Abstract
Background
Electroencephalographic (EEG) sleep slow wave activity (SWA; EEG power between 0.6–4 Hz) has been proposed as a marker of central synaptic plasticity. Decreased generation of sleep slow waves—a core feature of sleep in depression—indicates underlying plasticity changes in the disease. Various measures of SWA have previously been used to predict antidepressant treatment response. This study examined the relationship between baseline patterns of SWA in the first two NREM episodes and antidepressant response to an acute infusion of the N-methyl-D-aspartate (NMDA) antagonist ketamine.
Methods
Thirty patients (20M, 10F, 18–65) fulfilling DSM-IV criteria for treatment-resistant major depressive disorder (MDD) who had been drug-free for two weeks received a single open-label infusion of ketamine hydrochloride (.5 mg/kg) over 40 minutes. Depressive symptoms were assessed with the Montgomery-Asberg Depression Rating Scale (MADRS) before and after ketamine infusion. Sleep recordings were obtained the night before the infusion and were visually scored. SWA was computed for individual artifact-free NREM sleep epochs, and averaged for each NREM episode. Delta sleep ratio (DSR) was calculated as SWANREM1 / SWANREM2.
Results
A significant positive correlation was observed between baseline DSR and reduced MADRS scores from baseline to Day 1 (r=.414, p=.02).
Limitations
The sample size was relatively small (N=30) and all subjects had treatment-resistant MDD, which may limit the generalizability of the findings. Further studies are needed to replicate and extend this observation to other patient groups.
Conclusions
DSR may be a useful baseline predictor of ketamine response in individuals with treatment-resistant MDD.
Keywords: biomarker, delta sleep ratio (DSR), major depressive disorder (MDD), N-methyl-D-aspartate (NMDA) receptor, slow wave activity (SWA)
1. Introduction
Mood disorders are among the most debilitating and widespread psychiatric illnesses. Novel and more effective therapeutics are urgently needed, given the delayed onset and poor response associated with currently available monoamine-based therapies. Towards this end, the development of new treatments for mood disorders would be greatly facilitated by the identification of biomarkers to predict treatment outcomes and assist in treatment selection (Li et al., 2012; Wiedemann, 2011).
Recent studies have shown that the N-methyl-D-aspartate (NMDA) antagonist ketamine produces significant antidepressant effects in patients with treatment-resistant major depressive disorder (MDD), with the largest effect observed within one day (Ibrahim et al., 2012; Zarate et al., 2006). Several neurobiological correlates have been associated with ketamine response, including markers of pre-treatment prefrontal glutamine levels and neural activity of the anterior cingulate cortex (Salvadore et al., 2009; Salvadore et al., 2010; Salvadore et al., 2011).
Slow wave sleep has long been observed to be decreased in individuals with depression (Ehlers et al., 1996; Gillin et al., 1979; Kupfer and Ehlers, 1989; Reynolds et al., 1993). Delta Sleep Ratio (DSR)—the ratio of sleep slow wave activity (SWA; or wave count) between the first two non-REM sleep episodes—is lower in depressed patients than healthy controls (Kupfer et al., 1990). Furthermore, some traditional antidepressant treatments have been found to normalize slow wave sleep and DSR (Argyropoulos et al., 2009; Ehlers et al., 1996; Jindal et al., 2003). Relatedly, preclinical EEG studies have shown that the NMDA antagonists ketamine and dizocilpine-maleate (MK-801) both increased SWA in rats (Feinberg and Campbell, 1993), and clinical studies have identified DSR as a useful predictor of treatment outcome in individuals with MDD (Ehlers et al., 1996; Nissen et al., 2001).
This study examined whether baseline SWA (and DSR specifically) would predict rapid response to ketamine infusion in individuals with treatment-resistant MDD. We hypothesized that pre-treatment DSR would be associated with improved depressive symptoms in response to ketamine.
2. Methods
2.1 Patients
Thirty patients (20M, 10F, 18–65 years (mean age=47.3 SE±13.3)) participated in the study from January 2007 through December 2010 at the National Institute of Mental Health (NIMH) Clinical Research Center (CRC) Mood Disorders Research Unit in Bethesda, MD. All patients met DSM-IV criteria for treatment-resistant MDD as assessed by the Structured Clinical Interview for Axis I DSM-IV Disorders (SCID; (First et al., 2001)) and the Antidepressant Treatment History Form (ATHF)-modified (Sackeim, 2001). Inclusion criteria included a Montgomery Asberg Depression Rating Scale (MADRS) score ≥ 22 at baseline and previous failure to respond to at least two antidepressants. Exclusion criteria included a diagnosis of substance abuse or dependence in the last 90 days as determined by the SCID, or unstable medical illness. All patients were free of all psychotropic medications for at least two weeks (five weeks for fluoxetine) prior to ketamine infusion. Patients’ average MADRS score at baseline was 32.5±4.8 (SEM). The study was approved by the Combined Neuroscience Institutional Review Board (IRB) of the National Institutes of Health (NIH). All subjects provided written informed consent before entry into the study.
2.2 Infusion
Patients underwent a single open-label, 40-minute infusion of saline solution and ketamine hydrochloride (.5 mg/kg), followed by the double-blind administration of riluzole (100mg/day; 50 mg BID) or placebo four to six hours post-infusion. These patients were a subgroup of a larger, previously published study investigating the ability of riluzole (another glutamatergic modulator) to maintain ketamine’s initial antidepressant response (ketamine/placebo, n=11; ketamine/riluzole, n=19) (Ibrahim et al., 2012). That study found no difference in efficacy between the ketamine/placebo and ketamine/riluzole groups (Ibrahim et al., 2012), nor was any difference observed in sleep EEG variables between the groups (Duncan Jr. et al., in press). In the current study, group differences in correlations were examined to exclude the possibility of an early interactive effect between the two compounds.
2.3 Mood Assessment
For the initial analysis, depressive symptoms were assessed via the MADRS at baseline (60 minutes prior to ketamine infusion) and Day 1 post-infusion. Patients exhibiting a ≥50% reduction in MADRS scores on Day 1 post-infusion were classified as ketamine responders (n=12); non-responders (n=18) showed less than 50% improvement. Day 1 was selected as the primary endpoint for this analysis as it represents the timepoint with the largest clinical effect (Ibrahim et al., 2012).
In addition, daily MADRS ratings for the next seven days were obtained from the larger, previously published study (Ibrahim et al., 2012) and used to examine the relationship between baseline DSR and prolonged response to ketamine.
2.4 EEG Analysis
Whole-night sleep recordings were taken following an adaptation night on the baseline night before ketamine infusion. Two electroencephalograms (C3/A2 and C4/A1), two electrooculograms (EOGs), and one submental electromyogram (EMG) were recorded using a Nihon-Khoden system (Neurofax v. 05–50) and Polysmith Acquisition and Review software (v. 4.0.25.0). EEG readings were scored in 30 second epochs according to criteria established by Rechtschaffen and Kales (Rechtschaffen and Kales, 1968) by reviewers blind to night of study.
EEG signals were first filtered from .5–30 Hz. Spectral density analysis (Welch’s averaged modified periodogram with a Hamming window, averages of six five-second epochs) was performed on C3/A2 and C4/A1 derivations. Awake, REM, Movement Time, and NREM epochs that exceeded eight times the mean power values in the .75 to 4.5 Hz and 20 to 30 Hz bands were excluded from the analysis. Because SWA values were identical at both C3 and C4, averaged SWA values were used. For each subject the total night SWA was calculated. For each NREM period throughout the night, SWA was normalized using individual total night EEG power estimates. The DSR (Jindal et al., 2003; Nissen et al., 2001) was calculated as the quotient of normalized SWA in the first to the second NREM episode.
2.5 Statistical Analysis
Pearson correlations were used to examine the relationship between the baseline DSR and change in depression rating scales from 60 minutes pre-infusion to Day 1. Partial correlations and Fisher r-z transformations controlling for the effect of age and drug (riluzole administered post-ketamine infusion) were also completed. Paired t-tests were used to compare baseline sleep data for the responder and non-responder groups. ANOVA was used to examine the predictive effects of baseline DSR on mood response during the first week after dividing the patient group based on DSR threshold ≤ 1.
3. Results
3.1 Baseline sleep measures between ketamine responders and non-responders
No significant group differences were observed for sleep variables (Table 1), although sleep quality was slightly reduced in ketamine responders (n=12) versus non-responders (n=18), i.e., less total sleep, reduced sleep efficiency, and lower SWA. There was a trend (p=.067) for non-responders to have lower baseline NREM1 SWA and DSR than responders.
Table 1.
Baseline Sleep in Ketamine Responders (R) and Non-Responders (NR)
| WHOLE NIGHT SLEEP |
Non-Responder | Responder | t=; p= |
|---|---|---|---|
| Total Sleep Time (mins) |
386.1944* (25.15797) |
359.5417 (12.21531) |
0.819; 0.420 |
| Sleep Latency (mins) |
25.8056 (5.30971) |
18.2917 (3.22541) |
1.067; 0.295 |
| REM Latency (mins) |
43.3056 (6.15634) |
57.4583 (8.88957) |
−1.354; 0.187 |
| Wake % | 9.9000 (2.78342) |
13.0083 (3.13196) |
−0.729; 0.472 |
| REM % | 23.4944 (2.05545) |
21.0750 (1.90673) |
0.816; 0.421 |
| SWS % | 3.4444 (.81446) |
2.8417 (1.15243) |
0.440; 0.663 |
| Sleep Efficiency % | 89.1256 (2.86797) |
86.0083 (3.00316) |
0.727; 0.473 |
| SWA MEASURES | |||
| Total SWA | 49.3805 (6.9482) |
35.9261 (4.5727) |
1.443; 0.160 |
| NREM1 SWA | 60.4827 (7.8037) |
41.3455 5.7701) |
1.791; 0.084 |
| NREM2 SWA | 56.4102 (8.8214) |
43.3941 4.7298) |
1.130; 0.268 |
| Delta Sleep Ratio (DSR) |
1.1775 (.06354) |
.9799 (.08407) |
1.905; 0.067 |
Mean ± (SEM)
3.2 Baseline DSR was associated with changes in depression rating scores after a night of sleep
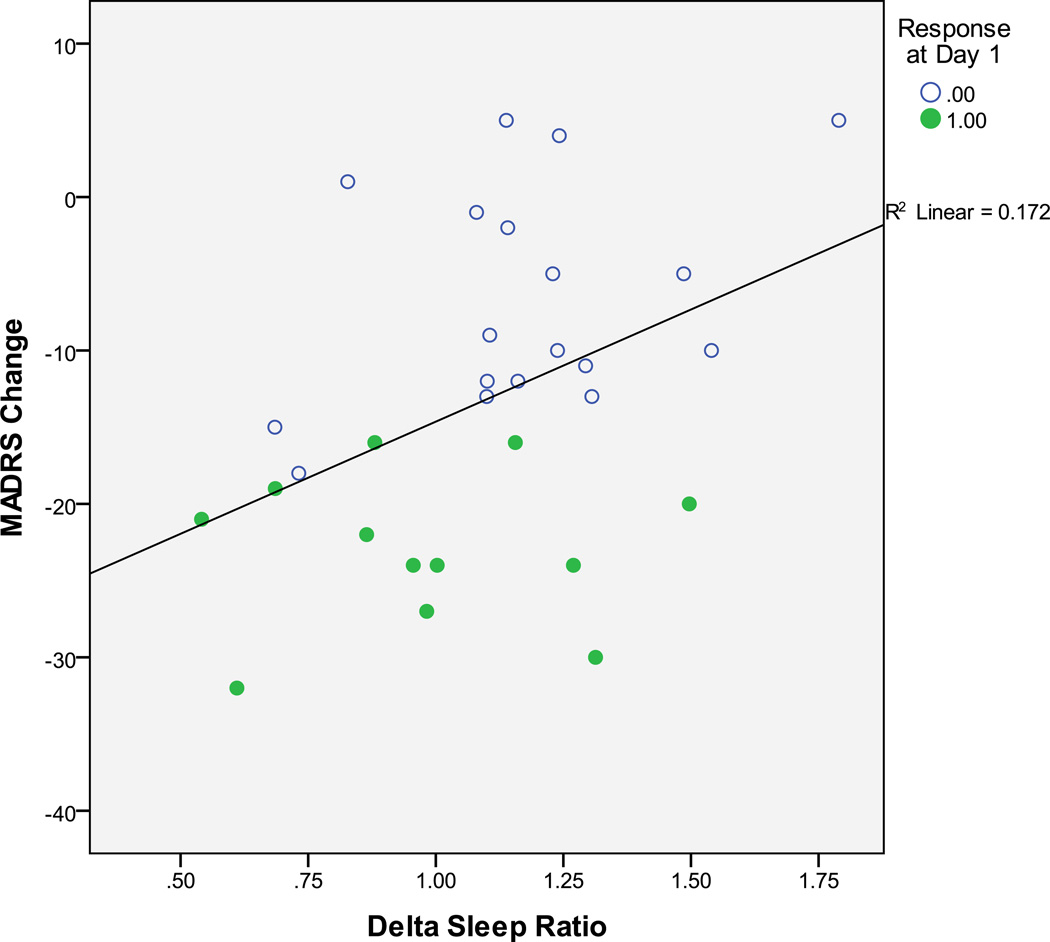
No significant correlation was observed between baseline DSR and change in MADRS scores from baseline to 230 minutes post-infusion (Pearson’s r=.217, p=.259). However, after a night of sleep following ketamine infusion, a significant positive correlation (r=.414, p=.02) was observed between baseline DSR and change in MADRS scores (from baseline to Day 1; Figure 1). No Day 1 differences were found in mood or gender between the ketamine/placebo and ketamine/riluzole subgroups, and the Fisher r-to-z transformation (Rosenthal, 1991) found no differences in DSR-MADRS correlations between these two groups. Correlations were significant when controlling for age (Day 1 change: r=.431, p=.02). Visual inspection of the relationship between baseline DSR and change in MADRS scores at Day 1 indicated that eight of 12 ketamine responders had DSRs ≤ 1, and 15 of 18 non-responders had DSRs > 1 (Chi-square=7.75; p=.0054).
Figure 1.
The delta sleep ratio (DSR: quotient of the SWA from the first to the second NREM episode) during the baseline night correlated with change in Montgomery Asberg Depression Rating Scale (MADRS) score from baseline to Day 1 (r=.414, p=.02) for MDD patients in the previously published studies investigating riluzole administration post-ketamine infusion. Ketamine responders are indicated by filled circles.
In order to examine the contribution of the MADRS sleep item to these relationships, correlations between baseline DSR and change in MADRS scores were examined after omitting MADRS Item 4 (Reduced Sleep). Correlations remained significant on Day 1 (r=.432, p=.017), even without Item 4.
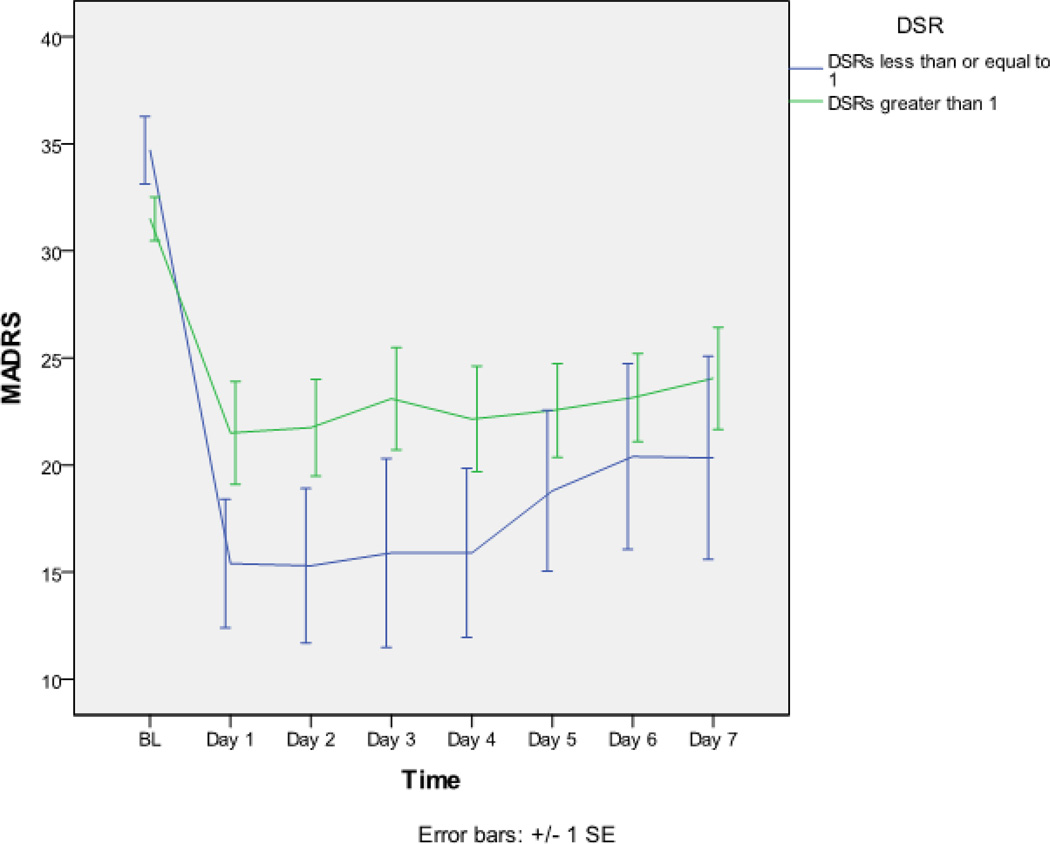
After dividing the patient group based on a threshold DSR ≤ 1 (SWANREM1 = SWANREM2), MADRS ratings over seven days showed a significant interaction between patients with low and high DSR scores (F=2.573; df =7, 195; p=.015). Individuals with low DSR scores showed a greater and more sustained clinical improvement than individuals with higher DSR scores (Figure 2).
Figure 2.
Average Montgomery Asberg Depression Rating Scale (MADRS) ratings from baseline until Day 7 for patients separated into two groups based on a baseline threshold value of delta sleep ratio (DSR) ≤ 1. MADRS ratings over the seven days showed a significant interaction between patients with low and high DSR scores (F=2.573; df =7, 195; p=.015); individuals with low DSR scores showed a greater and sustained clinical improvement in response to ketamine than individuals with higher DSR scores.
4. Discussion
This is the first study to demonstrate that DSR may be a useful predictor of rapid antidepressant treatment response to the NMDA receptor antagonist ketamine. Specifically, baseline DSR was significantly correlated with change in MADRS scores on Day 1 after ketamine infusion; low baseline DSR scores predicted better mood response, and high scores predicted poor response (Figure 1). Consistent with this correlation, more ketamine responders were found to have DSR scores ≤ 1. Furthermore, low baseline DSR scores predicted better mood response during the following week, indicating that the Day 1 rating preceded a more prolonged response (Figure 2).
Normal sleep patterns are characterized by elevated SWA in NREM1, followed by successive SWA declines in NREM2 and NREM3. This normal decline typically yields a DSR score > 1. In the two-process model of sleep regulation (Borbely, 1982), the normal decline in SWA represents the homeostatic component of sleep regulation that discharges during sleep until waking begins; the homeostatic component then accumulates during the waking phase until an upper threshold initiates a new sleep cycle. In contrast, sleep patterns in depressed individuals are often characterized by a blunted early SWA, followed by increased SWA, and DSR scores ≤ 1. Thus, DSR scores ≤ 1 suggest disrupted homeostatic regulation of sleep, and the possibility of normalization by successful clinical intervention.
The relationship between DSR and change in MADRS score was present on Day 1 (the day after ketamine infusion and after a night of sleep) but not on the baseline day (four hours after the ketamine infusion) when a rapid improvement in mood was first observed. This suggests that ketamine’s effects on subsequent sleep (induced during waking and affecting synaptic potentiation, or later during sleep and affecting synaptic downscaling [see below]) could contribute to next-day mood improvement. This possibility is particularly interesting because previous studies found that ketamine infusion increased total sleep time, reduced waking, and increased SWA in the first non-REM episode on the night prior to Day 1 (Duncan Jr. et al., in press). It is also interesting to note that the correlation between baseline DSR and change in MADRS score on Day 1 occurred independently of the MADRS sleep item. It is possible that small subclinical changes in sleep quality not evident between responders and non-responders (Table 1), or non-visible changes in sleep fragmentation (Martin et al., 1997), contributed to lowered scores on non-sleep items, and the correlation between DSR and change in MADRS score. Additional rating instruments would be required to evaluate the possible contribution of sleep change to ketamine’s effects on mood.
Several prior studies used DSR to predict treatment outcome in mood disorders. Some studies used baseline DSR to predict outcome, while others used DSR as a mediator of treatment outcome. Consistent with the results of the present study, Ehlers and colleagues reported that clomipramine infusion increased “next day” DSR in subsequent responders, but not non-responders (Ehlers et al., 1996). In that study, clomipramine responders—but not non-responders—had decreased early levels of SWA at baseline, suggesting that low baseline DSR was associated with response to clomipramine. Other treatments have also been reported to mediate DSR increases, but with no difference between responders and non-responders to treatment (Argyropoulos et al., 2009; Jindal et al., 2003); this suggests that for some treatments, increased DSR may not be sufficient to produce a beneficial treatment response. In contrast to the current study, increased baseline DSR predicted next day sleep deprivation response (Nissen et al., 2001), as well as maintenance response to interpersonal psychotherapy (Kupfer et al., 1990; Spanier et al., 1996), suggesting that the relationship between baseline DSR and clinical response may be specific to treatment method and patient history. Nevertheless, those findings are not at odds with the results of the present study, which are specific to a small group of patients with treatment-resistant MDD as well as ketamine treatment. Indeed, the two main limitations of the present study are the small sample size and the fact that patients had been diagnosed with treatment-resistant MDD. Larger studies are needed to replicate the finding, and to determine if the observation extends to other patient groups.
The synaptic homeostasis hypothesis argues that the discharge of SWA during sleep corresponds to synaptic downscaling (Tononi, 2006), a process with restorative and cognitive benefits. In contrast, the waking homeostatic component corresponds to synaptic strengthening, a process controlled in part by plasticity-related genes (e.g., brain derived neurotrophic factor (BDNF), activity-regulated cytoskeleton-associated protein (Arc), nerve growth factor 1-alpha (NGF1a)), and is expressed by the early discharge of sleep slow waves. The fact that ketamine responders had reduced early discharge of sleep slow waves (as indicated by their low SWA and DSR scores) is consistent with the view that ketamine responders—like other patients with MDD (Goldstein et al., 2011; Landsness et al., 2011)—exhibit decreased synaptic homeostasis at baseline. Furthermore, the ability of ketamine to increase SWA (Feinberg and Campbell, 1993) is consistent with both synaptic strengthening and antidepressant efficacy, a property shared by other interventions with rapid antidepressant effects, such as sleep deprivation (Duncan Jr. et al., 1980; Gillin, 2001; Landsness et al., 2011).
Overall, these preliminary data suggest that DSR may be a useful baseline biomarker of response to ketamine, with important implications for understanding the pathophysiology of MDD and identifying biomarkers of treatment response in the quest to develop novel and more effective therapeutics for this disorder.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argyropoulos SV, Hicks JA, Nash JR, Bell CJ, Rich AS, Nutt DJ, Wilson S. Redistribution of slow wave activity of sleep during pharmacological treatment of depression with paroxetine but not with nefazodone. J Sleep Res. 2009;18:342–348. doi: 10.1111/j.1365-2869.2008.00724.x. [DOI] [PubMed] [Google Scholar]
- Borbely A. Two process model of sleep regulation. Hum Neurobiology. 1982;1:195–204. [PubMed] [Google Scholar]
- Duncan WC, Jr, Gillin JC, Post RM, Gerner RH, Wehr TA. Relationship between EEG sleep patterns and clinical improvement in depressed patients treated with sleep deprivation. Biol Psychiatry. 1980;15:879–889. [PubMed] [Google Scholar]
- Duncan WC, Jr, Sarasso S, Ferrarelli F, Selter J, Riedner BA, Hejazi NS, Yuan P, Brutsche N, Manji HK, Tononi G, Zarate CA., Jr Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharmacol. doi: 10.1017/S1461145712000545. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Havstad JW, Kupfer DJ. Estimation of the time course of slow-wave sleep over the night in depressed patients: effects of clomipramine and clinical response. Biol Psychiatry. 1996;39:171–181. doi: 10.1016/0006-3223(95)00139-5. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Campbell IG. Ketamine administration during waking increases delta EEG intensity in rat sleep. Neuropsychopharmacology. 1993;9:41–48. doi: 10.1038/npp.1993.41. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams AR. Structured Clinical Interview for DSM-IV TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: New York State Psychiatric Institute, Biometrics Research; 2001. [Google Scholar]
- Gillin JC, Buchsbaum M, Wu J, Clark C, Bunney W. Sleep deprivation as a model experimental antidepressant treatment: findings from functional brain imaging. Depress Anxiety. 2001;14:37–49. doi: 10.1002/da.1045. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Duncan W, Pettigrew KD, Frankel BL, Snyder F. Successful separation of depressed, normal, and insomniac subjects by EEG sleep data. Arch Gen Psychiatry. 1979;36:85–90. doi: 10.1001/archpsyc.1979.01780010091010. [DOI] [PubMed] [Google Scholar]
- Goldstein MR, Plante DT, Hulse BK, Sarasso S, Landsness EC, Tononi G, Benca RM. Overnight changes in waking auditory evoked potential amplitude reflect altered sleep homeostasis in major depression. Acta Psychiatr Scand. 2011 Nov 19; doi: 10.1111/j.1600-0447.2011.01796.x. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA., Jr Course of Improvement in Depressive Symptoms to a Single Intravenous Infusion of Ketamine vs Add-on Riluzole: Results from a 4-Week, Double-Blind, Placebo-Controlled Study. Neuropsychopharmacology. 2012 Feb 1; doi: 10.1038/npp.2011.338. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal RD, Friedman ES, Berman SR, Fasiczka AL, Howland RH, Thase ME. Effects of sertraline on sleep architecture in patients with depression. J Clin Psychopharmacol. 2003;23:540–548. doi: 10.1097/01.jcp.0000095345.32154.9a. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Ehlers CL. Two roads to rapid eye movement latency. Arch Gen Psychiatry. 1989;46:945–948. doi: 10.1001/archpsyc.1989.01810100087016. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, McEachran AB, Grochocinski VJ. Delta sleep ratio. A biological correlate of early recurrence in unipolar affective disorder. Arch Gen Psychiatry. 1990;47:1100–1105. doi: 10.1001/archpsyc.1990.01810240020004. [DOI] [PubMed] [Google Scholar]
- Landsness EC, Goldstein MR, Peterson MJ, Tononi G, Benca RM. Antidepressant effects of selective slow wave sleep deprivation in major depression: a high-density EEG investigation. J Psychiatr Res. 2011;45:1019–1026. doi: 10.1016/j.jpsychires.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Frye MA, Shelton RC. Review of pharmacological treatment in mood disorders and future directions for drug development. Neuropsychopharmacology. 2012;37:77–101. doi: 10.1038/npp.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SE, Wraith PK, Deary IJ, Douglas NJ. The effect of nonvisible sleep fragmentation on daytime function. Am J Respir Crit Care Med. 1997;155:1596–1601. doi: 10.1164/ajrccm.155.5.9154863. [DOI] [PubMed] [Google Scholar]
- Nissen C, Feige B, Konig A, Voderholzer U, Berger M, Riemann D. Delta sleep ratio as a predictor of sleep deprivation response in major depression. J Psychiatr Res. 2001;35:155–163. doi: 10.1016/s0022-3956(01)00021-8. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington, D.C.: US Government Printing Office; 1968. [Google Scholar]
- Reynolds CF, 3rd, Hoch CC, Buysse DJ, Houck PR, Schlernitzauer M, Pasternak RE, Frank E, Mazumdar S, Kupfer DJ. Sleep after spousal bereavement: a study of recovery from stress. Biol Psychiatry. 1993;34:791–797. doi: 10.1016/0006-3223(93)90068-o. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. Meta-Analytic Procedures for Social Research (Revised Edition) Newbury Park: Sage Publications; 1991. [Google Scholar]
- Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–17. [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, Jr, Manji HK. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009;65:289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, Carver F, Holroyd T, DiazGranados N, Machado-Vieira R, Grillon C, Drevets WC, Zarate CA., Jr Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35:1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, van der Veen JW, Zhang Y, Marenco S, Machado-Vieira R, Baumann J, Ibrahim LA, Luckenbaugh DA, Shen J, Drevets WC, Zarate CA. An investigation of amino-acid neurotransmitters as potential predictors of clinical improvement to ketamine in depression. Int J Neuropsychopharmacol. 2011:1–10. doi: 10.1017/S1461145711001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanier C, Frank E, McEachran AB, Grochocinski VJ, Kupfer DJ. The prophylaxis of depressive episodes in recurrent depression following discontinuation of drug therapy: integrating psychological and biological factors. Psychol Med. 1996;26:461–475. doi: 10.1017/s0033291700035546. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Wiedemann K. Biomarkers in development of psychotropic drugs. Dialogues Clin Neurosci. 2011;13:225–234. doi: 10.31887/DCNS.2011.13.2/kwiedemann. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]