm; 273 volumes per run.
2.4.2 Structural
T1-weighted 3D MP-RAGE sequence, TI = 1100ms, TR = 2500ms, TE = 4.4ms, 176 transverse slices 1mm thick, 256 × 176 matrix FOV = 256mm.
2.4.3 Post-scan ratings
Mothers rated cry and control sounds on Zeskind and Lester’s (1978) scales of subjective response to cry. Using a 5-point Likert scale, they rated each sound on the following qualities: urgent, grating, arousing, piercing, discomforting, aversive, distressing, and soothing. No differences by infant attachment security (either categorical or continuous scale) were found. Mothers of infants showing more behaviors characteristic of attachment anxiety tended to rate their infant’s cry as less arousing (r = −.41, p = .06 with contact maintenance; r = −.38, p = .08 with resistance) and piercing (r = −.46, p = .03 with contact maintenance; r = −.45, p = .04 with resistance), whereas mothers of infants showing more avoidance behaviors tended to rate their infant’s cry as more arousing (r = .36, p = .10). No attachment-related differences in response to the other infant’s cry sound were found. Differing subjective maternal responses related to infant anxiety vs. avoidance underlined the advisability of investigating these insecurity dimensions separately. Finally, mothers were asked to indicate which cry sound belonged to their own infant; all 22 mothers correctly identified their own infant’s cry.
2.5 Data Analysis
Functional imaging data were analyzed with tools from the fMRIB Software Library (FSL v4.1). Preprocessing steps included motion correction with MCFLIRT, non-brain structure removal with BET, spatial smoothing using Gaussian kernel 5mm FWHM, intensity normalization using grand mean scaling and high-pass temporal filtering (sigma = 65s). Within-participant time series data were analyzed using FILM with local autocorrelation correction, and boxcar models indicating onset-offset of each sound stimulus were convolved with a double-gamma basis function. Functional data were registered to the participant’s own high-resolution structural image (6 df) and to a standard brain (Montreal Neurological Institute template; 12 df) using FLIRT. All data were checked for excessive motion (> 1mm) and artefacts.
Within-participant and group-level analyses were carried out using FEAT v.5.98. For each mother, three explanatory variables (EVs) modeled signal associated with own cry, other cry, and control sound; zero for all three stimulus EVs corresponded to rest. Contrasts of parameter estimates (COPEs) for own cry > control sound and own cry > other cry tested primary hypotheses regarding response to own infant distress (each sound > rest was also examined to describe signal change relative to baseline). First-level COPE images were averaged across runs using fixed-effects analysis. These served as inputs to higher-level group analyses, conducted using FLAME to model random-effects components of mixed-effects variance. AlphaSim was used to determine cluster size needed, in conjunction with intensity threshold p < .005, to achieve a false discovery rate (FDR) of .05 for whole-brain analyses (Cox, 1996). Using these criteria, activation clusters exceeding 16 voxels, or 615 mm3, were considered significant in group analyses.
At the group level, infant attachment EVs were tested in relation to mothers’ neural response to own infant cry (> control sound or > other cry) using the General Linear Model. Attachment-related differences in mothers’ neural response were tested in three ways. First, group differences were addressed by creating dichotomous (0 or 1) EVs for secure and insecure classifications, as well as organized and disorganized, then testing secure > insecure and organized > disorganized contrasts (the opposite direction, i.e. secure < insecure, was also tested; n’s for specific organized-insecure groups were too small to justify separate tests). Second, continuous (centered) security scores were tested as an EV to identify activation related to higher or lower likelihood of infant secure attachment. Finally, specific attachment behavior scores were entered as a set of simultaneous EVs, meaning that any effects for a particular behavior were controlling for the effects of the other behaviors. Both positive and negative contrast weights were tested for each continuous predictor to determine whether it related to increased or decreased neural response. To visualize data driving continuous attachment predictor effects, but not to run additional tests, spherical ROI’s (r = 4mm) centered on activation peaks were used to compute percent signal change associated with sound stimuli (compared to rest) and generate illustrative figures.
3. Results
3.1 Associations among Behavioral Measures
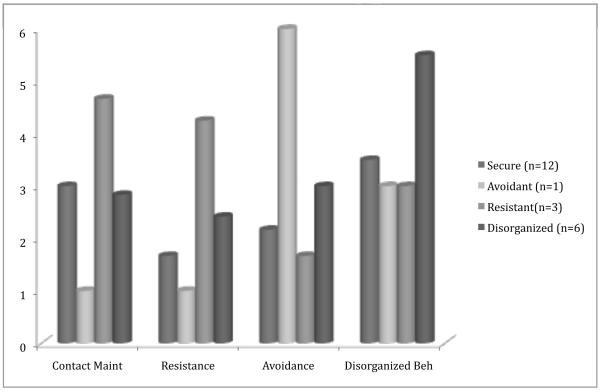
As expected, infants classified as Resistant (n = 3) displayed higher levels of contact maintenance and resistance, the infant classified as Avoidant (n = 1) displayed higher levels of avoidance, and infants classified as Disorganized (n = 6) displayed higher levels of disorganization, compared to Secure (n = 12; Figure 1). At the same time, substantial overlap in behavior ranges between categories suggested that infant behavior ratings were not wholly redundant with classification. The continuous security score correctly classified 82% of cases as secure vs. insecure and showed a significant inverse association with resistance (r = −.57), but not with the other behavior codes. Consistent with attachment theory, contact maintenance was positively correlated with resistance (r ‘s = .58-.63 across reunions) and negatively correlated with avoidance (r’s = −.52 across reunions) in this sample. Still, substantive unique variability was found for each behavior (>50% unexplained by the other behaviors), supporting their examination as simultaneous predictors.
Figure 1.
Infant attachment behaviors by classification.
To distinguish infant attachment behavior effects from those related to temperament, we also examined correlations with mother-reported temperament scales (negative affect, surgency, effortful control). Nonsignificant associations suggested that insecure attachment behaviors were not a proxy for negative affectivity and/or underregulation more generally in this sample. Separability of attachment from temperament effects was further confirmed by testing temperament scales in fMRI analysis models and finding no overlap with attachment results reported below.
Maternal depressive symptoms were not significantly associated with any attachment variables. Still, models including depressive symptom scores as a covariate were tested to definitively rule out maternal mood influences on attachment results.
3.2 Functional MRI Data Analysis
3.2.1 Maternal response related to infant attachment classification and likelihood of secure vs. insecure attachment
Comparisons of maternal response in secure vs. insecure and organized vs. disorganized groups yielded no significant differences in neural activity (to either own cry > control sound or > other cry) by attachment classification.
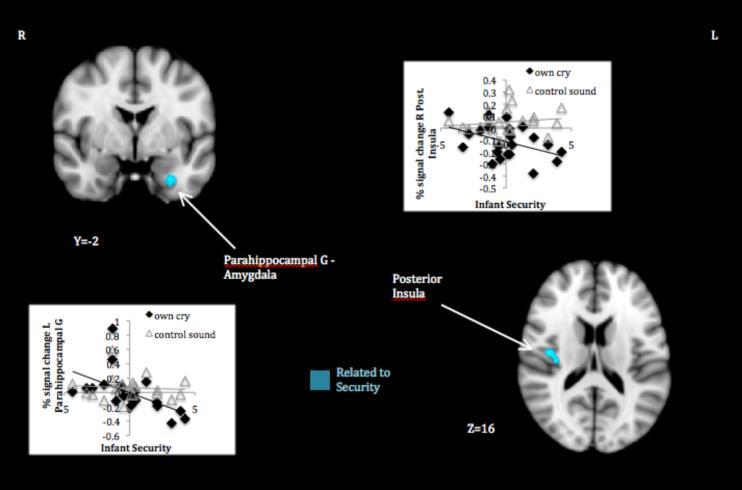
Mothers of infants with lower continuous security scores—which meant a greater likelihood of insecure attachment—responded more to their infant’s cry relative to control sound in the left parahippocampal gyrus extending to ventral amygdala and in the right posterior insula/parietal operculum (Table 1, panel A; Figure 2). Plots of signal change to own infant cry and control sound by security scores confirmed that differences in maternal response were attributable to the own cry stimulus (i.e., increasing security scores related to decreasing activation to own infant cry, ns association with control sound). Whereas mothers of infants more likely to be secure tended to deactivate in these regions when they heard their infant’s cry, mothers of infants more likely to be insecure failed to do so, or even showed activation. No security-related differences in maternal response to their own infant’s cry relative to that of another infant were found.
Table 1.
Mothers’ Neural Response to Own Infant Cry Related to Infant Attachment
| Peak Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Brain Area | BA | R/L | Volume (mm3) |
Peak Z | X | Y | Z |
| A. Own Cry > Control Sound Response Related to | |||||||
| Infant Security Scores | |||||||
| Inversely Related to Continuous Security | |||||||
| Parahippocampal Gyrus – Ventral Amygdala | 36 | L | 628 | 3.53 | −28 | −1 | −32 |
| Posterior Insula – Parietal Operculum | 41 | R | 718 | 3.56 | 36 | −18 | 15 |
| B. Own Cry > Control Sound Response Related to | |||||||
| Infant Behaviors | |||||||
| 1. Inversely Related to Contact Maintenance | |||||||
| Ventrolateral Prefrontal - Orbitofrontal Cortex | 11/47 | L | 1115 | 3.72 | −36 | 56 | −10 |
| Inferior Frontal Gyrus | 45 | L | 855 | 3.64 | −54 | 26 | 16 |
| Middle Frontal Gyrus | 46 | R | 932 | 3.54 | 40 | 23 | 35 |
| Premotor Cortex | 6 | R | 2000 | 3.76 | 45 | 4 | 42 |
| 6 | L | 836 | 3.78 | −33 | −2 | 48 | |
| Posterior Parietal Cortex | 7/40 | L | 2224 | 3.76 | −37 | −51 | 46 |
| Superior Occipital Cortex | 7/19 | R | 18619 | 4.32 | 26 | −82 | 45 |
| 19 | L | 8135 | 3.89 | −30 | −87 | 31 | |
| Lingual Gyrus | 19 | R | 967 | 3.71 | 19 | −62 | −7 |
| Occipital Pole | 17 | R/L | 937 | 3.57 | −3 | −90 | 10 |
| Cerebellum – Crus I | L | 1256 | 3.79 | −38 | −79 | −30 | |
| Cerebellum -- Vermis | R/L | 1088 | 3.83 | 2 | −76 | −26 | |
| 2. Inversely Related to Avoidance | |||||||
| Ventrolateral Prefrontal Cortex | 10/47 | L | 1274 | 3.99 | −38 | 46 | −1 |
| Posterior Parietal Cortex | 7 | L | 1178 | 3.55 | −40 | −57 | 56 |
| Cerebellum – Crus I | L | 886 | 3.89 | −37 | −81 | −30 | |
| 3. Inversely Related to Disorganization | |||||||
| Posterior Middle Temporal Gyrus – Superior | 21/22 | R | 1266 | 3.65 | 58 | −24 | −13 |
| Temporal Sulcus | |||||||
| 21/22 | L | 972 | 4.18 | −59 | −17 | −8 | |
| Anterior Middle – Superior Temporal Gyri | 22 | R | 919 | 3.39 | 50 | 0 | −16 |
| 22 | L | 930 | 4.02 | −55 | −6 | −12 | |
| Subcallosal Cortex | 25 | L | 824 | 3.89 | −10 | 10 | −18 |
| C. Own Cry > Other Cry Response Related to Infant | |||||||
| Behaviors | |||||||
| Inversely Related to Contact Maintenance | |||||||
| Temporal – Occipital Fusiform to Lingual Gyri | 37/19 | L | 4005 | 3.83 | −33 | −58 | −14 |
| 37/19 | R | 3353 | 3.47 | 19 | −59 | −7 | |
| Lateral Occipital Cortex | 18/19 | R | 5899 | 3.76 | 23 | −87 | 35 |
| Precuneus – Cuneus | 7 | L | 2643 | 3.31 | −15 | −75 | 41 |
| Superior Frontal Gyrus | 8 | R | 1348 | 3.74 | 25 | 25 | 57 |
Note. Clusters met thresholding criteria (> 615 mm3 p < .005) based on whole-brain FDR .05. Coordinates based on Montreal Neurological Institute template. BA = putative Brodmann’s Area.
Figure 2.
Maternal response to own infant cry > control sound associated with infant likelihood of secure attachment.
Note. Activations thresholded at whole brain FDR .05. Scatter plots depict signal change (compared to resting baseline) associated with own infant cry and control sound in areas showing attachment-related differences.
3.2.2 Maternal response related to infant insecure behaviors
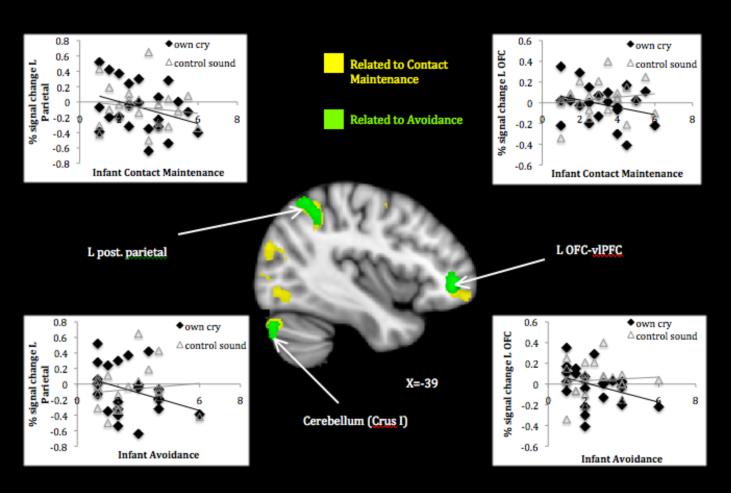
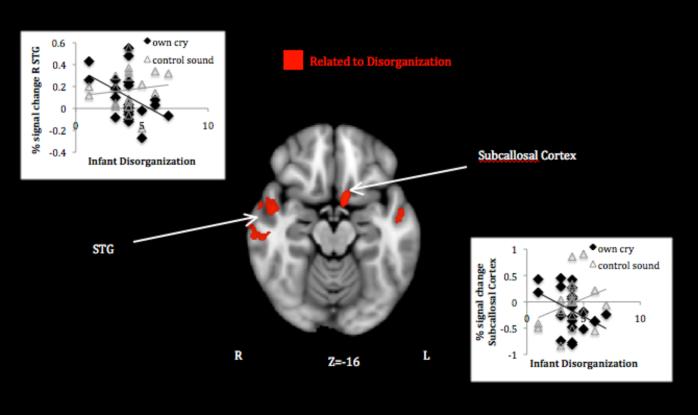
Mothers of infants exhibiting higher contact maintenance activated less to their own infant’s cry relative to control sound in several prefrontal clusters including left OFC extending to ventrolateral prefrontal cortex (vlPFC) and inferior frontal gyrus, right middle frontal gyrus, and bilateral premotor cortex. They also activated less in widespread posterior clusters involving parietal and occipital cortices and cerebellum (Table 1, panel B1; Figure 3). Higher levels of infant avoidance also related to decreased maternal activation in several clusters overlapping with those found for contact maintenance—left vlPFC, posterior parietal cortex, and cerebellar Crus I (Table 1, panel B2; Figure 3). Finally, mothers of infants who showed more disorganized behavior activated less in bilateral temporal areas around the superior temporal sulcus (STS), as well as left subcallosal (subgenual anterior cingulate) cortex (Table 1, panel B3; Figure 4).
Figure 3.
Maternal response to own infant cry > control sound associated with infant insecure-organized behaviors.
Note. Activations thresholded at whole brain FDR .05. Scatter plots depict signal change (compared to resting baseline) associated with own infant cry and control sound in areas showing attachment-related differences.
Figure 4.
Maternal response to own infant cry > control sound associated with infant insecure-disorganized behavior.
Note. Activations thresholded at whole brain FDR .05. Scatter plots depict signal change (compared to resting baseline) associated with own infant cry and control sound in areas showing attachment-related differences.
Plots of signal change to own infant cry and control sound by the above attachment behaviors again confirmed that differential response was attributable to the own cry stimulus. For the subcallosal cluster, increasing disorganization related not only to decreased own cry-related activity, but also to increased control sound-related activity, a reversal of the usual maternal response. The one Avoidant infant constituted an outlier on the continuous avoidance scale; examining associations with and without that data point suggested that whereas OFC and parietal clusters related reliably to avoidance, the cerebellum cluster should be interpreted with greater caution. Removal of this case also failed to substantively alter findings for the other attachment behaviors.
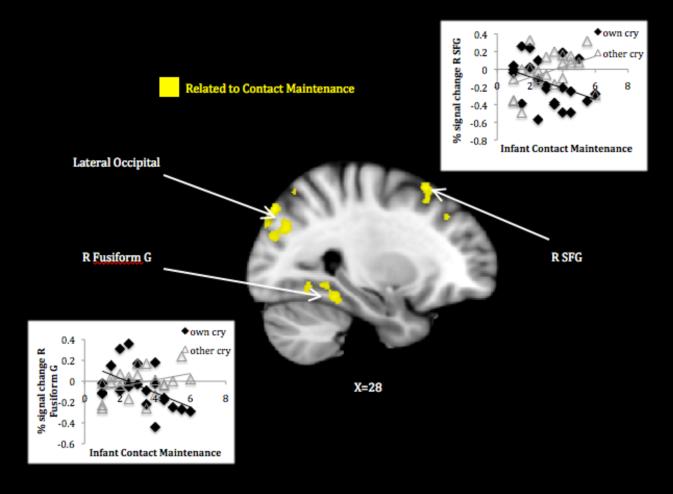
Mothers’ response to their own infant’s cry relative to that of another infant related to infant contact maintenance (no other behaviors related to this contrast). Mothers of infants exhibiting greater contact maintenance activated less in several visual processing areas—temporal-occipital fusiform and lingual gyri, lateral occipital cortex—and the cuneus/precuneus, as well as in the right superior frontal gyrus (see Table 1, panel C; Figure 5). Plots of signal change to own and other infant cry by contact maintenance revealed that mothers of highly contact maintaining infants tended to deactivate to their own infant’s cry while activating to the unfamiliar cry in these regions.
Figure 5.
Maternal response to own > other infant cry associated with infant contact maintenance.
Note. Activations thresholded at whole brain FDR .05. Scatter plots depict signal change (compared to resting baseline) associated with own and other infant cry in areas showing attachment-related differences.
3.2.3 Impact of maternal depressive symptoms
Finally, the above contrasts were tested while controlling for current maternal depressive symptoms. Whereas the continuous security score-related effects became nonsignificant, the attachment behavior (contact maintenance, avoidance, disorganization) effects remained essentially unchanged.
4. Discussion
In this study we found evidence for altered processing of infant distress cues in mothers of infants displaying more evidence of insecure attachment. Broadly, mothers of infants more likely to be insecurely attached maintained greater activation (failure to deactivate) to their infant’s cry in neural circuits involved in painful or emotionally laden memories. At the level of specific behaviors, mothers of infants exhibiting more insecure-organized behaviors (avoidance, contact maintenance) showed diminished activity in circuits involved in attending to sensory cues and cognitive regulation of emotion, whereas mothers of infants exhibiting more insecure-disorganized behavior showed lower activity in circuits involved in understanding social information and basic modulation of emotion. These findings illustrate and extend speculations about neurocognitive bases for transmission of attachment insecurity; the first pattern is consistent with priming negative schematic memories, and the latter two with suppressed processing of infant negative emotion information. Each of these patterns should lead to less accurate representations of and response to the infant’s emotional state, impairing maternal sensitivity. Although further study involving both mother and infant attachment measures will be needed to fully appreciate their relevance, analysis of these patterns and how they arise holds promise for understanding the transmission of insecurity.
Mothers whose infants were more likely to be securely attached based on behavior in the Strange Situation deactivated more to their infant’s cry in the left parahippocampal gyrus extending to ventral amygdala and right posterior insula. The parahippocampal and connected hippocampal regions serve important memory functions and may indicate the cueing of (negative) attachment-related schemas, as suggested by attachment transmission theory (Dykas & Cassidy, 2011). This line of reasoning also fits with previous findings of higher left hippocampal activation in adults with insecure attachment representations (Buchheim et al., 2006; Vrticka et al., 2008), as well as less deactivation of the region to cry sound in mothers reporting poor parental care histories (Kim et al., 2010). Although we cannot know the content of such memory processes, the amygdala component of this cluster is consistent with emotional hyperreactivity to attachment stimuli thought to mark insecurity throughout life (Lemche et al., 2006). Furthermore, the posterior insula has been implicated in both externally and internally generated pain (e.g., Berman et al., 2008; Isnard, Magnin, Jung, Mauguiere, & Garcia-Larrea, 2011) and could be interpreted as a felt component of aversive schemas cued by the cry sound. These results provide preliminary support for the contention that negatively biased responses to their infant’s distress prevent mothers from serving as a secure base. At the same time, they do not tell the whole story; maternal neural correlates of particular insecure behaviors suggested these are linked to different aspects of restricted attachment information processing.
Mothers of infants demonstrating behaviors characteristic of both attachment anxiety (i.e., contact maintenance) and avoidance (i.e., avoidance) responded less to their cry in left ventrolateral prefrontal, parietal, and cerebellar regions. Reduced left OFC activity to negative emotion cues has been previously implicated in adult attachment anxiety (Gillath et al., 2005) and avoidance (Strathearn et al., 2009), and development of this area may depend on positive early care experiences (Kim et al., 2010). More broadly, left ventrolateral prefrontal (including OFC) activity is implicated in successful emotion regulation using reappraisal (Kanske et al., 2010). Together with the current findings, this suggests that a problematic attachment history inhibits the development of prefrontal regulatory capacities that, in turn, make it difficult for a parent to adequately respond to their own infant’s distress and foster secure attachment behaviors. The posterior parietal cortex is also recognized as part of an attentional network with connections to affective experience. Proposed as part of a dorsal stream of phasic emotion regulation (i.e., modulating happy vs. sad mood; Liotti et al., 2000; Liotti & Tucker, 1995), posterior parietal areas are active during cognitive emotion regulation strategies and deactivated during sadness (Kanske, et al., 2010; Heissler, Schonfelder, Bongers, & Wessa, 2010).
Finally, the cerebellar region related to both contact maintenance and avoidance is known to be involved in cortical loops important for working memory and cognitive control (Habas et al., 2009); as such, it may serve to support and direct the task engagement suggested by prefrontal and parietal activations. Although the specific localization of effects varies, there is growing evidence that cerebellar development and function relate to parenting history (Kim et al., 2010) and current bonding with one’s infant (Kim et al., 2011), which this research further supports. It should be noted that these are unique effects for more anxious and avoidant behaviors (controlling for one another), meaning that despite the negative association between these types of insecurity, they relate to some common component of maternal underresponse. Even though the behaviors on both mothers’ and infants’ parts differ, as do the subjective perceptions of cry that likely contribute to different forms of maternal insensitivity, a core deficit in cognitive-affective regulation may help to explain insufficient responses to negative emotion in both varieties of organized insecurity.
In addition to the regions described above, mothers of more contact maintaining infants responded less across visuomotor integration and action planning circuits. Activation in both dorsal and ventral visual processing streams may reflect enhanced sensory awareness of and readiness to reach for and soothe their infants, a response chain supported by premotor activity that would allow mothers to execute the proper behaviors (Koch et al., 2008). Unique response to her own infant’s cry (vs. that of another infant) across dorsal and ventral visual association areas also related to reduced infant contact maintenance. Even though the infant stimulus was auditory and not visual, this fits with previous research showing visual activation in response to cry among mothers with better parental care experiences (Kim et al., 2010) and suggests such cross-modal response is beneficial for parenting. Further lateral prefrontal activity—particularly in the inferior frontal gyrus area associated with “mirror” functions (Molnar-Szakacs, Iacoboni, Koski, & Mazziotta, 2005)—suggests a connection between interpreting the distress cue, generating an empathic response, and enacting a behavioral plan. Although speculative at this point, these differences in activation may shed further light on how and why infants’ contact maintaining behaviors develop; if the mother fails to respond to cry with an accurately guided behavioral plan, due to her own defensive suppression of the cry information, the infant may attempt to compensate by staying close by and soliciting care through physical contact. The finding of more extensive response differences associated with contact maintenance vs. avoidance may mean the former signals a more generalized disturbance in maternal processing, or it may simply reflect differences in observed variability in this sample (more limited for the latter), and further replication is needed to determine its significance.
Diminished temporal and subcallosal response to cry in mothers of infants showing more disorganized behavior represents a novel finding, but it fits broadly with social and emotional processing disturbances identified previously in trauma research. The superior temporal sulcus and surrounding temporal regions have been commonly associated with social cognitive function, including using autobiographical memory to judge others’ intentions (Spreng & Mar, 2010) and perspective taking (Hooker, Verosky, Germine, Knight, & D-Esposito, 2010). The hypoactivation found here diverges from several studies of women responding to attachment trauma cues demonstrating increased activity with incoherent attachment or borderline personality features (Buchheim et al., 2006; 2008) and fits more with observations of post-traumatic stress-related dissociation (Hopper, et al., 2007). Although none of the mothers of disorganized infants in this sample met criteria for PTSD, they did report subthreshold trauma symptoms, and these may have contributed to observed neural patterns. It is also possible that the type of response task determines whether temporal hyper-vs. hypoactivation is found; as suggested by Dykas and Cassidy (2011), insecure working models of attachment should cause suppression of attachment-relevant social information when the task requires passive/automatic processing (as in our instruction to listen to the cry) but negatively biased schematic processing when the task requires explicit attention to attachment information (as in the attachment story tasks used by Buchheim). Again, development and infant-related activation of superior temporal regions appear to depend on quality of early care experiences and relate to current mother-infant bonding (Kim et al., 2010; 2011), underlining their importance in the transmission of competent parenting.
The subcallosal cortex—closely connected to the amygdala—has been widely implicated in emotional dysregulation, with decreased function related to depression (Hamani et al., 2011) and post-traumatic avoidance symptoms (Hopper et al., 2007). Inadequate inhibitory control of negative emotions, combined with inadequate interpretation of the situation itself (suggested by the above), could contribute to mothers’ disconnected and/or grossly insensitive responses to their infant’s distress that foster disorganization. Interestingly, mothers of more disorganized infants not only responded less to their infant’s cry, but responded more to the control sound, in this area. Similar to the interpretation of insecure adults’ failure to deactivate subcallosal areas following thought suppression in previous research (Gillath et al., 2005), this pattern reflects not universal nonresponse, but rather an inefficient or improperly targeted response. These mothers may have learned to shut down response to the cue actually requiring action (infant cry) while maintaining hypervigilance to an innocuous cue (control sound). More work with clinical samples is needed to disentangle trauma-related from disorganization-specific neural processing of negative emotional information, but the higher than usual occurrence of disorganization in this sample allowed for at least an initial observation of overlap.
Despite these differences in brain response related to attachment behaviors, we were unable to detect differences by classification. This may be because we did not have sufficient numbers of dyads within each insecure-organized category, forcing a combination of heterogenous groups for comparison. A larger sample capturing a more complete range of insecure variants, particularly avoidance, would be needed to fully characterize maternal response profiles. Still, the present results illustrate the utility of considering continuous variability in attachment behaviors and likelihood of secure vs. insecure attachment, and not simply categorical differences. The fact that different neural dysregulation components were detected at different levels of analysis—i.e., overactive negative processing related to overall likelihood of insecure attachment, underactive regulatory processing related to specific insecure behaviors—further suggests that attachment should be analyzed at multiple levels to fully appreciate common vs. distinct bases of insecure phenotypes. A final point is that the influence of parental psychopathology on attachment may depend on the level of analysis; in this study, maternal depressive symptom effects overlapped substantially with those related to infant likelihood of insecure attachment, whereas they did little to explain specific insecure infant behaviors. Maternal depression may do more to shape the neural responses supporting overall sensitivity with infants than those dictating the specific form of insensitivity. These and other issues could become clearer with further investigation of attachment as a multilevel construct.
Another area requiring more attention is the importance of a mother’s unique response to her own infant’s distress cues. This study demonstrated attachment-related differences in maternal response primarily for the own infant cry > control sound contrast, with few differences related to own > other infant cry activity. It may be that the mother’s receptivity to cry information and ability to regulate her own response typically applies more broadly to infant cry and is not unique to her own infant’s vocalizations. An alternative is that the limited form of stimulus presentation—i.e., passively listening to a cry sound known to be prompted by an injection, as opposed to interpreting and deciding how to respond to a more complex audiovisual infant distress scenario—made it difficult to detect actual differences in the maternal brain that guide her interactions with her own infant. This possibility should be explored with neuroimaging paradigms that include both simpler standardized infant stimulus tasks, and more complex naturalistic ones.
Further limitations of the current study should be used to guide future research in this area. Although our findings are consistent with a model by which parents’ attachment-influenced processing of negative infant emotional information contributes to their infant’s attachment to them, we did not have measures of mothers’ attachment to examine the full attachment transmission model, and a logical next step would be to investigate both parent and infant attachment in relation to neural response. Another important step involves translating maternal neural response into behavioral responses to infants’ positive and negative emotional signals. We have interpreted neural activations related to infant security as contributors to sensitive, accurate maternal responses, but definitive statements cannot be made without testing maternal behavior predictors of neural response. This process is underway in our lab at a relatively global level of behavioral analysis (Musser, Laurent, & Ablow, in press) and should be extended with more refined coding of emotional response sequences. Finally, while we have interpreted maternal brain response as the driver (via behavioral interactions) of infant attachment, it is possible that infant attachment behaviors shape maternal brain responses. We were able to rule out infant temperament as an explanation for these patterns, so it is unlikely that mothers simply respond less to more generally difficult infants. Still, longitudinal research is needed to examine which attachment-relevant differences in women’s response to cry sound precede becoming a mother, and which develop through mother-infant interaction experiences.
Limitations notwithstanding, this research adds a neural component to previous cognitive/behavioral observations of security-enhancing maternal responses and builds a bridge with previous neuroimaging research on adult attachment. It suggests a common negative response bias in mothers of infants more likely to be insecurely attached but differing sources of response difficulties—weakened cognitive regulation vs. basic social-emotional processing of negative emotional input—in mothers of infants showing insecure-organized and disorganized behaviors. These observations, and the neurobehavioral models they scaffold, should help to advance both practical (clinical treatment targets) and theoretical considerations in the field of parent-infant attachment.
Highlights.
We related maternal brain response to own infant cry to infant attachment behaviors
Parahippocampal, amygdala and insula deactivation related to infant security
Lower prefrontal and parietal activity related to insecure-organized behaviors
Lower temporal and subcallosal activity related to insecure-disorganized behavior
Inadequate response to infant cry may impair the developing attachment relationship
Acknowledgments
This work was supported by the National Science Foundation [0643393]; a National Institute of Mental Health postdoctoral fellowship [F32MH083462-02] to HL; and a pilot grant from the University of Oregon Brain Biology Machine Initiative. The authors also wish to thank the families who participated in this study and the research assistants who helped with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Adult attachment research reviewed here includes findings based on both adults’ representations of their own attachment histories with their parents (Adult Attachment Interview; AAI) and reports on current romantic attachment security (Experiences in Close Relationships; ECR). Given the paucity of neuroimaging research using the AAI—i.e., only Strathearn and colleagues’ 2009 study—and the convergence of available findings across these definitions of attachment, they are presented together. It should be noted that these measures tap both common and distinct aspects of adult attachment (see Shaver, Belsky, and Brennan, 2000), and further work distinguishing neural correlates associated with each is needed.
Contributor Information
Heidemarie K. Laurent, University of Wyoming
Jennifer C. Ablow, University of Oregon
References
- Ainsworth MDS, Blehar MC, Water E, Walls S. Patterns of attachment: A psychological study of the strange situation. Erlbaum; Hillsdale, NJ: 1978. [Google Scholar]
- Barrett J, Fleming AS. All mothers are not created equal: Neural and psychobiological perspectives on mothering and the importance of individual differences. Journal of Child Psychology and Psychiatry. 2010;52:368–397. doi: 10.1111/j.1469-7610.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- Benetti S, McCrory E, Arulanantham S, De Sanctis T, McGuire P, Mechelli A. Attachment style, affective loss and gray matter volume: A voxel-based morphometry study. Human Brain Mapping. 2010;31:1482–1489. doi: 10.1002/hbm.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Ohning G, Kilpatrick L, Bueller JA, Ruby K, Jarcho J, Mayer EA. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. Journal of Neuroscience. 2008;28:349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J. Attachment and loss. Vol 1, Attachment. Basic Books; New York: 1969. [Google Scholar]
- Buchheim A, Erk S, George C, Kachele H, Kircher T, Martius P, Pokorny D, Ruchsow M, Spitzer M, Walter H. Neural correlates of attachment trauma in borderline personality disorder: A functional magnetic resonance imaging study. Psychiatry Research. 2008;163:223–235. doi: 10.1016/j.pscychresns.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Buchheim A, Erk S, George C, Kachele H, Ruchsow M, Spitzer M, Kircher T, Walter H. Measuring attachment representation in an fMRI environment: A pilot study. Psychopathology. 2006;39:144–152. doi: 10.1159/000091800. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavior Sciences. 2nd ed Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dykas MJ, Cassidy J. Attachment and the processing of social information across the life span: Theory and evidence. Psychological Bulletin. 2011;137:19–46. doi: 10.1037/a0021367. [DOI] [PubMed] [Google Scholar]
- De Wolff MS, van Ijzendoorn MH. Sensitivity and attachment: A meta-analysis on parental antecedents of infant attachment. Child Development. 1997;68:571–591. [PubMed] [Google Scholar]
- Fortuna K, Roisman GI. Insecurity, stress, and symptoms of psychopathology: Contrasting results from self-reports versus interviews of adult attachment. Attachment and Human Development. 2008;10:11–28. doi: 10.1080/14616730701868571. [DOI] [PubMed] [Google Scholar]
- Fraley RC, Spieker SJ. Are infant attachment patterns continuously or categorically distributed? A taxometric analysis of strange situation behavior. Developmental Psychology. 2003;39:387–404. doi: 10.1037/0012-1649.39.3.387. [DOI] [PubMed] [Google Scholar]
- Gillath O, Bunge SA, Shaver PR, Wendelken C, Mikulincer M. Attachment-style differences in the ability to suppress negative thoughts: Exploring the neural correlates. Neuroimage. 2005;28:835–847. doi: 10.1016/j.neuroimage.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Goldberg S, MacKay-Soroka S, Rochester M. Affect, attachment, and maternal responsiveness. Infant Behavior and Development. 1994;17:335–339. [Google Scholar]
- Grossmann KE, Grossmann K, Kindler H, Zimmermann P. A wider view of attachment and exploration: The influence of mothers and fathers on the development of psychological security from infancy to young adulthood. In J. 2008 [Google Scholar]
- Cassidy, Shaver P, editors. Handbook of attachment theory and research. Guilford; New York: [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckman CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. Journal of Neuroscience. 2009;29:8588–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biological Psychiatry. 2011;69:301–308. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Germine LT, Knight RT, D’Esposito M. Neural activity during social signal perception correlates with self-reported empathy. Brain Research. 2010;1308:100–113. doi: 10.1016/j.brainres.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper JW, Frewen PA, van der Kolk BA, Lanius RA. Neural correlates of re-experiencing, avoidance, and dissociation in PTSD: Symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. Journal of Traumatic Stress. 2007;20:713–725. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- Isnard J, Magnin M, Jung J, Mauguiere F, Garcia-Larrea L. Does the insula tell our brain that we are in pain? Pain. 2011;152:946–951. doi: 10.1016/j.pain.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schonfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex. 2010 doi: 10.1093/cercor/bhq216. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, Swain JE. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. Journal of Child Psychology and Psychiatry. 2011 doi: 10.1111/j.1469-7610.2011.02406.x. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Newman M, Feldman R, Swain JE. Perceived quality of maternal care in childhood and structure and function of mothers’ brain. Developmental Science. 2010;13:662–673. doi: 10.1111/j.1467-7687.2009.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch F, Fernandez Del Omo M, Cheeran Bn., Schippling S, Caltagirone C, Driver J, Rothwell JC. Functional interplay between posterior parietal and ipsilateral motor cortex revealed by twin-coil transcranial magnetic stimulation during reach planning toward contralateral space. Journal of Neuroscience. 2008;28:5944–5953. doi: 10.1523/JNEUROSCI.0957-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G, Woodard J, Kim S, Koenig JL, Yoon JE, Barry RA. Positive socialization mechanisms in secure and insecure parent child dyads: Two longitudinal studies. Journal of Child Psychology and Psychiatry. 2010;51:998–1009. doi: 10.1111/j.1469-7610.2010.02238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC. A cry in the dark: Depressed mothers show reduced neural activation to their own infant’s cry. Social Cognitive and Affective Neuroscience. 2011;7:125–134. doi: 10.1093/scan/nsq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemche E, Giampietro VP, Surguladze SA, Amaro EJ, Andrew CM, Williams SC, Brammer MJ, Lawrence N, Maier MA, Russell TA, Simmons A, Ecker C, Joraschky P, Phillips ML. Human attachment security is mediated by the amygdala: Evidence from combined fMRI and psychophysiological measures. Human Brain Mapping. 2006;27:623–635. doi: 10.1002/hbm.20206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT. Differential limbic-cortical correlates of sadness and anxiety in healthy subjects: Implications for affective disorder. Biological Psychiatry. 2000;48:30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- Liotti M, Tucker DM. Emotion in asymmetric cortico-limbic networks. In: Davidson RJ, Hugdahl K, editors. Brain Asymmetry. MIT Press; Cambridge, MA: 1995. pp. 389–423. [Google Scholar]
- Main M, Solomon J. Procedures for identifying infants as disorganized/disoriented during the Ainsworth Strange Situation. In: Greenberg MT, Cicchetti D, Cummings EM, editors. Attachment in the preschool years: Theory, research, and intervention. University of Chicago Press; Chicago: 1990. pp. 121–160. [Google Scholar]
- Manassis K, Owens M, Adam KS, West M, Sheldon-Keller AE. Assessing attachment: Convergent validity of the adult attachment interview and the parental bonding instrument. Australian and New Zealand Journal of Psychiatry. 1999;33:559–567. doi: 10.1080/j.1440-1614.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- Meins E, Fernyhough C, Fradley E, Tuckey M. Rethinking maternal sensitivity: Mothers’ comments on infants’ mental processes predict security of attachment at 12 months. Journal of Child Psychology and Psychiatry. 2001;42:637–648. [PubMed] [Google Scholar]
- Molnar-Szakacs I, Iacoboni M, Koski L, Mazziotta JC. Functional segregation within pars opercularis of the inferior frontal gyrus: Evidence from fMRI studies of imitation and action. Cerebral Cortex. 2005;15:986–994. doi: 10.1093/cercor/bhh199. [DOI] [PubMed] [Google Scholar]
- Musser E, Laurent HK, Ablow JC. Neural correlates of maternal sensitivity, intrusiveness, and mother-infant dyadic harmony: An fMRI study. Developmental Cognitive Neuroscience. doi: 10.1016/j.dcn.2012.04.003. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Senoo A. The functional neuroanatomy of maternal love: Mother’s response to infant’s attachment behaviors. Biological Psychiatry. 2008;63:415–423. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Out D, Bakermans-Kranenburg MJ, van Ijzendoorn MH. The role of disconnected and extremely insensitive parenting in the development of disorganized attachment: Validation of a new measure. Attachment and Human Development. 2009;11:419–443. doi: 10.1080/14616730903132289. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Gartstein MA, Rothbart MK. Measurement of fine-grained aspects of toddler temperament: The Early Childhood Behavior Questionnaire. Infant Behavior and Development. 2006;29:386–401. doi: 10.1016/j.infbeh.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Richters JE, Waters E, Vaughn BE. Empirical classification of infant-mother relationships from interactive behavior and crying during reunion. Child Development. 1988;59:512–522. [PubMed] [Google Scholar]
- Shah PE, Fonagy P, Strathearn L. Is attachment transmitted across generations? The plot thickens. Clinical Child Psychology and Psychiatry. 2010;15:329–345. doi: 10.1177/1359104510365449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaver PR, Belsky J, Brennan KA. The adult attachment interview and self-reports of romantic attachment: Associations across domains and methods. Personal Relationships. 2000;7:25–43. [Google Scholar]
- Spreng RN, Mar RA. I remember you: A role for memory in social cognition and the functional neuroanatomy of their interaction. Brain Research. 2010 doi: 10.1016/j.brainres.2010.12.024. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslow T, Kugel H, Rauch AV, Dannlowski U, Bauer J, Konrad C, Arolt V, Heindel W, Ohrmann P. Attachment avoidance modulates neural response to masked facial emotion. Human Brain Mapping. 2009;30:3553–3562. doi: 10.1002/hbm.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ijzendoorn M, Kroonenberg PM. Cross-cultural consistency of coding the Strange Situation. Infant Behavior and Development. 1990;13:469–485. [Google Scholar]
- Vrticka P, Andersson F, Grandjean D, Sander D, Vuilleumier P. Individual attachment style modulates human amygdala and striatum activation during social appraisal. PLoS ONE. 2008;3:e2868. doi: 10.1371/journal.pone.0002868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeskind PS, Lester BM. Acoustic features and auditory perception of the cries of newborns with prenatal and perinatal complications. Child Development. 1978;49:580–89. [PubMed] [Google Scholar]