Abstract
Objective
To understand how regional body composition affects bone mineral density (BMD) in HIV-infected and uninfected women.
Methods
Dual energy X-ray absorptiometry was used to measure regional lean and fat mass and BMD at lumbar spine (LS), total hip (TH), and femoral neck (FN) in 318 HIV-infected and 122 HIV-uninfected Women's Interagency HIV Study participants at baseline and 2 and 5 years later. Total lean and fat mass were measured using bioimpedance analysis. Multivariate marginal linear regression models assessed the association of HIV status and body composition on BMD change.
Results
Compared to HIV-uninfected women, HIV-infected women were older (44 vs. 37 yrs), more likely to be HCV-infected (32% vs. 14%), and post-menopausal (26% vs. 3%), and had lower baseline total fat mass, trunk fat and leg fat. In multivariate models, increased total lean mass was independently associated with increased BMD at LS, TH and FN and total fat mass was associated with increased BMD at TH and FN (all p<0.05). When total fat was replaced in multivariate models with trunk fat and leg fat, increased trunk fat (and not leg fat) was associated with increased TH and FN BMD (p<0.001).
Conclusions
Total fat and lean mass are strong, independent predictors of TH and FN BMD, and lean mass was associated with greater LS BMD. Regardless of HIV status, greater trunk fat (and not leg fat) was associated with increased TH and FN BMD, suggesting that weight bearing fat may be a more important predictor of BMD in the hip.
Keywords: Body composition, fat redistribution, bone mineral density, HIV, women
Introduction
Long-term consequences of HIV infection and its treatment, particularly disturbances of bone metabolism, are emerging concerns given the growing numbers of older adults living with HIV. We previously demonstrated that HIV infection was associated with lower bone mineral density (BMD) in predominantly premenopausal women and there was little difference in the rate of decline in HIV-infected compared to HIV-uninfected women.1,2 Changes in body composition may be a strong component of bone health, particularly among HIV-infected patients, who frequently develop a fat redistribution syndrome. We reported that HIV-infected women were at greater risk of peripheral lipoatrophy than HIV-uninfected women.3 By contrast, HIV-infected women had a similar risk of central lipohypertrophy. How these regional changes in fat may affect bone metabolism is unknown in this population at increasing risk for osteoporosis and possibly fracture. We undertook this study to examine how body composition changes including lean mass and regional body fat affect BMD in HIV-infected and HIV-uninfected women.
Methods
Study Population
The Women's Interagency HIV Study (WIHS) is an ongoing, multicenter observational study of HIV infection in women. A total of 3,766 women (2,791 HIV-infected and 975 HIV-uninfected) were enrolled in 1994–95 (n=2,623) or 2001–02 (n=1,143) from six sites (Bronx/Manhattan, Brooklyn, Chicago, Los Angeles, San Francisco and Washington DC). WIHS methods and baseline cohort characteristics have been described previously.4,5 At each semi-annual visit, participants completed a physical examination and provided biological specimens and information on demographics, disease characteristics, and antiretroviral therapy (ART) use. Starting from April 2001, 440 WIHS women enrolled in the Metabolic Substudy of the WIHS from three sites (San Francisco, Bronx and Chicago) underwent dual X-ray absorptiometry (DXA) scanning for BMD and fat distribution at baseline and at follow up visits 2 and 5 years later. Eligibility criteria included women age ≤65 years old, weight <264 pounds (119.7 kg), height less < 6'1" (1.85 m), who were not pregnant or breast feeding in the past six months. Exclusion criteria included type I diabetes, use of corticosteroids, use of exogenous hormones including growth hormone and hormonal contraceptives in the past 12 months, and drugs used to treat osteoporosis. Informed consent was obtained in accordance with procedures approved by the committees on human research at each of the collaborating institutions.
Body Composition and bone mineral density assessment
Regional fat mass in the trunk and leg and bone mineral density (BMD) of the lumbar spine (LS), total hip (TH), and femoral neck (FN) were measured by DXA scans (GE/Lunar Prodigy, Madison, WI, USA) at the index visit and subsequent 2-year follow-up visit, and 5 year follow-up visit. Established instrument calibration and quality control procedures were used for accurate comparisons of BMD data between subjects measured at different times. The visit when the first DXA scan was performed was referred to as the index visit. Body composition at index visit and subsequent visits included trunk fat and leg fat which were measured in kilograms by DXA. Fat free mass (FFM), total body fat (TBF), and percent body fat (PBF) were calculated based on height, weight, resistance and reactance which were measured by bioimpedance analysis (BIA) (RJL Systems, Inc, Detroit, MI, USA).6,7 There were no technology changes in DXA or BIA used during the study period.
Statistical Analysis
The primary outcome of interest was BMD measured at the LS, TH and FN at the index visit and 2 year and 5 year follow-up visits. Exposures of interest included: (1) HIV status at index visit. HIV infection was defined as a positive HIV EIA confirmed by Western Blot. (2) Body composition measurements at index visit and subsequent visits, which included trunk fat, leg fat, FFM, TBF, and PBF. Covariates included: (1) Demographics including age and ethnicity (African American, Hispanic, Caucasian, and other) (2) Hepatitis C virus (HCV) infection which was defined as positive HCV antibody and positive HCV RNA at baseline enrollment; (3) Serum free testosterone level (ng/dL) at index visit;(4) Menopause status at index visit and subsequent visit, which was defined by self-reported menopause at 2 consecutive visits for women aged >=45 years old; (5) Behavioral factors: Self-reported cigarette smoking status which was defined by three categories: never smoked, current smoking and ever smoked; The amount of smoking (pack/day) for current smokers at each visit; Self-reported alcohol use status which was defined by four categories: abstainer, light (<3 drinks/week), moderate (3–13 drinks/week) and heavy(>=14 drinks/week); The amount of current alcohol use(drinks/week) at each visit; self-reported ever opiate use (including methadone) before index visit, self-reported opiate use at each visit and the duration of opiate use from enrollment to the index visit; (6) Use of calcium, vitamin D supplement or multi-vitamin at index visit. In the analysis limited to HIV-infected women, the following HIV-related factors were also included in the model: self-reported time since HIV infection diagnosis, self-reported AIDS status at index and subsequent visit; plasma HIV-RNA viral load and CD4 cell count at index and subsequent visit, and nadir CD4 count which was defined as the lowest CD4 count available prior to index visit; antiretroviral therapy (ART) use and highly active antiretroviral therapy (HAART) use at index and subsequent visit, and cumulative exposure to HAART, type of HAART regimen, and tenofovir (in months) prior to index visit; the type of HAART was categorized as Protease inhibitor (PI)-based HAART, Non-nucleoside reverse transcriptase inhibitor (NNRTI)-based HAART and other (including HAART regimens which included neither PI or NNRTI or both PI and NNRTI.)
The basic characteristics of HIV-infected and HIV-uninfected women were compared at the index visit. Binary and categorical characteristics in HIV-infected and HIV-uninfected women were compared by chi-squared tests; continuous covariates were compared by 2-sample t test if they were normally distributed, or by Wilcoxon rank sum test if they were not normally distributed. Age-standardized BMD of LS, TH and FN were compared between HIV-infected and HIV-uninfected women and across three measured times. Marginal linear regression models that account for within-person correlation in repeated measurements were applied to assess the effect of body components and HIV status on BMD of LS, TH and FN over three DXA scans, by adding (adjusting for) each of the following potential confounders into the model individually: demographic covariates including age at index visit, race/ethnicity, study center and enrollment cohort; behavioral factors including cigarette use, alcohol use, opiate use and vitamin D, calcium or multivitamin use; and other factors such as menopause status at visit, serum testosterone at index visit, HCV infection at enrollment. The following potential confounders were evaluated in two separate models as time- fixed (at index visit) and time-updated covariates (lagged by 1 visit), respectively: smoking status, amount of smoking among current smokers, alcohol use, amount of alcohol use, opiate use, serum CD4 cell count and HIV viral load, ART use and HAART use. In the multivariate model, all continuous covariates were centered at the mean or median of HIV-uninfected women; CD4 cell count, CD4 nadir and HIV viral load (in log scale) were centered at the median in HIV- infected women. The interactions between years since index visit and HIV status, body components, age at index visit were assessed individually, and the significant interactions were included in the model. Subgroup analysis were performed among HIV- infected women and HAART users to assess the association between BMDs and HAART use, type of HAART regimen, tenofovir use, CD4 nadir, CD4 cell count, and plasma HIV RNA level. All analyses were performed using SAS Version 9.2 (SAS Institute, Cary, North Carolina, USA).
Results
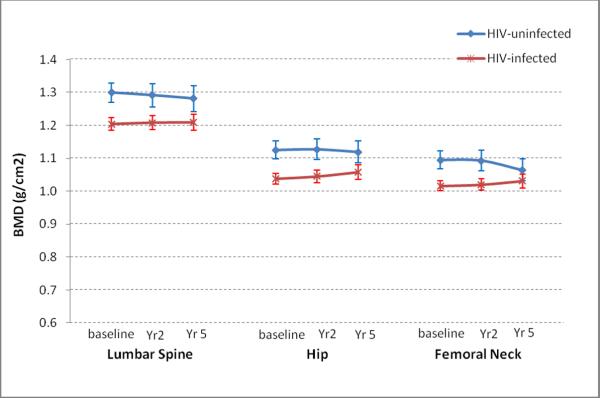
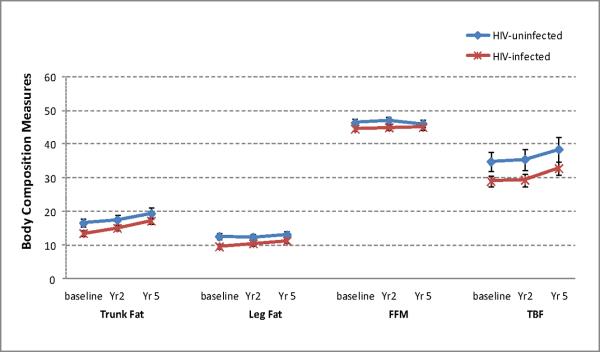
By September 30, 2009, 440 women (318 HIV+, 122 HIV−) had completed up to three DXA scans with 947 person-visits. Median time between two adjacent scans was 2.6 years (2.0 years between 1st and 2nd scans and 3.4 years between 2nd and 3rd scans, respectively). Participant characteristics are shown in Table 1. Compared with HIV-uninfected women, HIV-infected women were older (44 vs. 37 yrs), more likely to be HCV-infected (32% vs. 14%) and post-menopausal (26% vs.3%). HIV-infected women also had lower BMI, and lower trunk, leg and total body fat than HIV-uninfected women (Table 1). Rate of decline in BMD for HIV-infected and HIV-uninfected women is shown in Figure 1. After adjustment for demographic and clinical factors, HIV-infection was associated with decreased BMD at all 3 sites. There was little difference in absolute changes over time for trunk fat, leg fat, FFM, TBF, and PBF between HIV-uninfected and HIV-infected women (Figure 2). Body composition measures were stable or increasing over time both HIV-infected and HIV-uninfected women (Figure 2).
Table 1.
Characteristics of HIV uninfected women and HIV infected women at index visit**
| HIV-uninfected (N=122) | HIV-infected (N=318) | |
|---|---|---|
| Age (yr), mean ± SD * | 37 ± 8.6 | 44 ± 8.1 |
|
| ||
| Race, n(%) | ||
| African American | 70 (57) | 186 (58) |
| Hispanic | 36 (30) | 81 (26) |
| Caucasian & other | 16 (13) | 51 (16) |
|
| ||
| Study center, n(%) | ||
| Bronx/Manhattan | 49 (40) | 128 (40) |
| San Francisco | 44 (36) | 119 (37) |
| Chicago | 29 (24) | 71 (22) |
|
| ||
| Enrollment cohort, n (%) * | ||
| Original (1994–95) | 45 (37) | 211 (66) |
| 2nd enrollment (2001–02) | 77 (63) | 107 (34) |
|
| ||
| Smoking status, n(%) | ||
| Never smoker | 26 (21) | 57 (18) |
| Current smoker | 80 (66) | 189 (60) |
| Ever smoker | 16 (13) | 68 (22) |
|
| ||
| Type of alcohol use, n (%) * | ||
| Abstainer | 43 (35) | 167 (53) |
| Light (<3 drinks/wk) | 42 (34) | 106 (34) |
| Moderate (3–13) | 31 (25) | 33 (10) |
| Heavy (≥14 drink/wk) | 6 (5) | 10 (3) |
|
| ||
| Opiate use at index visit, n (%) | ||
| No | 101 (83) | 264 (84) |
| Yes | 21 (17) | 52 (16) |
|
| ||
| Opiate use from enrollment to index visit, n (%) | ||
| No | 91 (75) | 223 (70) |
| Yes | 31 (25) | 95 (30) |
|
| ||
| Calcium/Vitamin D/Multivitamin use, n (%) | 4 (3) | 26 (8) |
|
| ||
| Post-menopausal status, n (%) * | 4 (3) | 81 (26) |
|
| ||
| Free Testosterone (ng/dL), median (IQR) * | 41.2 (26.6–55.4) | 23.3 (19.0–34.1) |
|
| ||
| BMI (kg/m2), median (IQR) * | 30.2 (25.1–36.0) | 27.0 (23.4–30.9) |
| Waist circumference (cm), median (IQR) | 92.2 (81.6–103.3) | 88.9 (80.1–98.4) |
|
| ||
| Body components, median (IQR) | ||
| Trunk fat (kg)* | 15.6 (10.7–22.0) | 12.7 (9.1–16.8) |
| Leg fat (kg)* | 11.9 (8.4–16.0) | 8.7 (5.6–12.6) |
| Fat free mass (kg) | 45.9 (42.3–49.0) | 44.0 (40.7–47.8) |
| Total body fat (kg)* | 32.1 (21.7–46.1) | 26.4 (18.6–35.2) |
| Percent body fat (%)* | 41.8 (34.9–48.6) | 37.4 (30.8–43.6) |
|
| ||
| Hepatitis C virus seropositive, n (%) * | 17 (14) | 102 (32) |
|
| ||
| AIDS diagnosis, n (%) | 146 (46) | |
|
| ||
| CD4 count (cells/ml), median (IQR) * | 1060 (796–1236) | 399 (262–598) |
| CD4 nadir (cells/ml), median (IQR) * | 800 (662–1036) | 237 (123–346) |
| HIV RNA viral load, median (IQR) | 575 (80–6900) | |
| HIV RNA viral load(log10scale), median (IQR) | 6.4 (4.4–8.8) | |
|
| ||
| On ART, n (%) | 265 (83) | |
|
| ||
| On HAART, n (%) | 179 (56) | |
|
| ||
| On tenofovir, n (%) | 71 (22) | |
|
| ||
| Cumulative HAART exposure (months), median (IQR) | 48 (30–72) | |
|
| ||
| Type of HAART, n (%) | ||
| PI only | 85 (47) | |
| NNRTI only | 66 (37) | |
| dual/other | 28 (16) | |
P<0.05 for Chi-squared test, two sample t-test (for age) or Wilcoxon rank-sum test (for other continuous variables that are not normally distributed)
Index visit refers to the first visit when BMD/DXAs were measured.
Abbreviations: HIV: human immunodeficiency virus; BMI: body mass index; ART: antiretroviral therapy; HAART: highly active antiretroviral therapy; PI: protease inhibitor; NNRTI: non-nucleoside analog reverse transcriptase inhibitor
Figure 1.
Age-standardizedBMD in the Lumbar Spine, Hip, and Femoral Neck over 5 years in HIV-infected and uninfected Women
Abbreviations: BMD: bone mineral density; HIV: human immunodeficiency virus
Figure 2.
Age-standardized Body Composition Measures over 5 years in HIV-infected and uninfected Women
Abbreviations: BMD: bone mineral density; HIV: human immunodeficiency virus; FFM: fat free mass; TBF: total body fat
Body composition and BMD in HIV-infected and uninfected women
We found that greater total FFM (or lean mass) was independently associated with increased LS, TH, and FN BMD; greater TBF was independently associated with increased TH and FN BMD (Table 2). Their inclusion in the model did not significantly alter the relationship of HIV infection with decreased BMD (based on the intermediate model results). In addition to HIV infection and body composition factors, being post-menopausal and HCV-infected was associated with decreased BMD at all 3 sites. Older age was associated with a significantly decreased TH BMD; there was a significant HIV by age interaction for FN BMD. Hispanic race was significantly associated with decreased LS BMD. Greater testosterone level was associated with greater BMD at all 3 sites and heavy alcohol use was significantly associated with greater FN BMD.
Table 2.
Effect of Fat Free Mass and Total Body Fat on BMD in HIV-infected and uninfected women
| Lumbar spine BMD | Total hip BMD | Femoral neck BMD | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| ß(SE) | P value | ß(SE) | P value | ß(SE) | P value | |
| Intercept | 1.3180 (0.0254) | <.001 | 1.1324 (0.0214) | <.001 | 1.1114 (0.0219) | <.001 |
|
| ||||||
| HIV(+) at index visit | −0.0643 (0.0202) | <.01 | −0.0471 (0.0171) | 0.01 | −0.0376 (0.0170) | 0.03 |
| Years since index visit | 0.0052 (0.0031) | 0.09 | −0.0002 (0.0025) | 0.93 | 0.0107 (0.0052) | 0.04 |
| HIV* Years index visit (interaction) | −0.0038 (0.0018) | 0.04 | −0.0043 (0.0013) | <.01 | −0.0179 (0.0063) | 0.01 |
|
| ||||||
| Body Components | ||||||
| Fat free mass (FFM) | 0.0023 (0.0007) | <.01 | 0.0030 (0.0006) | <.001 | 0.0040 (0.0007) | <.001 |
| Total body fat (TBF) | −0.0003 (0.0003) | 0.32 | 0.0010 (0.0003) | <.001 | 0.0010 (0.0003) | <.001 |
| TBF*Year since index visit (interaction) | 0.0002 (0.0001) | <.01 | 0.0001 (0.0000) | <.01 | . | |
|
| ||||||
| Age at index visit | −0.0008 (0.0004) | 0.08 | −0.0010 (0.0004) | 0.01 | −0.0001 (0.0005) | 0.79 |
| HIV* Age at index visit (interaction) | . | . | −0.0012 (0.0005) | 0.02 | ||
|
| ||||||
| Race/Ethnicity | ||||||
| African American (Ref) | ||||||
| Hispanic | −0.0536 (0.0202) | 0.01 | −0.0043 (0.0171) | 0.80 | −0.0252 (0.0169) | 0.14 |
| Caucasian & Other | −0.0294 (0.0259) | 0.26 | −0.0061 (0.0210) | 0.77 | −0.0290 (0.0207) | 0.16 |
|
| ||||||
| Study Center | ||||||
| Bronx (Ref) | ||||||
| Chicago | 0.0589 (0.0231) | 0.01 | 0.0093 (0.0194) | 0.63 | 0.0107 (0.0192) | 0.58 |
| San Francisco | −0.0049 (0.0213) | 0.82 | −0.0015 (0.0178) | 0.93 | 0.0094 (0.0176) | 0.59 |
|
| ||||||
| Original cohort | 0.0340 (0.0184) | 0.07 | −0.0002 (0.0156) | 0.99 | −0.0106 (0.0154) | 0.49 |
|
| ||||||
| Calcium or vitamin D use at index visit | 0.0396 (0.0365) | 0.28 | 0.0073 (0.0291) | 0.80 | −0.0063 (0.0286) | 0.83 |
|
| ||||||
| Opiate use at last visit | −0.0139 (0.0094) | 0.14 | 0.0006 (0.0074) | 0.93 | 0.0033 (0.0089) | 0.71 |
|
| ||||||
| Smoking status at last visit | ||||||
| Never smoked (Ref) | ||||||
| Current smoker | −0.0278 (0.0139) | 0.05 | −0.0146 (0.0117) | 0.21 | −0.0162 (0.0134) | 0.23 |
| Ever smoked | −0.0229 (0.0142) | 0.11 | −0.0036 (0.0118) | 0.76 | −0.0061 (0.0136) | 0.65 |
|
| ||||||
| Alcohol use at last visit | ||||||
| Abstainer (Ref) | ||||||
| Light | 0.0013 (0.0055) | 0.81 | 0.0063 (0.0044) | 0.16 | 0.0098 (0.0055) | 0.08 |
| Moderate | −0.0031 (0.0075) | 0.69 | 0.0052 (0.0060) | 0.39 | 0.0051 (0.0075) | 0.50 |
| Heavy | −0.0034 (0.0108) | 0.76 | 0.0136 (0.0088) | 0.12 | 0.0226 (0.0110) | 0.04 |
|
| ||||||
| HCV status at enrollment | −0.0664 (0.0200) | <.01 | −0.0455 (0.0167) | 0.01 | −0.0416 (0.0165) | 0.01 |
|
| ||||||
| Testosterone at index visit | 0.0011 (0.0004) | <.01 | 0.0010 (0.0003) | <.01 | 0.0012 (0.0003) | <.001 |
|
| ||||||
| Menopause at visit | −0.0546 (0.0088) | <.001 | −0.0410 (0.0069) | <.001 | −0.0358 (0.0083) | <.001 |
Baseline age was centered at mean age for HIV-uninfected women, all other continues covariates were centered at median for HIV-uninfected women (as shown in Table 1).
Abbreviations: BMD: bone mineral density; HIV: human immunodeficiency virus; HCV: hepatitis C virus
We next examined the association of regional fat with BMD by replacing total fat with trunk fat and leg fat (the sites most commonly affected by HIV infection) in the model (Table 3). We found that greater trunk fat was associated with increased TH and FN BMD. We did not find an association of leg fat with BMD at any site. There was little change in the inferences for all other demographic and clinic factors when total fat was replaced with trunk and leg fat. In analyses limited to HIV-infected women only, all inferences remained similar to those in the pooled cohort with the exception that older age was significantly associated with decreased BMD at all 3 sites (data not shown). Among the HIV-related factors, we found that greater log HIV RNA was associated with greater BMD at all 3 sites, (LS β=0.0028, p= 0.03; TH β 0.0033, p=0.02; FN β= 0.0032 p=0.009.) We also found a significant association between cumulative NNRTI use and increased BMD at the TH (β=0.0015, p= 0.004) and FN (β=0.0010, p= 0.035), but not the LS (β 0.0011, p=0.093). We did not find an association between recent or nadir CD4 count, or cumulative use of tenofovir, PI- based HAART, other HAART (including HAART regimens which included neither PI or NNRTI or both PI and NNRTI), or any HAART with BMD at any site.
Table 3.
Effect of Regional Fat Mass on BMD in HIV-infected and Uninfected Women
| Lumbar spine BMD | Total hip BMD | Femoral neck BMD | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| ß(SE) | P value | ß(SE) | P value | ß(SE) | P value | |
| Intercept | 1.3187 (0.0255) | <.001 | 1.1359 (0.0216) | <.001 | 1.1148 (0.0221) | <.001 |
|
| ||||||
| HIV(+) at index visit | −0.0648 (0.0202) | <.01 | −0.0496 (0.0173) | <.01 | −0.0394 (0.0171) | 0.02 |
| Years since index visit | 0.0050 (0.0031) | 0.11 | −0.0010 (0.0025) | 0.69 | 0.0107 (0.0052) | 0.04 |
| HIV* Years index visit (interaction) | −0.0039 (0.0018) | 0.03 | −0.0044 (0.0013) | <.01 | −0.0183 (0.0063) | <.01 |
|
| ||||||
| Body Components | ||||||
| Fat free mass | 0.0023 (0.0008) | <.01 | 0.0025 (0.0006) | <.001 | 0.0037 (0.0008) | <.001 |
| Leg fat | −0.0008 (0.0014) | 0.55 | −0.0012 (0.0011) | 0.29 | −0.0009 (0.0013) | 0.52 |
| Trunk fat | −0.0001 (0.0010) | 0.90 | 0.0022 (0.0008) | 0.01 | 0.0022 (0.0009) | 0.02 |
| Trunk fat*Years since index visit (interaction) | 0.0003 (0.0001) | 0.01 | 0.0003 (0.0001) | <.001 | . | |
|
| ||||||
| Age at index visit | −0.0008 (0.0004) | 0.08 | −0.0010 (0.0004) | 0.01 | −0.0002 (0.0005) | 0.77 |
| Age * HIV (interaction) | . | . | −0.0012 (0.0005) | 0.01 | ||
|
| ||||||
| Race/Ethnicity | ||||||
| African American (Ref) | ||||||
| Hispanic | −0.0554 (0.0203) | 0.01 | −0.0106 (0.0174) | 0.54 | −0.0297 (0.0171) | 0.08 |
| Caucasian & Other | −0.0303 (0.0259) | 0.24 | −0.0103 (0.0212) | 0.63 | −0.0322 (0.0208) | 0.12 |
|
| ||||||
| Study Center | ||||||
| Bronx (Ref) | ||||||
| Chicago | 0.0576 (0.0231) | 0.01 | 0.0092 (0.0196) | 0.64 | 0.0103 (0.0193) | 0.59 |
| San Francisco | −0.0060 (0.0213) | 0.78 | −0.0016 (0.0179) | 0.93 | 0.0100 (0.0176) | 0.57 |
|
| ||||||
| Original cohort | 0.0334 (0.0184) | 0.07 | −0.0018 (0.0158) | 0.91 | −0.0118 (0.0154) | 0.44 |
|
| ||||||
| Calcium or vitamin D use at index visit | 0.0394 (0.0364) | 0.28 | 0.0104 (0.0293) | 0.72 | −0.0040 (0.0287) | 0.89 |
|
| ||||||
| Opiate use at last visit | −0.0143 (0.0095) | 0.13 | −0.0008 (0.0075) | 0.91 | 0.0031 (0.0089) | 0.73 |
|
| ||||||
| Smoking status at last visit | ||||||
| Never smoked (Ref) | ||||||
| Current smoking | −0.0270 (0.0139) | 0.05 | −0.0138 (0.0118) | 0.24 | −0.0162 (0.0135) | 0.23 |
| Ever smoked | −0.0216 (0.0142) | 0.13 | −0.0017 (0.0119) | 0.88 | −0.0050 (0.0136) | 0.71 |
|
| ||||||
| Alcohol use at last visit | ||||||
| Abstainer (Ref) | ||||||
| Light | 0.0014 (0.0056) | 0.81 | 0.0058 (0.0045) | 0.20 | 0.0082 (0.0056) | 0.14 |
| Moderate | −0.0032 (0.0077) | 0.68 | 0.0044 (0.0061) | 0.48 | 0.0036 (0.0076) | 0.64 |
| Heavy | −0.0037 (0.0109) | 0.74 | 0.0126 (0.0089) | 0.16 | 0.0208 (0.0111) | 0.06 |
|
| ||||||
| HCV status at enrollment | −0.0656 (0.0200) | <.01 | −0.0462 (0.0168) | 0.01 | −0.0419 (0.0166) | 0.01 |
|
| ||||||
| Testosterone at index visit | 0.0011 (0.0004) | <.01 | 0.0011 (0.0003) | <.01 | 0.0012 (0.0003) | <.001 |
|
| ||||||
| Menopause at visit | −0.0557 (0.0089) | <.001 | −0.0419 (0.0070) | <.001 | −0.0366 (0.0083) | <.001 |
Baseline age was centered at mean age for HIV-uninfected women, all other continues covariates were centered at median for HIV-uninfected women (as shown in Table 1).
Abbreviations: BMD: bone mineral density; HIV: human immunodeficiency virus; HCV: hepatitis C virus
Discussion
Using a large longitudinal cohort of BMD in HIV-infected and uninfected women, we found that HIV-infected women had decreased BMD over the 5-year period compared to HIV-uninfected women. As expected, greater total fat and greater lean mass were associated with increased BMD. It is noteworthy that regardless of HIV status, greater trunk fat was associated with greater FN and TH BMD and there was little association of leg fat with BMD. These findings suggest that weight-bearing trunk fat is an important predictor of increased BMD in our cohort of predominantly African American women. While HIV infection affects subcutaneous fat and particularly loss of fat in the leg, these alterations appear to have little impact on BMD.
In the general population, several studies have found that bone mass is positively associated with body weight. One possible reason for this includes increased mechanical loading on the skeleton with higher body weight. Fat mass is also thought to secrete bone-active hormones from the pancreatic β-cell, and secretion of bone-active factors from adipocytes (adipokines) such as leptin and adiponectin.8 There is debate as to whether lean body mass or fat mass most determines BMD. Some studies have found that fat mass is either not associated with, or is negatively associated with bone mass.9–13 We found not only an association of total fat with increased BMD, but also that increased trunk fat (and not leg fat) was associated with increased BMD. These findings are consistent with the hypothesis that increased weight bearing (from excess fat in the trunk) leads to an increase weight load in the lower limbs and thus increased BMD in the hip and femoral neck.
Because fat tissue is metabolically active, its effect on the skeleton may be influenced not only by weight-bearing effects but also by other non-weight bearing effects, including the hormonal metabolism of adipocytes. Adipokine levels may differ according to fat depot and mediate the relationship between regional distribution and BMD. Most studies have assessed only total fat mass, but the pattern of fat distribution in the subcutaneous and visceral compartments may also be an important predictor of disease risk. Gilsanz et al reported that visceral and subcutaneous fat had opposite effects on femoral bone structure and strength among healthy young women, and suggested that while subcutaneous fat may be beneficial to bone, visceral fat serves as a unique pathogenic fat depot.14 Adiponectin, which is thought to be protective against bone loss, may have a lower level of expression in visceral than subcutaneous fat,15 and leptin, levels of which generally relate to the amount of total adiposity, may also have lower levels in visceral than subcutaneous tissue.16,17 While our women had a median waist circumference greater than 88 cm, which has been used as a criteria for visceral obesity, it is unclear whether subcutaneous adipose tissue in the abdomen or visceral adipose tissue was a greater contributor to this measure. African American women have been shown to have greater amounts of subcutaneous fat and lower amounts of visceral fat than Caucasian women.18 This could explain why we found that greater trunk fat was associated with greater BMD in our population; adiponectin levels may not have been significantly affected if the increase was predominantly in abdominal subcutaneous fat.
The lack of an association between leg fat and BMD was somewhat surprising particularly in HIV. Reduced leg fat is thought to be associated with decreased BMD, and perhaps related to bone marrow fat levels. Both osteoblasts and adipocytes are derived from mesenchymal cells and thus share a common embryonic progenitor.19 Increased intravertebral bone marrow fat measured by magnetic resonance spectroscopy has been associated with osteopenia and osteoporosis in elderly women, as well as bone weakening, and increased risk of vertebral compression fracture in older women with osteopenia.20−22 It has been suggested that bone loss with aging results from preferential differentiation into the adipocyte lineage, such that the increased number of adipocytes occurs at the expense of the production of osteoblasts, resulting in osteoporosis.23–25 Few studies have investigated marrow fat in HIV-infected persons, and results have varied.26,27 The subjects in our study were predominantly overweight and obese racial and ethnic minority women, which might explain why we did not find an association between leg fat and BMD. HIV-infected women in our analyses had a median BMI of 27 kg/m2 and median leg fat of 8.7 kg, which contrasts sharply from HIV-infected lipoatrophic men described in the studies of marrow adiposity and BMD. The association of regional body fat and its potential mediators with BMD is complex and needs study, including whether the relationship between adiposity and BMD is similar in overweight or obese adults compared with those who are lean or normal weight.
Unexpectedly, we found an association of greater HIV RNA with increased BMD among HIV-infected women, and additionally we found that NNRTI use was associated with an increase in BMD over time. Prior studies in HIV-infected adults have shown a 2–6% decline in BMD upon initiation of HAART in antiretroviral naïve patients in the first year of therapy.28–32 However BMD generally appears stable in chronically HIV-infected individuals maintained on established HAART.2, 33–36 Based upon this existing literature, we would have expected to find either an inverse association, or perhaps a lack of association between HIV RNA and BMD, rather than the positive association which we observed at each of the three sites, LS, TH and FN. In the SMART study, an unfavorable effect of continuous ART on BMD was reported- such that BMD steadily declined in the group receiving continuous ART, while BMD remained stable or increased in the first year of intermittent, CD4 cell count-guided ART.37 While the significance of this relationship between HIV RNA and BMD we described remains unclear, it may indirectly reflect a negative effect of ART on BMD similar to that found in the SMART study, although we did not actually measure a detrimental effect of HAART on BMD, rather we found an association between NNRTI use and increase in TH and FN BMD.
We found that consumption of 14 or more drinks per week was associated with increased BMD at the FN. We did not find any significant associations between heavy alcohol consumption and BMD at TH or LS, nor with lesser amount of alcohol and BMD at any site. In general, the effects of alcohol on bone health depend on the dose and duration of alcohol consumed.38 Consumption of one glass of alcohol per day or less for women and two for men (often considered light consumption) has been associated with no effect or beneficial effects on BMD in a number of studies, while consumption of more than four glasses of per day of alcohol is detrimental to bone.39–42 Data on the effects on bone of consumption of two or three glasses per day of alcohol are inconsistent, and are dependent on the age, sex, and menopausal status (for women) of the subject. In a study by Tucker, intake of >2 drinks per day was associated with increased BMD in postmenopausal women; however in men high liquor intake (>2 drinks/d) was associated with significantly lower BMD, and no effect was seen for premenopausal women.43 The EPIDOS study in France reported an increase in trochanteric BMD in elderly women who drank 11–29 g/d of alcohol, or 1–3 glasses of wine per day.44 Definitions of light, moderate and heavy alcohol consumption vary in the literature, and the definition of a standard drink, generally between 8 and 12g of ethanol, differs between countries. Few women in our study (<4%) consumed 14 or more drinks per week, which by many standards would be considered moderate alcohol consumption at an average or two drinks per day, therefore we could not examine whether alcohol effects varied according to menopause or HIV status. Possible explanations of a beneficial effect of light or moderate alcohol consumption on BMD in women include an increase in calcitonin, which inhibits bone resorption,45 an increase in estrogen level,46,47 and lower bone remodeling with decreased levels of serum osteocalcin and N and C terminal telopeptides of type I collagen.48,49
Our study has limitations. We did not measure amount of regional subcutaneous or visceral fat. BIA was used to assess total fat and lean mass, whereas DXA was used to assess regional body fat and lean mass. Given the increased BMI of our population, in some cases, the arm fell out of the field of view of the DXA scan. Thus, we were not able to accurately assess the amount of total fat and lean mass measured by DXA, nor were we able to directly correlate BIA measures of total fat and total lean mass to DXA measures in our cohort. Nevertheless, good agreement has been reported for body composition measurements between BIA and DXA including fat free mass, fat mass and percentage body fat among overweight and obese women.50,51 We were not able to assess the biological mechanisms underlying the relationship between body composition and BMD, such as measurement of adipokines, reproductive hormones, or biological markers of bone turnover, although these analyses are planned. Additionally, the majority of women in WIHS are African American and overweight, therefore our results may not be generalizable to all women. There are also several strengths to our study. The WIHS cohort has a well-matched comparison group of HIV-uninfected women with similar risk factors for bone disease, and is representative of the HIV epidemic among US women. Data on traditional risk factors for osteoporosis have been collected at regular intervals in addition to repeated measures of bone mineral density and body composition, and participant retention is excellent.
In conclusion, in this cohort of HIV-infected and uninfected women, total fat and lean mass are independently associated with increased BMD, regardless of HIV status. Greater trunk fat but not leg fat was associated with increased BMD, suggesting different mechanisms by which regional body fat may affect BMD, and future studies should include measurement of regional subcutaneous and visceral adipose tissue. Clarification of the mechanisms underlying these associations may have important implications for bone density screening and preventive interventions for reduced BMD in HIV-infected women, particularly those with alterations in body composition.
Acknowledgments
Conflicts of Interest and Source of Funding: Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the National Institute of Child Health and Human Development (UO1-HD-32632). The study is co- funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Dr. Yin is supported by NIH (AI-095089). Dr. Sharma is supported by the Robert Wood Johnson Foundation Physician Faculty Scholars Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anastos K, Lu D, Shi O, et al. The association of bone mineral density with HIV infection and antiretroviral treatment in women. Antivir Ther. 2007;12(7):1049–1058. doi: 10.1177/135965350701200701. [DOI] [PubMed] [Google Scholar]
- 2.Yin MT, Lu D, Cremers S, Tien PC, Cohen MH, Shi Q, Shane E, Golub ET, Anastos K. Short-term bone loss in HIV-infected premenopausal women. J Acquir Immune Defic Syndr. 2010 Feb 1;53(2):202–8. doi: 10.1097/QAI.0b013e3181bf6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tien PC, Cole SR, Williams CM, Li R, Justman JJ, Cohen MH, Young M, Rubin N, Augenbraun M, Grunfeld C. Incidence of lipoatrophy and lipohypertrophy in the Women's Interagency HIV Study. J Acquir Immune Defic Syndr. 2003 Dec;34(5):461–466. doi: 10.1097/00126334-200312150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Bacon M, et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Am Soc Microbiol. 2005:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barkan S, et al. The Women's Interagency HIV Study. Epidemiology. 1998;9(2):117. [PubMed] [Google Scholar]
- 6.Kotler D, et al. Prediction of body cell mass, fat-free mass, and total body water with bioelectrical impedance analysis: effects of race, sex, and disease. American Journal of Clinical Nutrition. 1996;64(3):489S. doi: 10.1093/ajcn/64.3.489S. [DOI] [PubMed] [Google Scholar]
- 7.Tien P, et al. Antiretroviral therapies associated with lipoatrophy in HIV-infected women. AIDS Patient Care and STDs. 2007;21(5):297–305. doi: 10.1089/apc.2006.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reid IR. Relationships among body mass, its components, and bone. Bone. 2006;31:547–555. doi: 10.1016/s8756-3282(02)00864-5. [DOI] [PubMed] [Google Scholar]
- 9.Janicka A, Wren TA, Sanchez MM, Dorey F, Kim PS, Mittelman SD, Gilsanz V. Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab. 2007;92:143–147. doi: 10.1210/jc.2006-0794. [DOI] [PubMed] [Google Scholar]
- 10.Lazcano-Ponce E, Tamayo J, Cruz-Valdez A, Díaz R, Hernández B, Del Cueto R, Hernández-Avila M. Peak bone mineral area density and determinants among females aged 9 to 24 years in Mexico. Osteoporos Int. 2003;14:539–547. doi: 10.1007/s00198-002-1363-2. [DOI] [PubMed] [Google Scholar]
- 11.Lee K, Lee S, Kim YJ, Kim YJ. Waist circumference, dual-energy X-ray absortiometrically measured abdominal adiposity, and computed tomographically derived intra-abdominal fat area on detecting metabolic risk factors in obese women. Nutrition. 2008;24:625–631. doi: 10.1016/j.nut.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Travison TG, Araujo AB, Esche GR, Beck TJ, McKinlay JB. Lean mass and not fat mass is associated with male proximal femur strength. J Bone Miner Res. 2008;23:189–198. doi: 10.1359/JBMR.071016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiler HA, Janzen L, Green K, Grabowski J, Seshia MM, Yuen KC. Percent body fat and bone mass in healthy Canadian females 10 to 19 years of age. Bone. 2000;27:203–207. doi: 10.1016/s8756-3282(00)00314-8. [DOI] [PubMed] [Google Scholar]
- 14.Gilsanz V, Chaifant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab. 2009;94(9):3387–3393. doi: 10.1210/jc.2008-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006:235–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 16.Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Kim CA, et al. Serum leptin is a regulator of bone mass. Proc Natl Acad Sci USA. 2004;101:3258–3263. doi: 10.1073/pnas.0308744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamauchi M, Sugimoto T, Yamaguchi T, Nakaoka D, Kanzawa M, Yano S, Ozuru R, Sugishita T, Chihara K. Plasma leptin concentrations are associated with bone mineral density and the presence of vertebral fractures in postmenopausal women. Clin Endocrinol (Oxf) 2001;55:341–347. doi: 10.1046/j.1365-2265.2001.01361.x. [DOI] [PubMed] [Google Scholar]
- 18.Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL, Ravussin E, Ryan DH, Smith SR, Bouchard C. Racial Differences in abdominal depot-specific adiposity in white and African-American Adults. American Journal of Clinical Nutrition. 2010;91:7–15. doi: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gimble JM, et al. The function of adipocytes in the bone marrow stroma: an update. Bone. 1996;19:421–428. doi: 10.1016/s8756-3282(96)00258-x. [DOI] [PubMed] [Google Scholar]
- 20.Yeung DK, et al. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging. 2005;22:279–285. doi: 10.1002/jmri.20367. [DOI] [PubMed] [Google Scholar]
- 21.Schellinger D, et al. Potential value of proton MR spectroscopy in determining bone weakness. Am J Neuroradiology. 2001;22:1620–1627. [PMC free article] [PubMed] [Google Scholar]
- 22.Wehrli FW, et al. Cross-sectional study of osteopenia with quantitative MR imaging and bone densitometry. Radiology. 2000;217:527–538. doi: 10.1148/radiology.217.2.r00nv20527. [DOI] [PubMed] [Google Scholar]
- 23.Quarto R, Thomas D, Liang CT. Bone progenitor cell deficits and the age-associated decline in bone repair capacity. Calcif Tissue Int. 1995;56:123–129. doi: 10.1007/BF00296343. [DOI] [PubMed] [Google Scholar]
- 24.Mullender MG, van der Meer DD, Huiskes R, Lips P. Osteocyte density changes in aging and osteoporosis. Bone. 1996;18:109–113. doi: 10.1016/8756-3282(95)00444-0. [DOI] [PubMed] [Google Scholar]
- 25.Chan GK, Duque G. Age-related bone loss: old bone, new facts. Gerontology. 2002;48:62–71. doi: 10.1159/000048929. [DOI] [PubMed] [Google Scholar]
- 26.Huang JS, Mulkern RV, Grinspoon S. Reduced intravertebral bone marrow fat in HIV-infected men. AIDS. 2002;16:1265–1269. doi: 10.1097/00002030-200206140-00009. [DOI] [PubMed] [Google Scholar]
- 27.Wiercinska-Drapalo A, Jaroszewicz J, Tarasow E, Siergiejczyk L, Prokopowicz D. The possible association between serum cholesterol concentration and decreased bone mineral density as well as intravertebral marrow fat in HIV-1 infected patients. Infection. 2007;35:46–48. doi: 10.1007/s15010-007-5033-3. [DOI] [PubMed] [Google Scholar]
- 28.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs. stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. Journal of the American Medical Association. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 29.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. Journal of Acquired Immune Deficiency Syndrome. 2009;51:554–561. doi: 10.1097/QAI.0b013e3181adce44. [DOI] [PubMed] [Google Scholar]
- 30.Duvivier C, Kolta S, Assoumou L, Ghosn J, Rozenberg S, Murphy LR, et al. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS. 2009;23:817–824. doi: 10.1097/QAD.0b013e328328f789. [DOI] [PubMed] [Google Scholar]
- 31.van Vonderen MG, Lips P, van Agtmael MA, Hassink EA, Brinkman K, Geerlings SE, et al. First line zidovudine/lamivudine/lopinavir/ritonavir leads to greater bone loss compared to nevirapine/lopinavir/ritonavir. AIDS. 2009;23:1367–1376. doi: 10.1097/QAD.0b013e32832c4947. [DOI] [PubMed] [Google Scholar]
- 32.Mallon PWG. HIV and bone mineral density. Current Opinion in Infectious Diseases. 2010;23:1–8. doi: 10.1097/QCO.0b013e328334fe9a. [DOI] [PubMed] [Google Scholar]
- 33.Bolland MJ, Grey AB, Horne AM, et al. Bone mineral density is not reduced in HIV-infected caucasian men treated with highly active antiretroviral therapy. Clin Endocrinol (Oxf) 2006;65(2):191–197. doi: 10.1111/j.1365-2265.2006.02572.x. [DOI] [PubMed] [Google Scholar]
- 34.Dolan SE, Kanter JR, Grinspoon S. Longitudinal analysis of bone density in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 2006;91(8):2938–2945. doi: 10.1210/jc.2006-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mondy K, Yarasheski K, Powderly WG, et al. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin Infect Dis. 2003;36(4):482–490. doi: 10.1086/367569. [DOI] [PubMed] [Google Scholar]
- 36.Sharma A, Cohen HW, Freeman R, Santoro N, Schoenbaum EE. Prospective Evaluation of Bone Mineral Density among Middle-Aged HIV-Infected and Uninfected Women: Association between Methadone Use and Bone Loss. Maturitas. 2011 Nov;70(3):295–301. doi: 10.1016/j.maturitas.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grund B, Peng G, Gibert CL, Hoy JF, Isaksson RL, Shlay JC, et al. Continuous antiretroviral therapy decreases bone mineral density. AIDS. 2009;23:1519–1529. doi: 10.1097/QAD.0b013e32832c1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maurel DB, Boisseau N, Benhamou CL, Jaffre C. Alcohol and bone: review of dose effects and mechanisms. Osteoporosis Int. 2012;23:1–16. doi: 10.1007/s00198-011-1787-7. [DOI] [PubMed] [Google Scholar]
- 39.Ilich JZ, Brownbill RA, Tamborini L, Crncevic-Orlic Z. To drink or not to drink: how are alcohol, caffeine and past smoking related to bone mineral density in elderly women? J Am Coll Nutr. 2002 Dec;21(6):536–44. doi: 10.1080/07315724.2002.10719252. [DOI] [PubMed] [Google Scholar]
- 40.Pedrera-Zamorano JD, Lavado-Garcia JM, Roncero-Martin R, Calderon-Garcia JF, Rodriguez-Dominguez T, Canal-Macias ML. Effect of beer drinking on ultrasound bone mass in women. Nutrition. 2009 Oct;25(10):1057–63. doi: 10.1016/j.nut.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Williams FM, Cherkas LF, Spector TD, MacGregor AJ. The effect of moderate alcohol consumption on bone mineral density: a study of female twins. Ann Rheum Dis. 2005 Feb;64(2):309–10. doi: 10.1136/ard.2004.022269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venkat KK, Arora MM, Singh P, Desai M, Khatkhatay I. Effect of alcohol consumption on bone mineral density and hormonal parameters in physically active male soldiers. Bone. 2009 Sep;45(3):449–54. doi: 10.1016/j.bone.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Tucker KL, Jugdaohsingh R, Powell JJ, Qiao N, Hannan MT, Sripanyakorn S, Cupples LA, Kiel DP. Effects of beer, wine, and liquor intakes on bone mineral density in older men and women. Am J Clin Nutr. 2009 Apr;89(4):1188–96. doi: 10.3945/ajcn.2008.26765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ganry O, Baudoin C, Fardellone P. Effect of alcohol intake on bone mineral density in elderly women: The EPIDOS Study. Epidémiologie de l'Ostéoporose. Am J Epidemiol. 2000 Apr 15;151(8):773–80. doi: 10.1093/oxfordjournals.aje.a010277. [DOI] [PubMed] [Google Scholar]
- 45.Vantyghem MC, Danel T, Marcelli-Tourvieille S, Moriau J, Leclerc L, Cardot-Bauters C, Docao C, Carnaille B, Wemeau JL, D'Herbomez M. Calcitonin levels do not decrease with weaning in chronic alcoholism. Thyroid. 2007 Mar;17(3):213–7. doi: 10.1089/thy.2006.0216. [DOI] [PubMed] [Google Scholar]
- 46.Jugdaohsingh R, O'Connell MA, Sripanyakorn S, Powell JJ. Moderate alcohol consumption and increased bone mineral density: potential ethanol and non-ethanol mechanisms. Proc Nutr Soc. 2006 Aug;65(3):291–310. doi: 10.1079/pns2006508. [DOI] [PubMed] [Google Scholar]
- 47.Chen JR, Lazarenko OP, Haley RL, Blackburn ML, Badger TM, Ronis MJ. Ethanol impairs estrogen receptor signaling resulting in accelerated activation of senescence pathways, whereas estradiol attenuates the effects of ethanol in osteoblasts. J Bone Miner Res. 2009 Feb;24(2):221–30. doi: 10.1359/jbmr.081011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rapuri PB, Gallagher JC, Balhorn KE, Ryschon KL. Alcohol intake and bone metabolism in elderly women. Am J Clin Nutr. 2000 Nov;72(5):1206–13. doi: 10.1093/ajcn/72.5.1206. [DOI] [PubMed] [Google Scholar]
- 49.Sripanyakorn S, Jugdaohsingh R, Mander A, Davidson SL, Thompson RP, Powell JJ. Moderate ingestion of alcohol is associated with acute ethanol-induced suppression of circulating CTX in a PTH-independent fashion. J Bone Miner Res. 2009 Aug;24(8):1380–8. doi: 10.1359/JBMR.090222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fakhrawi DH, Beeson L, Libanati C, Feleke D, Kim H, Quansah A, Darnell A, Lammi-Keefe CJ, Cordero-MacIntyre Z. Comparison of body composition by bioelectrical impedance and dual-energy x-ray absorptiometry in overweight/obese postmenopausal women. J Clin Densitom. 2009 Apr-Jun;12(2):238–44. doi: 10.1016/j.jocd.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 51.Thomson R, Brinkworth GD, Buckley JD, Noakes M, Clifton PM. Good agreement between bioelectrical impedance and dual-energy X-ray absorptiometry for estimating changes in body composition during weight loss in overweight young women. Clin Nutr. 2007 Dec;26(6):771–7. doi: 10.1016/j.clnu.2007.08.003. [DOI] [PubMed] [Google Scholar]