Abstract
Rationale
ATP-binding cassette transporter A1 (ABCA1) plays a critical role in eliminating excess free cholesterol (FC) from tissues by effluxing cellular FC and phospholipids to lipid-poor apolipoprotein A-I. Macrophage ABCA1 also dampens pro-inflammatory MyD88-dependent Toll-like receptor signaling by reducing cellular membrane FC and lipid raft content, indicating a role of ABCA1 in innate immunity. However, whether ABCA1 expression has a role in regulating macrophage function in vivo is unknown.
Objective
We investigated whether macrophage ABCA1 expression impacts host defense function, including microbial killing and chemotaxis.
Methods and Results
Myeloid cell-specific ABCA1 knockout (MSKO) vs. wild type (WT) mice were infected with Listeria monocytogenes (Lm) for 36h or 72h before sacrifice. Lm-induced monocytosis was similar for WT and MSKO mice; however, MSKO mice were more resistant to Lm infection, with significantly less body weight loss, less Lm burden in liver and spleen, and less hepatic damage 3 days post infection. In addition, Lm-infected MSKO mouse livers had: 1) greater MCP-1 and MIP-2 expression, 2) more monocyte/macrophage infiltration, 3) less neutral lipid accumulation, and 4) diminished expression of lipogenic genes. MSKO macrophages showed enhanced chemotaxis toward chemokines in vitro and increased migration from peritoneum in response to LPS in vivo. Lm infection of WT macrophages markedly reduced expression of ABCA1 protein as well as other cholesterol export proteins (such as ABCG1 and apoE).
Conclusions
Myeloid-specific ABCA1 deletion favors host response to and clearance of Lm. Macrophage Lm infection reduces expression of cholesterol export proteins, suggesting that diminished cholesterol efflux enhances innate immune function of macrophages.
Keywords: Macrophage, ABCA1, chemotaxis, bacterial killing
INTRODUCTION
ATP-binding cassette transporter A1 (ABCA1) is a plasma membrane protein that functions to eliminate excess free cholesterol (FC) from tissues by effluxing cellular FC and phospholipids to lipid-free apolipoprotein AI, forming nascent HDL particles1, 2. ABCA1 plays a critical role in the movement of cholesterol from peripheral tissues to the liver in a process known as reverse cholesterol transport. Mutations that inactivate the human ABCA1 gene result in Tangier disease, which is characterized by extremely low plasma HDL cholesterol concentrations, mildly elevated plasma triglyceride levels, and accumulation of cholesterol in macrophages3–5. ABCA1 protein is widely expressed in most cells in the body, and its expression is regulated by transcriptional activation and protein degradation6, 7. Generation of cell-specific Abca1 knockout mice has helped define the role of cell-specific ABCA1 expression in whole body HDL biogenesis as well as several unanticipated roles for the transporter. For example, hepatocyte and intestinal epithelial cell ABCA1 contribute 70–80% and 20–30% of the plasma HDL pool, respectively8, 9. Pancreatic β cell ABCA1 plays a role in insulin secretion10 and brain ABCA1 regulates neuronal structure and function11.
Macrophages protect the host against exogenous and endogenous dangers by killing invading microbes and phagocytosing apoptotic or dead cells, thereby acting as one of the primary immune cell types involved in innate immunity. To explore the specific role of ABCA1 in macrophages, we generated myeloid cell-specific ABCA1 knockout (MSKO) mice12. Using this unique mouse model, we demonstrated that macrophages from MSKO mice have a significant increase in FC and are more proinflammatory in vivo and in vitro in response to lipopolysaccharide (LPS) via Toll-like receptor 4 (TLR4) compared with wild type (WT) mice. This response was mediated through a myeloid differentiation primary-response protein 88 (MyD88)-dependent pathway and was independent of alterations in plasma lipid concentrations12. The hypersensitivity of MSKO macrophages to LPS was most likely due to increased lipid raft content, presumably caused by increased intracellular FC accumulation12. Yvan-Charvet et al. observed a similar inflammatory phenotype in Abca1−/− Abcg1−/− macrophages compared with WT mice13. More recently, we demonstrated that macrophage ABCA1 deficiency leads to selective lipid raft FC accumulation without alteration of PL composition14. Macrophage ABCA1 deficiency also results in more TLRs residing in lipid rafts, resulting in enhanced TLRs activation14. Taken together, these data suggest that ABC transporters down-regulate TLR signaling by reducing FC enrichment in lipid rafts and the trafficking of TLRs into rafts.
In spite of the down-regulation of TLR signaling by ABCA1, little is known about whether ABCA1 expression impacts macrophage function, such as microbial killing. In the present study, we describe the innate immune response of MSKO mice during infection with the Gram-positive, facultative intracellular bacterium Listeria monocytogenes (Lm)15, a widely used model of intracellular bacterial infection, known to require virtually all aspects of the innate and adaptive immune responses for effective control. We documented here that myeloid cell-specific deletion of ABCA1 leads to a greater resistance to Lm infection in mice and enhanced chemotaxis, relative to WT control mice.
METHODS
An expanded Methods section is available in the Online Data Supplement.
Animals
WT and MSKO (homozygous) mice were generated as described previously12. Mice were backcrossed to a C57BL/6 background for six generations before use in the studies. All animal procedures were approved by the Wake Forest School of Medicine Animal Care and Use Committee.
Cell culture
Thioglycollate-elicited peritoneal macrophages (PMs) and bone marrow-derived macrophages (BMDM) from WT and MSKO littermate mice were cultured as previously described12.
In vivo Lm clearance experiments
Female mice (20–30 weeks old) were infected intraperitoneally (IP) with Lm 10403S at a dose of 5 ×104 Listeria/mouse. Thirty-six or 72h post infection, mice were sacrificed. Concentrations of cytokines/chemokines in plasma or liver lysates were measured using ELISA or Bioplex assay according to the manufacturer's instructions. Liver protein concentration was measured by BCA protein assay. Bacterial burden in spleen and liver were assessed by culturing serial dilutions of tissue homogenate on BHI agar plates for bacterial colony formation. Small portions of liver were fixed in 10% formalin for hematoxylin and eosin or immunohistochemistry staining.
Immunohistochemistry staining
Livers sections were incubated with the primary antibodies to CD68 (abD Serotec), Ly6B.2 (abD Serotec), cleaved caspase-3 (Cell Signaling) and MCP-1 (Novus Biologicals), followed by the biotinylated secondary antibody. The staining was visualized using ABC reagent (ABC vector kit; Vector) and AEC (Dako; for CD68, Ly6B.2 and cleaved caspase-3) or DAB substrate chromogen (Dako; for MCP-1). The percentage of liver sections covered by CD68+ cells (% CD68+ area) was calculated to indicate the intensity of hepatic CD68+ cells.
TUNEL assay
In Situ Cell Death Detection Kit, Fluorescein (Roche) was used to detect hepatic apoptosis in Lm-infected mouse liver sections according to the manufacturer's instructions.
Macrophage migration assay
In vitro chemotaxis of macrophages in response to MCP-1 and MIP-1 was performed in a 48-well microtaxis chamber as described before16. The in vivo migration assay was performed as described previously with minor modifications17, 18. Briefly, WT mice were injected IP with 1 ml of 10% thioglycollate to elicit sterile peritonitis. Three days later, the mice were similarly injected with an equal number (5×106) of BMDM from WT (labeled with cell tracker green CMFDA) and MSKO (labeled with cell tracker red CMPTX; both from Molecular Probes) mice. Twenty hours later, 400 ng LPS was injected IP into the mice. Three h later, mice were sacrificed, peritoneal cells were harvested, and the proportion of fluorescent-labeled macrophages in all peritoneal cells was analyzed by flow cytometry.
Western blotting and real time PCR
WT BMDMS were infected with Lm 10403S for 0, 6, 12, and 24h before harvesting protein using RIPA buffer containing proteinase inhibitor cocktails (Roche) and RNA using TRIzol reagent (Invitrogen), respectively. Protein expression was examined using Western blotting and mRNA expression was examined using real-time PCR.
Flow cytometry
After blocking the Fcγ receptor with purified anti-mouse CD16/CD32 antibody (Fcγ receptor III/II; BD Biosciences), blood, bone marrow cells, or macrophages were incubated at 4 °C for 30 min with isotype controls or the following Abs: FITC-anti-CD11b (M1/70, BD Pharmingen), PE-anti-Ly6C (AL-21, BD pharmingen), APC-anti-CD45 (30-F11, BD Pharmingen), Percp-Cy5.5-anti-Ly6G (RB6-BC6, eBioscience) or APC- CCR2 (475301, R&D Systems). Cell fluorescence was measured and analyzed with FlowJo software.
Statistics
Data are presented as the mean ± SD unless indicated otherwise. Differences were compared with two-tailed Student's t-test or one-way ANOVA using GraphPad Prism software. P < 0.05 was considered statistically significant.
RESULTS
MSKO mice are more resistant to Lm infection
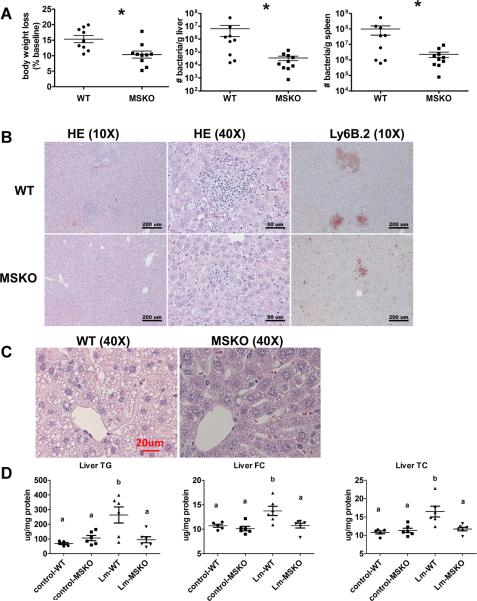
To investigate the potential involvement of macrophage ABCA1 in bacterial killing, we first challenged WT and MSKO mice with the gram-positive intracellular bacteria Lm; mice were sacrificed 3 days post infection. WT mice infected with Lm lost15% their body weight (Fig 1A). Weight loss was significantly less in Lm-infected MSKO mice, suggesting a milder effect of infection. Consistent with this finding, MSKO mice had significantly better clearance of Lm compared to WT mice, shown by an ~3-log lower liver Lm burden and a 2-log lower spleen Lm burden (Figure 1A). Livers of WT mice had numerous micro-abscesses consisting of a large number of neutrophils (Figure 1B, top panel); these were fewer and smaller in MSKO mice (Figure 1B, bottom panel). There were also multiple droplets in WT compared to MSKO livers (Figure 1C). Enzymatic lipid assays confirmed the marked lipid accumulation induced by Lm infection in WT liver, showing a significant increase in triglyceride, total cholesterol and free cholesterol concentrations in Lm-infected WT vs. MSKO or uninfected mouse liver (Figure 1D). Furthermore, this increased lipid accumulation induced by acute Lm infection was associated with decreased expression of lipogenic genes such as SREBP-1c and SCD1 and increased expression of the cholesterol esterification gene ACAT2 in infected WT liver (Online Figure I). Collectively, compared with WT mice, MSKO mice displayed a greater resistance to Lm infection with fewer bacteria in liver and spleen, and less hepatic histological damage and lipid accumulation, implying that myeloid cell-specific ABCA1 deletion protected mice from acute systemic Lm infection.
Figure 1. Myeloid cell-specific ABCA1 knockout (MSKO) mice are more resistant to Listeria Monocytogenes (Lm) infection.
Wild type (WT) and MSKO mice were i.p. infected with Lm (strain 10403S) for 3 days. (A) Body weight loss (left), and bacterial counts in liver (middle) and spleen (right) after 3 days of Lm infection. (B) Histological analysis of livers 3 days post Lm infection. Neutrophilic microabscesses are visualized by hematoxylin and eosin staining (objective magnification 10× and 40×) and immunohistochemical staining using an antibody against Ly6B.2 (neutrophil marker, objective magnification 40×). (C–D) Lipid analysis of livers 3 days post Lm infection. Hemtoxylin and eosin staining (objective magnification 40×) revealed massive accumulation of hepatic lipid droplets in Lm-infected WT mice relative to MSKO mice (C). Liver triglyceride (TG), free cholesterol (FC), and total cholesterol (TC) were measured by enzymatic assays (D). Data are expressed as mean ± SD. * P<0.05 and values with different letters are statistically different (P<0.05).
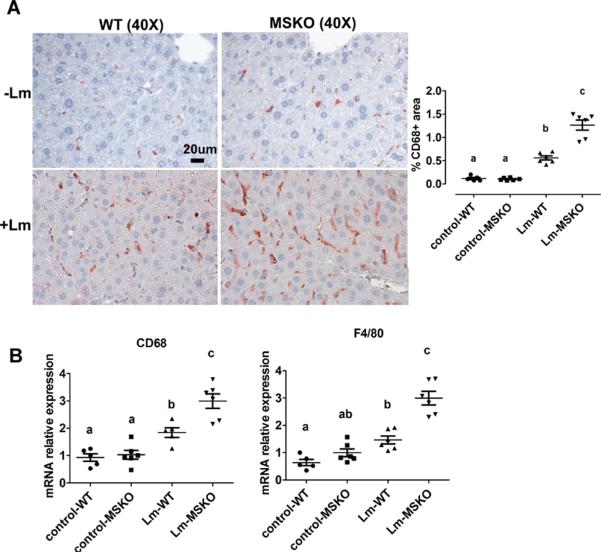
Increased hepatic monocyte/macrophage infiltration in Lm-infected MSKO mice
Macrophages and neutrophils are two major innate immune cells responsible for Lm clearance at the early stage of acute Lm infection. To examine whether the differences in Lm clearance between the two genotypes were attributable to differences in the number of immune cells in the liver, we first performed immunohistochemical staining to visualize monocyte/macrophages (using antibody against CD68) and neutrophils (using antibody against Ly6B.2) in livers of control (uninfected) and Lm-infected (3-day infection) mice. CD68+ cells in Lm-infected MSKO liver were greatly increased compared to WT and uninfected control mice, indicating significantly more macrophage infiltration in livers of MSKO mice (Figure 2A). Real-time PCR analysis confirmed the increased number of macrophages in livers of MSKO mice, shown by a significant greater expression of CD68 and F4/80 (macrophage markers) in MSKO liver compared to livers of uninfected and WT mice. There was also massive neutrophil influx in livers of both Lm-infected WT and MSKO mice (Online Figure II). Interestingly, the distribution of neutrophils in liver differed between two genotypes. Neutrophils formed large foci (abscesses) in livers of infected WT mice, and were almost absent outside of abscesses. However, in infected MSKO mice, neutrophils were more diffusely distributed throughout the liver (Figure 1B, right panel; Online Figure II). Since macrophages and neutrophils are central in Lm killing during early infection, the increased infiltration of monocytes/macrophages and more evenly distributed neutrophils in livers of MSKO mice may partially explain the significant improved Lm clearance.
Figure 2. MSKO mice had increased macrophage accumulation in liver 3 days post Lm infection.
WT and MSKO mice were i.p. infected with Lm (strain 10403S) for 3 days. (A) Monocytes/macrophages in liver were visualized by immunohistochemistry staining using antibody against CD68 (objective magnification 40×). CD68+ cells were quantified by Image Pro software. Data are presented as % CD68+ area to indicate the % liver sections occupied by CD68+ cells. (B) mRNA expression of CD68 and F4/80 was analyzed by real-time PCR. Data are expressed as mean ± SD. values with different letters are statistically different (P<0.05).
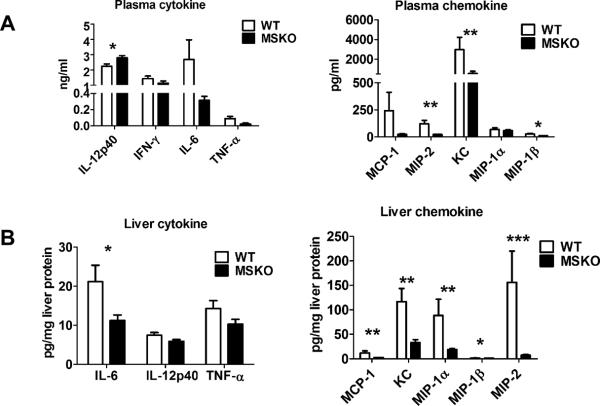
Reduced inflammatory responses in MSKO mice 3 days post Lm infection
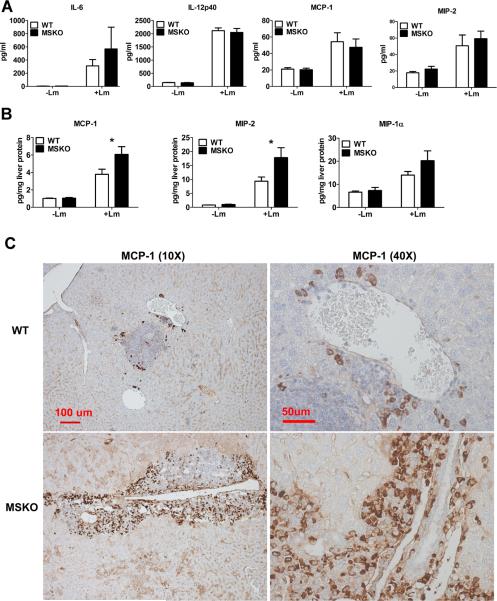
To further explore enhanced bacterial clearance in MSKO mice, we next assessed the expression of plasma and liver cytokines/chemokines in WT and MSKO mice three days after infection. Compared to WT, Lm-infected MSKO mice showed significantly increased plasma IL-12p40, but a trend towards decreased IFN-γ, IL-6, and TNF-α concentrations (Figure 3A, left panel). The levels of plasma chemokines (MCP-1, MIP-2, KC and MIP-1β) were also generally lower in MSKO vs. WT mice (Figure 3A, right panel). Furthermore, IL-6 was significantly reduced in livers of Lm-infected MSKO versus WT mice, but IL-12p40 and TNF-α production was similar. MSKO mice showed a significant reduction in liver chemokines compared to WT mice. Messenger RNA levels of liver cytokine/chemokine expression analyzed by real-time PCR were consistent with protein concentrations (Online Figure III), indicating less inflammation in the local microenvironment. The reduced Lm burden and the reduced plasma and liver cytokine/chemokine profile 3 days post infection suggests a more rapid and efficient resolution of infection and inflammation in MSKO vs. WT mice.
Figure 3. Cytokine/chemokine expression in mouse plasma and liver 3 days post Lm infection.
(A) WT and MSKO mice were infected intraperitoneally with Lm (strain 10403S) for 3 days. Plasma cytokine and chemokine concentrations measured by ELISA (left) and Bioplex assay (right), respectively, 3 days after infection. (B) Liver cytokine/chemokine concentrations were quantified by Bioplex assay. Liver protein concentrations were measured by BCA protein assay. Data are expressed as mean ± SD. * P<0.05, ** P<0.01, and *** P<0.001.
Apoptosis does not account for the differential macrophage accumulation in Lm-infected MSKO vs. WT mice
Expression of ABCA1 is highly regulated by LXR in macrophages19. LXR activation protects mice from Lm infection by regulating apoptosis inhibitor of macrophages (AIM, also known as SPα) to reduce macrophage apoptosis20, 21. In our study, SPα may have been induced in ABCA1-deficient macrophages due to sterol accumulation and LXR activation, resulting in the increased hepatic accumulation of monocytes/macrophages in Lm-infected MSKO vs. WT mice. However, although FC was slightly but significantly elevated in MSKO vs. WT macrophages, as shown previously12, macrophage oxysterol content did not differ between two genotypes (Online Figure IV A). Macrophage SPα expression was also indistinguishable between genotypes treated with or without LXR agonist (TO-901317) (Online Figure IV B). Finally, neither TUNEL assays nor immunohistochemical analysis of cleaved caspase-3 revealed a difference in apoptosis in immune cells in Lm-infected livers between the two genotypes (Online Figure IV C and D). Taken together, these data rule out a major role for apoptosis in the differential accumulation of hepatic monocytes/macrophages between genotypes.
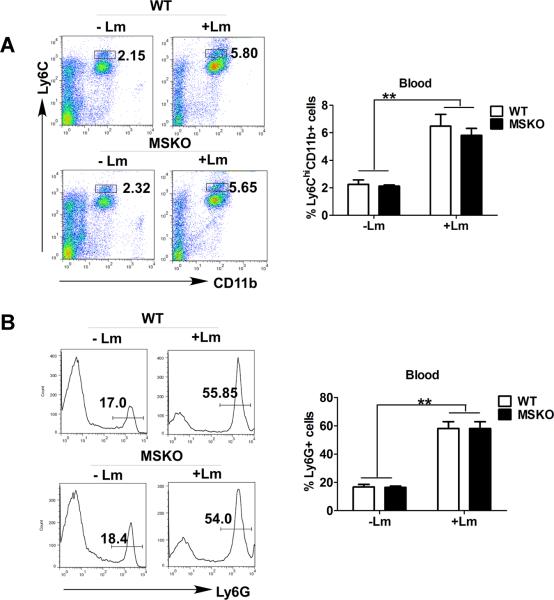
WT and MSKO mice had similar inflammatory monocyte egression from bone marrow to circulation
Infection of mice with Lm rapidly induces CCR2-dependent emigration of Ly6ChighCD11b+ monocytes from bone marrow to the circulation, which is essential for host defense against pathogens22. To determine whether the increased numbers of hepatic monocytes/macrophages in Lm-infected MSKO mice resulted from enhanced egress of Ly6Chigh monocytes from bone marrow to the circulation, we infected mice with Lm for 36 h and characterized the expression of the monocyte markers CD11b and Ly6C in blood and bone marrow leukocytes (defined as CD45+ cells) as described by Serbina and Pamer22. As shown in Figure 4A, compared to uninfected mice, Lm infection induced a significantly greater percentage of Ly6ChighCD11b+ cells in the bloodstream. There also were a significantly reduced percentage of Ly6ChighCD11b+ cells in bone marrow of Lm-infected mice versus controls (data not shown). In addition, we observed a marked increase in percentage of Ly6G+ cells (neutrophils) in blood circulation 36 h post Lm infection compared to uninfected mice (Figure 4B). However, the frequency distribution of circulating Ly6ChighCD11b+ cells and Ly6G+ cells did not differ between the two genotypes, suggesting indistinguishable egress of innate immune cells from bone marrow into bloodstream in response to acute Lm infection. Together, these data suggest that the greater accumulation of hepatic innate immune cells in MSKO mice 3 days post infection was likely not due to increased bone marrow egress of myeloid cells into blood.
Figure 4. Lm infection induced blood monocytosis and neutrophilia in mice.
WT (n=6) and MSKO (n=9) mice were infected intraperitoneally with Lm (strain 10403S) for 36h. (A) Lm infection resulted in increased Ly6Chigh (Ly6Chi) CD11b+ monocytes in peripheral blood relative to uninfected mice. Left panel: representative FACS plots of blood cells gated on CD45+ leukocytes and stained for CD11b and Ly6C; right panel: quantified data. (B) Lm infection resulted in increased Ly6G+ cells in peripheral blood. Left panels: representative FACS plots of blood cells gated on CD45+ leukocytes and stained for Ly6G; right panels: quantified data. Data are expressed as mean ± SD. ** P<0.01.
Increased chemokine expression in liver in MSKO mice at 36 h Lm infection
Since the profile of plasma and liver cytokines/chemokines three days post-infection suggested better resolved inflammation in MSKO mice, we measured plasma and liver cytokine/chemokine expression at an earlier time point (i.e., 36 h) after Lm infection. Lm-infected mice had increased plasma concentrations of cytokines (IL-6 and IL-12p40) and chemokines (MCP-1, MIP-2) compared to uninfected mice (Figure 5A), but there was no difference between genotypes, unlike results obtained three days after infection (Figure 3). However, Lm-infected MSKO mice had significantly greater hepatic expression of MCP-1 and MIP-2 and a trend towards increased MIP-1α expression (Figure 5B). Hepatic expression of cytokines (IL-6, TNF-α and IL-12p40) and other chemokines (MIP-1α and KC) was indistinguishable between the two genotypes (data not shown). MCP-1 was mainly expressed in perivascular infiltrated mononuclear leukocytes (Figure 5C). Of 6 liver sections from each genotype, only one WT mouse liver had MCP-1 positive perivascular inflammatory infiltrates, whereas 4 of 6 MSKO mouse livers showed massive perivascular leukocyte infiltrates with positive MCP-1 staining (Figure 5C). Since MCP-1 and MIP-2 are potent chemoattractants for macrophages and neutrophils, respectively23, increased hepatic MCP-1 and MIP-2 expression in Lm-infected MSKO mice during the early stages of infection may have promoted Lm clearance by rapid recruitment of myeloid cells into tissues involved in Lm clearance, such as the liver.
Figure 5. Cytokine/chemokine expression in mouse plasma and liver 36 h post Lm infection.
WT (n=6) and MSKO (n=9) mice were intraperitoneally infected with Lm (strain 10403S) for 36 h. (A) Plasma and liver (B) cytokine/chemokine concentrations were quantified by Bioplex assay. Liver protein concentration was measured by BCA protein assay. (C) MCP-1-positive cells in liver were visualized by immunohistochemical staining using antibody against MCP-1(objective magnification 10× and 40×). Data are expressed as mean ± SEM. * P<0.05.
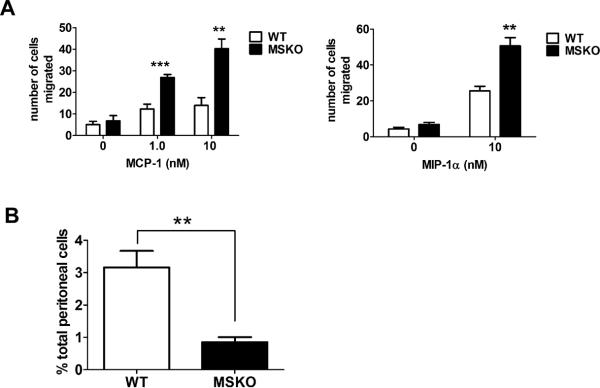
MSKO macrophages have enhanced chemotaxis
Whether ABCA1 is involved in regulation of macrophage chemotaxis is controversial16, 24. We first examined the chemotactic response of MSKO and WT macrophages to MCP-1 and MIP-1α using a 2-chamber chemotaxis assay. BMDM from MSKO mice showed significantly increased migration towards MCP-1 and MIP-1α gradients compared to WT mice (Figure 6A). This difference suggests that ABCA1 deficiency enhances macrophage migration in response to chemotactic factors, consistent with previous findings by Francone et al16. Similar data were obtained with thioglycollate-elicited peritoneal macrophages (Online Figure V). This enhanced chemotaxis to MCP-1 in MSKO macrophages was not associated with a difference in CCR2 surface expression (data not shown). Furthermore, the increased migration capacity of MSKO vs. WT macrophages was also observed in vivo, with more rapid egress of MSKO macrophages out of the peritoneum in response to LPS injection17, 18 (Figure 6B). Thus, the increased hepatic monocyte/macrophage infiltration in Lm-infected MSKO mice results both from enhanced chemokine expression in locally inflamed tissues, and from accelerated chemotaxis of ABCA1-deficient macrophages.
Figure 6. ABCA1-deficient macrophages display increased chemotaxis.
(A) Chemotactic response of bone marrow-derived macrophages from WT and MSKO mice to MCP-1 and MIP-1α was tested in a 48-well microchemotaxis chamber (see Methods). (B) Equal numbers of fluorescent-labeled BMDM from WT (cell tracker green CMFDA) and MSKO (cell tracker red CMPTX) mice were mixed and intraperitoneally injected into thioglycollate pre-conditioned WT mice followed by intraperitoneal injection of LPS. Three hours later, the proportion of fluorescent-labeled macrophages in peritoneal cells was analyzed by flow cytometry. Data are expressed as mean ± SD of at least 2 independent experiments. ** P<0.01 and *** P<0.001.
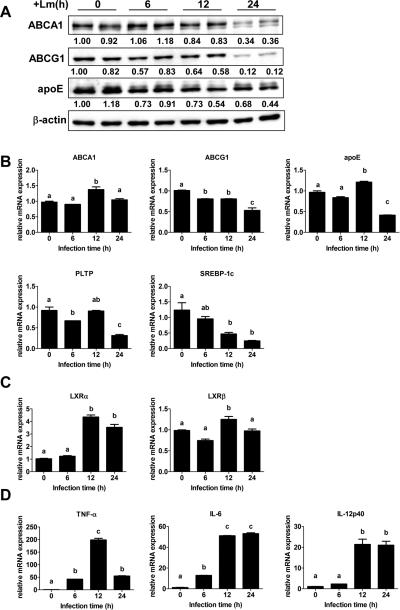
Lm infection down-regulates lipid export gene expression in WT macrophages
Gram negative bacterial (E. coli) or viral infection down-regulates LXR target gene expression, such as ABCA1 and apoE, via activation of transcription factor IRF3, but the physiological role of this down-regulation is unclear25. Lm up-regulates expression of the LXR target gene SPα, which inhibits macrophage apoptosis20, 21. To investigate whether Lm also regulates ABCA1 and other lipid metabolism related LXR target gene expression, we infected WT macrophages with Lm for 6, 12 and 24 h. Lm infection strongly inhibited the protein expression of ABCA1, ABCG1 and apoE, especially at 24 h post infection (Figure 7A). Lm infection also down-regulated mRNA expression of LXR target genes such as ABCG1, apoE, PLTP, and SREBP-1c, as measured by realtime PCR (Figure 7B). mRNA levels of ABCA1 were unchanged by Lm infection and ABCG1 mRNA expression was much less reduced compared to protein reduction induced by Lm, indicating that inhibition of these lipid export proteins induced by Lm infection most likely occurs at the post-transcriptional level. Interestingly, Lm infection significantly induced LXRα expression without alteration of LXRβ (Figure 7C), consistent with the findings of Joseph et al20. As expected, Lm infection induced expression of multiple inflammatory genes (Figure 7D). Since macrophages lacking ABCA1 more efficiently clear and eliminate Lm (Figure 1A), these results suggest that down-regulation of lipid export gene expression by Lm infection facilitates macrophage clearance of pathogens.
Figure 7. Lm infection inhibits cellular lipid export gene expression.
Bone marrow-derived macrophages from WT mice were infected with Lm for 0, 6, 12, and 24 h before analysis. (A) Protein expression in control and Lm-infected macrophages was analyzed by Western blots. β-actin was used as a loading control. The intensity of each target protein band was normalized to β-actin and the intensity of one uninfected sample was set to one; intensity of each protein band relative to the uninfected sample is shown under the gel bands. (B–D) mRNA expression of lipid export genes (B), LXRα, LXRβ (C) and inflammatory genes (D) were measured by real time PCR and was normalized to GAPDH. Data are expressed as mean ± SD of 2 independent experiments. Values with different letters are statistically different (P<0.05).
DISCUSSION
Macrophages play a critical role in innate immunity by killing invading pathogens and eliminating apoptotic cells. Macrophage ABCA1 expression dampens MyD88-dependent TLR signaling by facilitating FC efflux and reducing plasma membrane lipid rafts12–14. However, whether ABCA1 expression also impacts macrophage function, such as bacterial killing or chemotaxis, is less clear. In this study, we demonstrate that myeloid cell-specific ABCA1 deficiency renders mice more resistant to Lm infection and macrophage Lm infection down-regulates cholesterol export proteins. We also show that macrophage ABCA1 deletion accelerates macrophage migration toward chemoattractants, which may partially explain the enhanced bacterial killing in MSKO mice. Collectively, our data suggest that deletion of macrophage ABCA1 enhances macrophage function.
Gram-positive, facultative intracellular bacterium Lm is a widely used model of intracellular bacterial infection15. Bacteria are first internalized into a vacuole, also known as a phagosome. In the vacuoles, Lm secretes the pore-forming toxin Listeriolysin O and phospholipase C, which lyse phagosomal membranes and allow Lm to escape into cytosol. In the cytosol, the bacteria rapidly replicate, and recruit and polymerize host cell actin. The polymerization of actin at one pole of the cell produces energy to propel bacterial entry into neighboring cells. Lm infection stimulates macrophage MyD88-dependent secretion of IL-12 and TNF-α, which stimulates natural killer (NK) cells to generate IFN-γ, enhancing macrophage bactericidal action26, 27. Mice lacking MyD88, IL-12, or IFN-γ are more susceptible to Lm infection27, 28, indicating the important role of MyD88-dependent cytokine production in Lm clearance. Since our previous studies established that ABCA1-deficient macrophages are hyper-sensitive to MyD88-dependent TLR stimulation14, we hypothesized that MSKO mice may have enhanced Lm clearance with increased pro-inflammatory cytokine production due to exaggerated MyD88-dependent TLR response to Lm infection. In support of this hypothesis, we observed significantly better clearance of Lm in MSKO mice. However, the cytokine profile in mice at 36 h or 3 days post infection showed no differences in MSKO mice, suggesting that MyD88-dependent cytokine production induced by Lm infection does not play a significant role in the increased Lm clearance in MSKO mice. Alternatively, increased efficiency of Lm clearance in MSKO mice may have allowed a more rapid return of plasma cytokines to basal levels.
Compared to MSKO mice, WT mice infected with Lm showed marked hepatic triglyceride and cholesterol accumulation. In rodents, infection and inflammation stimulate adipose tissue lipolysis, and increase de novo hepatic fatty acid and cholesterol synthesis, coupled with suppression of fatty acid oxidation, decreased LDL clearance and conversion of cholesterol to bile acids29. In our study, the more severe Lm infection in WT vs. MSKO mice may have led to greater adipose tissue lipolysis, resulting in increased hepatic lipogenesis (fatty acid, triglyceride, and cholesterol) and lipid accretion. However, the marked hepatic lipid accumulation in Lm-infected mice resulted in down-regulation of de novo lipogenic genes (e.g. HMGCoA synthase, SREBP-1c, SCD1 and ACC1), and increased ACAT2 to reduce FC accumulation in liver by conversion of FC to CE (Online Figure I). The more efficient clearance of Lm in MSKO vs. WT mice reduced the inflammatory state of the liver and completely abrogated the increase in hepatic neutral lipid content (Figure 1 C and D).
Macrophages and neutrophils are two major innate immune cells responsible for Lm killing during the early phase of infection. Our results demonstrate that 3 days post infection, MSKO mice had significantly more monocytes/macrophages and neutrophils in the liver than WT mice, indicating an improved local microenvironment for bacterial clearance. We hypothesized that increased monocytes/macrophages in Lm-infected MSKO liver might result from: 1) decreased hepatic immune cell apoptosis, 2) increased migration of Ly6Chigh monocytes from bone marrow22, 3) increased hepatic chemokine production, and/or 4) increased chemotaxis of MSKO macrophages to the liver. Our data indicate that the increased macrophage and neutrophil accumulation in livers of MSKO mice is not due to the decreased apoptosis of hepatic leukocytes or enhanced recruitment of Ly6Chigh inflammatory monocytes from bone marrow to the bloodstream. We observed a significant elevation in hepatic MCP-1 and MIP-2 production and a marked increase in positive staining for MCP-1 in perivascular leukocyte infiltrates, in MSKO vs. WT mice at 36 h post Lm infection. MIP-2 is a potent neutrophil chemoattractant. Signaling via its receptor CCR2, MCP-1 is a potent monocyte/macrophage chemoattractant and is readily detected in liver and spleen of Lm-infected mice30. Mice lacking the CCR2 receptor are highly susceptible to Lm infection22, 31. Thus, increased hepatic MCP-1 and MIP-2 expression and increased MCP-1-producing leukocyte infiltration in MSKO mice may partially explain the more rapidly recruitment of monocytes/macrophages and neutrophils to the foci of infection, leading to a more efficient pathogen clearance. Macrophage MCP-1 production can be induced by WT Lm, but not by heat-killed or Listeriolysin O deficient Lm, even though macrophage TNF-α and IL-12p40 production is similar30, 32. These results suggest that Lm-induced MCP-1 production requires cytosolic invasion by bacteria, is independent of classic TLR-MyD88 signaling, and likely is mediated via unknown cytosolic receptors. If so, enhanced hepatic production of MCP-1, and perhaps MIP-2, in Lm-infected MSKO mice may result both from up-regulated MyD88-dependent TLR signaling and MyD88-independent cytosolic receptor signaling.
ABCA1 has been implicated in macrophage chemotaxis, although results have been conflicting16, 24. Using resident peritoneal macrophages, Francone et al16 observed a significant increase in chemotactic migration of Abca1−/−LDLr−/− vs. LDLr−/− macrophages, suggesting that ABCA1 expression limits macrophage migration toward chemokines. In contrast, using thioglycollate-elicited peritoneal macrophages, Pagler et al24 found no difference in macrophage migration toward chemokines between ABCA1-deficient and WT macrophages. Instead, they found that Abca1−/−Abcg1−/− vs. WT macrophages had increased plasma membrane FC and defective redistribution of sterol to the outer leaflet, which resulted in increased Rac1 signaling and impaired chemotaxis. Using both elicited PMs or BMDMs, we consistently observed a significant increase in chemotaxis of MSKO macrophages in response to MCP-1 and MIP-1α, consistent with Francone's finding. Meanwhile, our in vivo migration assay further supported enhanced chemotaxis of MSKO vs. WT macrophages. The enhanced chemotaxis in ABCA1-deficient macrophages provides another potential explanation for increased monocyte/macrophage infiltration in Lm-infected MSKO mice, although the underlying mechanism is unknown and still under investigation.
Expression of ABCA1, especially in macrophages, is highly regulated by LXR19, a nuclear receptor with an established role in lipid metabolism. Interestingly, one function of LXR is to regulate apoptosis inhibitor of macrophages (AIM, also known as SPα) to reduce macrophage apoptosis in response to Lm infection20, 21. Gram-negative bacteria, or bacterial products such as LPS, down-regulate LXR targeted gene expression related to lipid metabolism25, although the physiological role of this down-regulation remains unknown. In our current study, we observed that Lm infection, like Gram-negative bacterial infection, markedly reduced expression of ABCA1, ABCG1, apoE, and other LXR-responsive genes, mainly at the post-transcriptional level. Notably, macrophages lacking ABCA1 and/or ABCG1 are pro-inflammatory12–14. Thus, acute bacterial infection not only activates LXR to up-regulate SPα expression to prevent macrophages from apoptosis, but also lowers cholesterol transporter gene expression to mount a more robust inflammatory response that aids host defense against infections. However, infection may also worsen chronic inflammation-related diseases, such as atherosclerosis, by exaggerating foam cell formation due to down-regulation of cellular lipid export proteins.
In summary, we have shown that ABCA1 deficiency in myeloid cells protects the host against bacterial infection by accelerating macrophage chemotaxis and increasing chemokine expression in local infected tissue. Bacterial infection also down-regulates macrophage LXR-induced cholesterol export proteins. Thus, ABCA1 not only plays a key role in lipid metabolism, but also in innate immunity, suggesting a more diverse functional role for this transporter than originally envisioned. Our study also suggests that diminished myeloid cholesterol efflux enhances macrophage function and facilitates innate immunity against acute pathogen infection.
Supplementary Material
Novelty and Significance.
What Is Known?
ATP-binding cassette transporter A-I (ABCA1) is a plasma membrane protein that transports cellular free cholesterol (FC) and phospholipids to lipid-free apolipoprotein A-I, forming nascent HDL particles and eliminating excess FC from tissues.
ABCA1 attenuates macrophage inflammation by down-regulating Toll like receptor (TLR) signaling via reducing FC enrichment in membrane lipid rafts and the trafficking of TLRs into rafts.
What New Information Does This Article Contribute?
Myeloid cell–specific ABCA1 knockout (MSKO) mice are more resistance to acute infection with intracellular bacteria Listeria monocytogenes (Lm) compared to wild type (WT) mice.
MSKO mice infected with Lm have enhanced macrophage chemotaxis and increased hepatic chemokine expression, resulting in more rapid and efficient clearance and killing of Lm.
Lm infection reduces expression of macrophage cholesterol export proteins, suggesting that diminished myeloid cholesterol efflux enhances macrophage innate immune function.
Macrophages are one of the primary cell types involved in innate immunity and chronic inflammatory diseases, such as atherosclerosis, type 2 diabetes and metabolic syndrome. The membrane lipid transporter ABCA1 plays a key role in removing excess cholesterol from peripheral tissues for transport to liver for excretion. In macrophages, ABCA1 also attenuates inflammatory signaling by decreasing plasma membrane free cholesterol and lipid raft content. We evaluated the hypothesis that specific deletion of ABCA1 in myeloid cells (macrophages and neutrophils) protects mice from acute bacterial infection. We show that MSKO mice infected with Lm have less bacterial burden in liver and spleen concomitant with increased macrophage and neutrophil infiltration. Increased myeloid cell infiltration resulted from elevated tissue chemoattractant expression as well as enhanced migration of macrophages towards chemokine gradients in MSKO mice compared with WT controls. Bacterial infection markedly reduced expression of cellular lipid export proteins (e.g., ABCA1, ABCG1, apoE), mainly at the post-transcriptional level. Our data suggest that acute bacterial infection likely lowers cellular cholesterol export protein expression to mount a more robust inflammatory response that aids host defense against infections. However, acute infection or chronic low-grade inflammatory diseases, such as atherosclerosis, may exacerbate macrophage foam cell formation by down-regulating cholesterol export proteins, worsening outcome.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Karen Klein (Research Support Core, Wake Forest School of Medicine) for editing the manuscript and Dr. Michael J. Thomas (Department of Biochemistry, Wake Forest School of Medicine) and the Mass Spectroscopy Facility of the Comprehensive Cancer Center (5P30CA12197 and NCBI 2007-IDG-1021) of Wake Forest School of Medicine for the macrophage oxysterol measurements.
SOURCES OF FUNDING This study was supported by National Institutes of Health Grants HL49373, HL94525 (JSP) and by American Heart Association fellowship 09POST2250225 (XZ).
Non-standard Abbreviations
- ABC
ATP-binding cassette transporter
- BMDM
bone marrow-derived macrophages
- CE
cholesteryl ester
- FC
free cholesterol
- IP
intraperitoneally
- LM
Listeria monocytogenes
- LPS
lipopolysaccharide
- MSKO
myeloid cell-specific ABCA1 knockout
- MyD88
myeloid differentiation primary-response protein 88
- PL
phospholipids
- PM
peritoneal macrophages
- TLR4
Toll-like receptor 4
- WT
wild type
Footnotes
DISCLOSURES None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- (1).Attie AD, Kastelein JP, Hayden MR. Pivotal role of ABCA1 in reverse cholesterol transport influencing HDL levels and susceptibility to atherosclerosis. J. Lipid Res. 2001;42:1717–1726. [PubMed] [Google Scholar]
- (2).Oram JF, Lawn RM. ABCA1. The gatekeeper for eliminating excess tissue cholesterol. J. Lipid Res. 2001;42:1173–1179. [PubMed] [Google Scholar]
- (3).Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- (4).Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van DM, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J, Jr., Hayden MR. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- (5).Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denefle P, Assmann G. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- (6).Oram JF, Heinecke JW. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev. 2005;85:1343–1372. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- (7).Wang N, Chen W, Linsel-Nitschke P, Martinez LO, Agerholm-Larsen B, Silver DL, Tall AR. A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoA-I. J. Clin. Invest. 2003;111:99–107. doi: 10.1172/JCI16808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, Hussain MM, Parks JS, Kuipers F, Hayden MR. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 2006;116:1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, Hayden MR, Maeda N, Parks JS. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Brunham LR, Kruit JK, Pape TD, Timmins JM, Reuwer AQ, Vasanji Z, Marsh BJ, Rodrigues B, Johnson JD, Parks JS, Verchere CB, Hayden MR. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat. Med. 2007;13:340–347. doi: 10.1038/nm1546. [DOI] [PubMed] [Google Scholar]
- (11).Karasinska JM, Rinninger F, Lutjohann D, Ruddle P, Franciosi S, Kruit JK, Singaraja RR, Hirsch-Reinshagen V, Fan J, Brunham LR, Bissada N, Ramakrishnan R, Wellington CL, Parks JS, Hayden MR. Specific loss of brain ABCA1 increases brain cholesterol uptake and influences neuronal structure and function. J. Neurosci. 2009;29:3579–3589. doi: 10.1523/JNEUROSCI.4741-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J. Biol. Chem. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, Hiltbold EM, Fessler MB, Parks JS. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J. Lipid Res. 2010;51:3196–3206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Cossart P, Toledo-Arana A. Listeria monocytogenes, a unique model in infection biology: an overview. Microbes. Infect. 2008;10:1041–1050. doi: 10.1016/j.micinf.2008.07.043. [DOI] [PubMed] [Google Scholar]
- (16).Francone OL, Royer L, Boucher G, Haghpassand M, Freeman A, Brees D, Aiello RJ. Increased cholesterol deposition, expression of scavenger receptors, and response to chemotactic factors in Abca1-deficient macrophages. Arterioscler. Thromb. Vasc. Biol. 2005;25:1198–1205. doi: 10.1161/01.ATV.0000166522.69552.99. [DOI] [PubMed] [Google Scholar]
- (17).Cao C, Lawrence DA, Strickland DK, Zhang L. A specific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics. Blood. 2005;106:3234–3241. doi: 10.1182/blood-2005-03-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).van Gils JM, Derby MC, Fernandes LR, Ramkhelawon B, Ray TD, Rayner KJ, Parathath S, Distel E, Feig JL, Alvarez-Leite JI, Rayner AJ, McDonald TO, O'Brien KD, Stuart LM, Fisher EA, Lacy-Hulbert A, Moore KJ. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat. Immunol. 2012;13:136–143. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- (20).Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, Hogenesch J, O'connell RM, Cheng G, Saez E, Miller JF, Tontonoz P. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- (21).Valledor AF, Hsu LC, Ogawa S, Sawka-Verhelle D, Karin M, Glass CK. Activation of liver X receptors and retinoid X receptors prevents bacterial-induced macrophage apoptosis. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17813–17818. doi: 10.1073/pnas.0407749101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- (23).Wolpe SD, Davatelis G, Sherry B, Beutler B, Hesse DG, Nguyen HT, Moldawer LL, Nathan CF, Lowry SF, Cerami A. Macrophages secrete a novel heparin-binding protein with inflammatory and neutrophil chemokinetic properties. J. Exp. Med. 1988;167:570–581. doi: 10.1084/jem.167.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Pagler TA, Wang M, Mondal M, Murphy AJ, Westerterp M, Moore KJ, Maxfield FR, Tall AR. Deletion of ABCA1 and ABCG1 impairs macrophage migration because of increased Rac1 signaling. Circ. Res. 2011;108:194–200. doi: 10.1161/CIRCRESAHA.110.228619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Castrillo A, Joseph SB, Vaidya SA, Haberland M, Fogelman AM, Cheng G, Tontonoz P. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol. Cell. 2003;12:805–816. doi: 10.1016/s1097-2765(03)00384-8. [DOI] [PubMed] [Google Scholar]
- (26).Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc. Natl. Acad. Sci. U. S. A. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Seki E, Tsutsui H, Tsuji NM, Hayashi N, Adachi K, Nakano H, Futatsugi-Yumikura S, Takeuchi O, Hoshino K, Akira S, Fujimoto J, Nakanishi K. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J. Immunol. 2002;169:3863–3868. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- (28).Edelson BT, Unanue ER. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J. Immunol. 2002;169:3869–3875. doi: 10.4049/jimmunol.169.7.3869. [DOI] [PubMed] [Google Scholar]
- (29).Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- (30).Jia T, Serbina NV, Brandl K, Zhong MX, Leiner IM, Charo IF, Pamer EG. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J. Immunol. 2008;180:6846–6853. doi: 10.4049/jimmunol.180.10.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J. Exp. Med. 1997;186:1757–1762. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Serbina NV, Kuziel W, Flavell R, Akira S, Rollins B, Pamer EG. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity. 2003;19:891–901. doi: 10.1016/s1074-7613(03)00330-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.